Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
Xiaohu Zhang,Lixiao Han,Hao Chen,Shengyao Wang
College of Science,Huazhong Agricultural University,Wuhan 430070,China
Keywords:NO removal Photo-catalysis Electro-catalysis Thermo-catalysis Oxidation Reduction
ABSTRACT Considering the significant importance in both ecological and environmental fields,converting nitrogen oxide (NOx,especially NO) into value-added NH3 or harmless N2 lies in the core of research over the past decades.Exploring catalyst for related gas molecular activation and highly efficient reaction systems operated under low temperature or even mild conditions are the key issues.Enormous efforts have been devoted to NO removal by utilizing various driving forces,such as thermal,electrical or solar energy,which shine light on the way to achieve satisfying conversion efficiency.Herein,we will review the stateof-the-art catalysts for NO removal driven by the above-mentioned energies,including a comprehensive introduction and discussion on the pathway and mechanism of each reaction,and the recent achievements of catalysts on each aspect.Particularly,the progress of NO removal by environmentally friendly photocatalysis and electrocatalysis methods will be highlighted.The challenges and opportunities in the future research on the current topic will be discussed as well.
1.Introduction
The global environment and human’s respiratory system are being affected by NOx(NO and NO2) pollution,which is mainly produced from large utility boilers,industrial boilers,municipal waste plants,vehicles and incinerators [1–6].At present,the most popular way of NO removal is selective catalytic reduction (SCR) of NOxwith NH3(NH3-SCR) to harmless N2and H2O which was initially developed by Hitachi Zosen in Japan at ∼1970 [7–9].Generally,TiO2supported V-,W- or Mo-based oxides are the most widely used industrial catalyst under high temperature (>200 °C)and 80–100% NOxemissions can be reduced by this technology.Although some other types of catalyst,such as zeolite-supported Cu[1,10]and Mn,Cu-based oxides [11,12],can work under lower temperatures,dilute NO (ppb level) can not be reduced by this method effectively and consumption of valuable NH3makes it as an undesirable way.
Recently,environmentally friendly semiconductors-based photocatalysis (especially photocatalytic oxidation) using solar energy has received considerable attention in view of its potentiality for efficient pollutants [13–16]and dilute NO removal especially in ppb level (∼600 ppb) [17,18].Up till now,numerous photocatalytic materials,such as TiO2[19–21],Bi-based metal oxides [22–27],g-C3N4[28–33]and heterojunctions [34–37],all exhibit excellent NO removal efficiency and selectivity.In this process,NO removal is mostly supposed to take place via an Eley-Rideal mechanism,through which O2is firstly activated by photogenerated e−with formation of reactive oxygen species (ROS) and then NO will react with ROS [38].That is,NO is inactivated in this mechanism which is disadvantageous in photocatalytic NO oxidation.Generally,enhancing e−/h+separation through bandgap engineering,doping or heterojunction fabrication and facilitating O2activation by construction of surface oxygen vacancies (OVs) are two mainstream strategies for designing photocatalyst to improve NO removal activity.From the thermodynamically point of view,activated NO (NO+,a better electron acceptor) would strongly interact with the•O2−to produce nitrate via dual-site route through Langmuir-Hinshelwood(L-H) mechanism as compared with the inactivated NO [38].Inspired by the fact that Au nanoparticles with unfilled orbital electronic structure are capable of binding with NO possessing lone pair electrons,a bicomponent Au/CeO2photocatalyst with dualsites for adsorption/activation of O2and NO simultaneously was designed by Shanget al.to improve the photocatalytic NO purification efficiency [38].Compared with photocatalytic NO oxidation reaction,reducing NO to N2or even NH3synthesis through photocatalysis or electrocatalysis are rarely investigated [39–44].However,these types of reactions would be more promising in consideration of the high value of NH3and the lack of species such as NO3−occupying active sites on the surface of materials which often result in the inactivation of photocatalyst.

