Nanofluidics for single-cell analysis
Zengnan Wu,Ling Lin
a Department of Bioengineering,Beijing Technology and Business University,Beijing 100048,China
b State Key Laboratory of Chemical Resource Engineering,Beijing University of Chemical Technology,Beijing 100029,China
ABSTRACT Living single-cell analysis is vital for cell biology,disease pathology,drug discovery and medical treatment.It is of great significance to reveal the law of creature and to explore the mechanism of serious disease.The conventional single cell analysis focuses on a large number of cells or cell lysis,in order to obtain the average information about cells.Therefore,it fails to analyze the real-time and continuous data of differences between the individual cells,thus limiting the development of many fields,such as biomedical.Nanofluidics based biochemical analysis exhibits advantages over conventional methods in terms of small sample volume,rapid turnaround time,straightforward operation,and efficient processing,which has been widely used in complex operations such as single cell capture,separation and single cell detection.Here we review the recent developments of nanofluidic technologies for single-cell analysis,with emphasis on cell trapping,treatment,and biochemical studies.The potential of nanofluidics-based single-cell analysis is discussed.
Keywords:Nanofluidics Single cell Biochemical analysis Microfluidics
1.Introduction
The single-cell is the basic unit of human life which must be in detail to reveal the laws of life and explore the mechanism of major diseases.The individualized differences and behaviors of single cells play a vital role in various key life processes(such as embryonic development,tissue repair,and tumorigenesis)[1–3].Real-time detection of single cells with extremely small sizes(10–20 μm in diameter),volume(picoliter(pL)-femtoliter(fL)),and sample size femtomole(fmol)-zeptomole(zmol)can help capture the heterogeneity and individualized differences among cells[4–6];especially,for specific single cells among the same type of cells,the different changes in the internal environment of a single cell caused by the extracellular environment can be studied[7–9].Single-cell analysis is an essential feature of cancer research and biology for identifying genes in cells and proteomics and for understanding the spatial and temporal diversity of physiology and pathology[10–12].To study the characteristics of single cells,biomedical scientists have performed numerous studies on the signal response of intracellular proteins,ions,and genes during cell growth,metabolism,and reproduction.Limited by the accuracy of the cell-operation method and the sensitivity of the detection method in small spaces,traditional cell analysis methods mainly employ numerous mixed cell samples,and the average information of the cells is obtained through strict statistical calculation methods.Such methods conceal the differences between individual cells,restricting the development of many fields such as biomedicine[13,14].Many investigations have demonstrated that individual cells,even for those identical in appearance,show cellto-cell variability caused by genetic or microenvironment variations.Therefore,there is an urgent need to develop a highly sensitive and integrated real-time detection platform for single cells.
The micro/nanofluidic chip technology developed in recent years has gradually become a new generation of single-cell research platforms.Since the microfluidic chip analysis technology was proposed in the 1990s,it has been widely studied and applied in the fields of biology,chemistry,and medicine and has become one of the current research hotspots[15–18].Micro/nanofluidic chips have rapidly developed into a relatively independent field with a high degree of interdisciplinarity and occupy a leading position in fields such as analytical chemistry and life sciences[19–21].The effective combination of micro-nanofluidic technology can integrate single cell capture and highly sensitive real-time dynamic analysis on a chip.Recently,miniaturization research has reduced the analysis space from the 50–500 μm scale to the 10–1000 nm scale and has integrated micro/nano channel platforms in microfluidic chips(Fig.1).
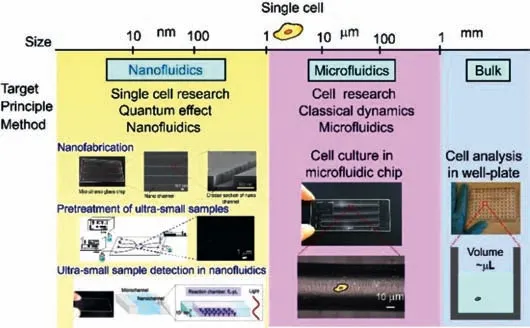
Fig.1.Comparison of microfluidics and nanofluidics technologies for single cell analysis.
The nanofluidic space is located between conventional nanotechnology and microtechnology,and the nano space is a transient space from single molecules to bulk condensed phase,and fluidics and chemistry are unknown.In this situation,a new discovery was made with specific phenomena within chemistry and fluidics by developing basic methodologies.This phenomenon was applied to specifically unique chemical operations,including ion selection and concentration.Nanofluidic technology is not only important for studying the unique phenomenon of liquid transport in nanochannels[22,23]but also provides a very small scale analysis space for the analysis of very small volume samples[24–27].In this review,we summarize the methods of cell analysis on nanofluidics and focus on several important analysis platforms.We also briefly discuss the analysis principles,applications,and prospects of nanofluidics in cell biology.
2.Fabrication of nanofluidic chip
Because of the small scale and unique properties of nanospaces(10–1000 nm),they have attracted the attention of the scientific community in recent years.Such nanospaces involve a transition from a single molecule to a condensed phase,exhibiting significant surface effects and material migration capabilities[28].The volume of the nanospace ranges from aL(=(100 nm)3)to fL(=(1000 nm)3),which is 104–103times smaller than the volume of a single cell;thus,it is a very suitable platform for performing single cell sample analysis.In existing studies,various manufacturing methods have been proposed to prepare nano-level channels;these methods are mainly divided into two types.One is the topdown preparation process.The nanochannel structure is directly prepared on a block substrate by cutting or grinding the substrate.The other is a bottom-up preparation process,which uses electron beam lithography to prepare nanochannels on quartz glass used to prepare nanochannels[29,30].The two substrates are bonded at a high temperature of 1080 °C by thermal bonding technology[31].In addition,to prevent the modified nanochannels from being destroyed at high temperatures,chemical bonding of silanol groups without high temperature bonding has also been reported[32–34].
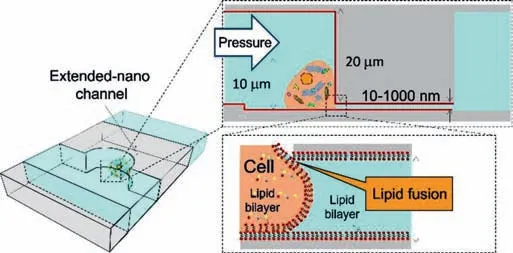
Fig.2.Concept of micro/nano sampling interface.A lipid bilayer was modified on the nano-channel by vesicle.The lipid bilayers on the cell and nano channel contact and form a new lipid bilayer after the fusion.A hole of the same size as the nano-channel is fusion,and the proteins inside the single cell can be sampled.Reproduced with permission[39].Copyright 2017,Royal Society of Chemistry.
3.Single cell sampling with nanochannels
Single cell analysis research is becoming increasingly important in numerous fields;however,the ultra-small size(e.g.,pL)of single cells makes the analysis operation process challenging.Generally,the diameter of a single cell is usually in the order of micrometers(μm),and the volume is on the order of pL.Therefore,it is necessary to analyze the specific analytes of a single cell at the level of a single molecule or several molecules.The analysis process includes the following three steps:(1)single cell sampling,(2)sample pretreatment,and(3)trace sample detection.Recently,numerous research groups have reported chemical treatments and detection methods[35–37],but the sampling of single-cell samples at the fL level is still difficult to control,and single-cell viability cannot be maintained under experimental operations.Sampling is the basic step of routine analytical chemistry.Precisely controlling the sample amount and maintaining cell viability is one of the interface conditions for the development of single-cell sampling.Although numerous studies have used nanotechnology for single-cell analysis,they are limited to those that have observed cell activity or morphological characterization.Sampling from living single cells will help expand the research of biomolecules in single cells,and it is expected that the number of molecules in the sample will appear at a low level of tens of thousands of molecules after sampling[38].Linet al.used nanochannels to form nanometer-sized sampling ports on the cell membrane to achieve a direct and tight connection between cells and nanochannels[39].A micro/nanofluidic sampling interface was developed.Single-cell chambers were designed and prepared on the chip,and single-cell separation and capture were achieved through microfluidic control.The nanochannels linked to the single-cell chambers were used as samplers for living single cells at the fL level.The micro/nanofluidic sampling interface of living single cells was realized by modifying the phospholipid bilayer in the nanochannel to fuse it with the phospholipid on the cell membrane(Fig.2)[39].Through micro/nanofluidic control,they successfully sampled human arterial endothelial living single cells(HAECs)in the nanochannel at the fL level.
4.Pretreatment of ultra-small samples
Constructing miniaturized liquid chromatography separation columns is an important trend in the process of single cell analysis.Kitamoriet al.used the micro/nanofluidic chromatographic separation platform to build a novel analysis platform that can separate samples much smaller than a single cell[40].As shown in Figs.3 and 4[40],to realize the separation mode of the sample in the micro/nano chip,an experimental device for the pressure drive and fluid control system was constructed(Fig.3)[40].On the micro/nanofluidic chromatographic separation platform,the sample solution was filled into the loading channel by applying pressure from the top,left,and right channels,and the mobile phase was filled into the separation channel(Fig.4a)[40];then,the pressure on the right side was turned off,and a small amount of sample was squeezed into the separation channel(Fig.4b)[40].After a while,the pressure at the top was turned off to cut off the sample in the separation channel(Fig.4c)[40],and the injected sample was detected downstream of the separation channel(Fig.4d)[40].Based on this principle,the liquid chromatography system can provide pressure-driven flow in the nanochannel in the micro/nanofluidic chip,which can efficiently separate molecules in the sample with ultra-small volume[41–44].
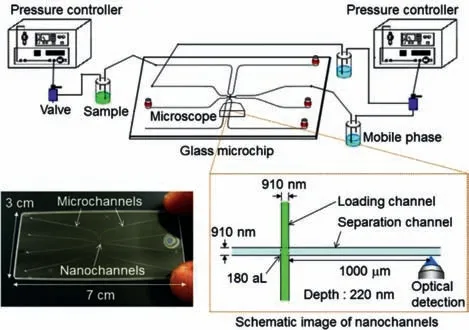
Fig.3.Overview of experimental setup for extended-nano chromatography.Two pressure controllers are used to push solutions of the sample and mobile phase in vials.The vials are connected to a glass microchip,which has micro-channels for introduction and nanochannels.Two nano-channels,the loading and separation channels,cross orthogonally at the center of the microchip.Reproduced with permission[40].Copyright 2017,Elsevier.
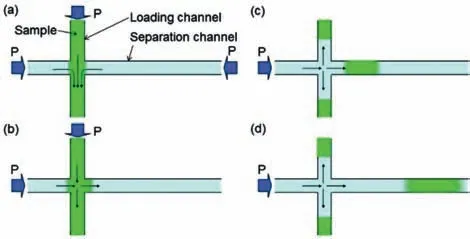
Fig.4.Flow control of sample injection by pressure switching.(a)Sample is loaded from the loading channel.Pressures from the top,left,and right sides are balanced at the intersection of nano-channels.(b)Sample is injected from the loading channel to the separation channel by switching off the pressure from the right side.(c)Sample is cut off by switching off the pressure from the top side after a time lag.(d)Sample diffuses in the separation channel.Reproduced with permission[40].Copyright 2017,Elsevier.
Recently,Smirnovaet al.reported,for the first time,mixedstep reversed-phase chromatographic separation achieved on the nanochannel[45].The chip platform was tested for the separation and analysis of various amino acids.As shown in Fig.5[45],the micro/nanofluidic chromatography platform completed the separation of 17 amino acids within 50 s.The unique separation characteristics in the nanospace can be used for the separation of proteins and macromolecules and the application of biological samples,especially for the analysis of living single cells[45].It has the potential to analyze amino acids in the cytoplasm and is expected to clarify the process of protein synthesis in the cell during transmembrane transport.
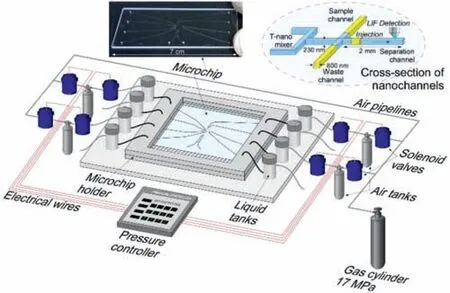
Fig.5.The nanofluidic device for handling and sorting samples at aL.Reproduced with permission[45].Copyright 2016,American Chemical Society.
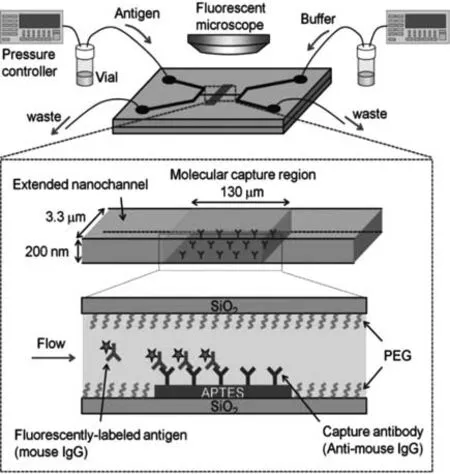
Fig.6.Schematic illustrating fluidic control and the immunochemical reaction in the molecular capture region.Reproduced with permission[47].Copyright 2014,John Wiley &Sons,Inc.
5.Ultra-small sample detection in nanofluidics
Recently,the researches of sample detection in single-cell have shown a trend of the smaller amount and higher concentrations[46];thus,precise processing and ultra-small volume sample detection are required.Kitamoriet al.reported a single-molecule enzyme-linked immunosorbent assay(ELISA)platform using micro/nanofluidic technology[47].On this micro/nanofluidic chip,sample processing was integrated into a nanochannel.Specific single molecules(proteins)were accurately captured and detected.In the chemical processing step,they developed a nanofluid immunoassay device that could perform an efficient immunochemical reaction(nearly 100% capture rate)in only a few seconds.The technical challenge in their study was ensuring that the correct antibody modification is performed on the inner surface of the nanochannel.Through the development of a chemical method of photolithography,the technical vacuum ultraviolet(VUV)light and low-temperature bonding technology were used to bond the modified glass chip.The modification of the antibody in the nanochannel was completed,and the integrity of the antibody after bonding was guaranteed.As shown in the schematic diagram of Fig.6[47],the nanofluid immunoassay device introduced and captured target molecules by adjusting the liquid volume exchange under the control of a pressure-driven fluid.In the chemical processing part,an efficient antigen–antibody reaction in the nanospace was developed,which could capture a very small amount of analyte without losing it.Nevertheless,the experimental results showed that the detection limit did not reach a single or countable molecular region.
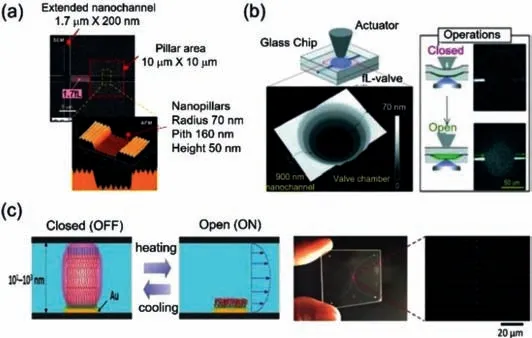
Fig.7.Nanofluidic for single cell analysis(a)SEM and AFM images of the nanovalve and nanopillar array.Reproduced with permission[49].Copyright 2012,American Chemical Society.(b)Schematic illustration of a nanochannel open/close valve.Image below shows the valve chamber with the four-stepped nanostructure,which is connected to the nanochannels,observed by an optical profiler).Reproduced with permission[50].Copyright 2019,Royal Society of Chemistry.(c)Nano-valve system in nano-space for sample injection control.Reproduced with permission[51].Copyright 2016,John Wiley &Sons,Inc.
To solve the problem of precise detection in the nanochannel,Kitamoriet al.used a combination of enzymatic reaction chemical amplification and differential interference contrast thermal penetration microscopy(DIC-TLM)ultra-high sensitivity detection to increase the detection limit to the single molecule level[48].The device could chemically process and detect specific single molecules,which is an indispensable function for single-molecule analysis in nanochannels.To be able to detect a single analyte molecule,the optimal channel size and enzymatic reaction time for DICTLM detection were designed.The detection signal of ELISA in the nanochannel was successfully obtained,so that single molecule detection could be performed on the nanochannel.The modified method can be used to analyze ultra-small samples(single cells,single bacteria,etc.),and the antigen–antibody reaction time scale will make ultra-fast immunoassays possible,which can greatly shorten the time required for clinical assays.
6.Other applications
Numerous studies have detailed the development of highly integrated and miniaturized nanofluidic single-cell analysis,singlecell manipulation and live single-cell sampling in the chip,and real-time monitoring of genes and proteins in the process of cell drug induction.Such platforms will play important roles in promoting the research on cell differentiation mechanisms.Therefore,the association with the detection instrument is particularly important for single-cell analysis platforms,especially the control operation of the nanospace fluid in the detection process is indispensable.Here,Mawatariet al.used the electron beam lithography and dry etching to embed nanopillar arrays into nanochannels and used the Laplace force principle to construct nanovalves for the control of fL droplets in the nanochannels,and successfully controlled 1.7 fL droplets(Fig.7a)[49].Kazoeet al.developed a deformable nanovalve fabricated of glass and other rigid materials(such as plastic)(Fig.7b)[50].The nanovalve had a fourlevel nanostructure,which was suitable for deflecting the arc shape of glass.The authors confirmed the stability and durability of 50 open/close operations and successfully stopped and allowed the solution to flow in the nanochannel under a pressure of 100 kPa,with a fast response time of ~0.65 s[50].An integrated thermal conduction valve switch system was developed in the nanospace for sample injection and manipulation,which transformed the traditional pressure control system(Fig.7c)[51].In addition,the basic measurement methods in the nanospace,including the flow rate method,were used to explain the behavior of molecules in nanochannels[52,53].In the future,nanochannels will facilitate micro-volume analysis and ultra-sensitive detection.
7.Conclusion and outlook
In summary,the introduction of micro/nanofluidic chip technology with international cutting-edge technology into the realtime analysis of drug-induced monomeric living cells can overcome problems such as difficult cell manipulation in routine evaluation and analysis,difficulty in cell survival after sampling,inability of detection in real time,low accuracy,and poor reliability of analysis results.In this review,we report the micro/nanofluidic chip technology used for single cell analysis in the past ten years,covering the preparation of micro/nano fluidic technology and its application in single cell research.For example,steps such as single-cell sample collection,sample pre-processing,and sample detection require complex and precise control of the fluid.Compared with porous materials and carbon nanotubes,micro/nanofluidic chips that can be designed to manipulate the size and function are suitable platforms.The indispensable steps of the single cell analysis process such as micro/nanofluidic single cell sampling ports,nanochannel ELISA,and nanochannel chromatography are integrated into the micro/nanofluidic chip platform to provide a new method for living single cell research.By using these new methods and combining them with microchemical processes,it will be possible to obtain new biological analysis tools that are difficult to obtain with traditional microtechnology.The successful development of this technology can provide new information and novel methods for the study of cell differentiation mechanisms,real-time detection of the process of gene expression changes in cells,highsensitivity detection of cell heterogeneity,and systematic identification of key parameters of potential states during cell differentiation,which has very important academic significance and application value for the study of living single cells.To fulfill this goal,sophisticated nanofluidic device fabrication techniques and advanced detection systems could be developed for more precise handling and more sensitive analysis of single cells.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China(Nos.82073816,21804026,and 21727814),Advanced Talents of Beijing Technology and Business University(No.19008021179).
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