Fig.1.NOx removal is driven by thermo-,photo- and electro-catalysis processes.
Although NH3-SCR has been successfully applied in industry for almost 50 years,photocatalytic NO oxidation has been investigated for more than 10 years,and some more interesting NO reduction reactions have emerged very recently,a systematic review on the field of NO removal has lacked as far as we know.Generally,the key scientific issues related to NO removal lies in: (1) Exploring more efficient catalyst working under ambient conditions along with high selectivity.(2) Mechanism investigation such as NO removal pathway and structure-activity relationship.(3) Active sites construction and reactant,especially NO molecule,activation.This short review mainly summarizes the reported literature in both NO oxidation and NO reduction reactions (Fig.1) by series of energy input.And then,some critical issues are discussed.Finally,some prospective strategies are highlighted.The objective of this review is to provide a clear horizon on this field and guide the rational design for more effective catalysts.
2.Overview on reactions of NO removal
2.1.Reduction of NO
2.1.1.Selective reduction of NO with NH3 (NH3-SCR)
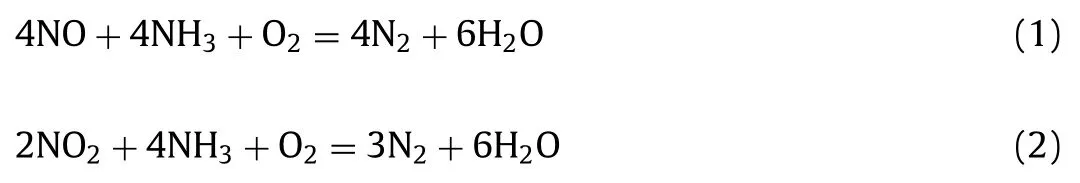
The NH3-SCR technology proceeds according to following Eqs.1 and 2:
Taking supported V2O5-WO3/TiO2catalysts which are the most widely used and investigated industrial catalysts for these SCR applications as for example,NH3-SCR proceedsviaa surface Marsvan Krevelen mechanism as follows (Eqs.3–8) involving the participation of weakly adsorbed NO with surface adsorbed NHxspecies(mostly NH4+∗species on Brønsted acid sites,and some surface NH3∗species can convert to surface NH4+∗species in the presence of moisture) [7]:

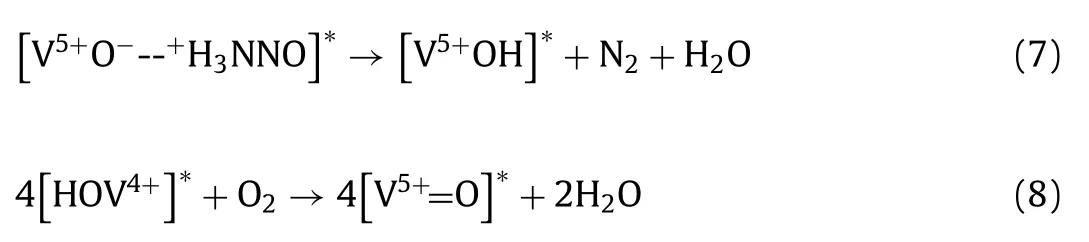
The rate-determining step investigated by density functional theory (DFT) methods involves breaking of an N−H bond during the course of formation or decomposition of the surface NO-NHxreaction intermediate complex.In addition,moisture that is the H2O concentration plays a significant role in the SCR reaction and inhibiting oxidation of SO2to SO3should be taken into consideration [7].
2.1.2.Electrocatalytic reduction reaction of NO (NORR)
Although NO can be converted into harmless N2by above SCR technology,valuable NH3(an essential chemical substance to produce fertilizers) is consumed.Electrochemical ammonia synthesis from N2(NRR) or NO3−reduction has been widely reported [45],and it is found that NO can be reduced from exhausted gas to ammonia by coupling NO removal and ammonia synthesis electrochemically [41,42].In addition,the NH3synthesis activity and selectivity in NORR is higher than that of NRR which is usually limited by N2dissociation and competitive HER (hydrogen evolution reaction).Generally,NORR follows proton-coupled electron transfer process reported in literature (Eqs.9–18):
Route 1 [43]:

Route 2 [44]:
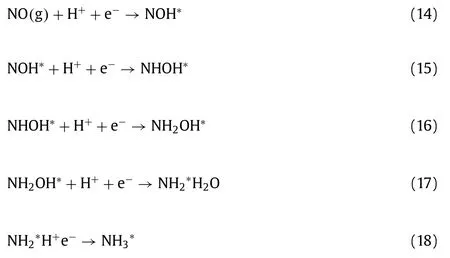
2.1.3.Photocatalytic NORR
Compared with NH3-SCR and electrocatalytic NORR,direct dissociation of NO into N2and O2by semiconductor based photocatalyst such asTiO2is promising.Generally,metal doping or oxygen vacancies formation is necessary for photocatalytic NO reduction[39,40].
2.2.Oxidation of NO
2.2.1.Oxidation of NO by strong oxidants
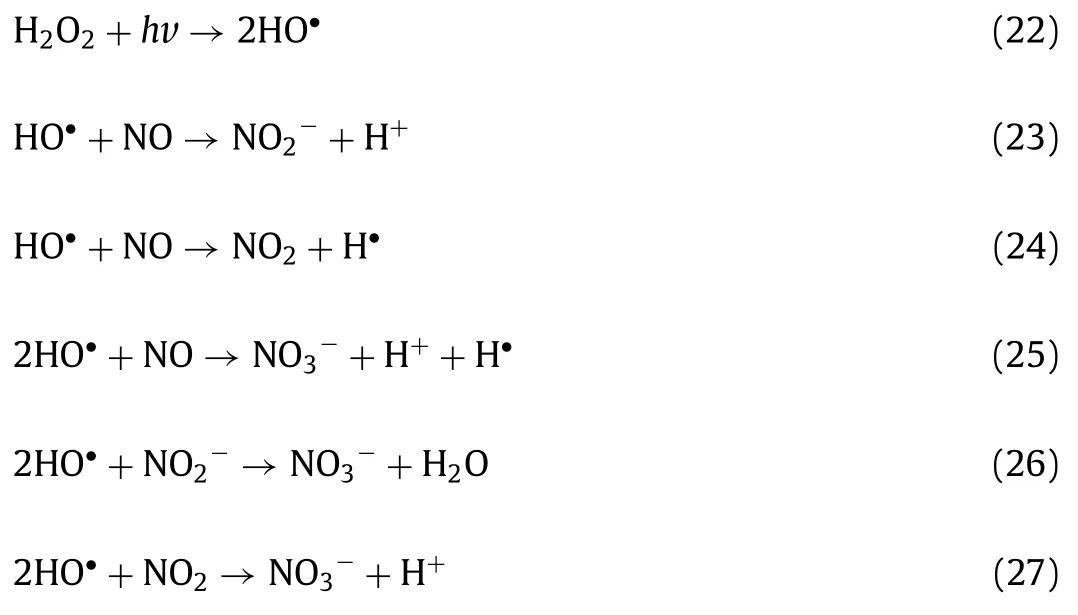
Generally,NO can be oxidized into NO2and NO3−by strong oxidants such as H2O2and O3[46–48].Take H2O2as example,the standard electrode potentials of H2O2(1.770 V) and HO•(2.800 V,produced from H2O2with UV light irradiation) are far higher than those of NO2/NO (1.049 V),NO3−/NO (0.957 V),NO2−/NO(0.460 V),and NO3−/NO2−(0.835 V),which demonstrates the feasibility of NO oxidation according to the following Eqs.19–27:
Route 1: without irradiation

Route 2: with irradiation
2.2.2.Photocatalytic oxidation of NO
Reactive oxygen species (ROS,such as•OH,•O2−,1O2) and holes(h+or O−) play significant roles in semiconductor-based photocatalytic oxidation of NO.Up till now,two representative mechanisms are proposed as follows: (1)•O2−originated from the reaction of oxygen and photogenerated electrons mainly participated in the oxidation of NO to NO2,which was further oxidized to NO3−by•OH and holes (Eqs.28-33);(2) NO was directly oxidized to NO3−by•O2−,and partial NO oxidation for NO2generation was normally attributed to•OH and holes (Eqs.28,34-35).Generally,tuning the formation of oxygen reactive species in catalyst design is significant.
Route 1 [23,49,50]:
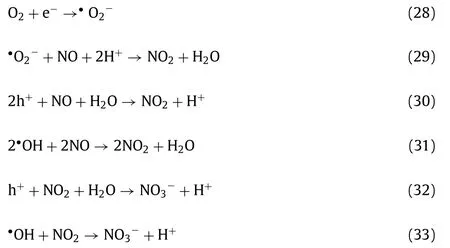
Route 2 [17,18]:

3.Recent developments in NO removal
3.1.NH3-SCR
As mentioned above,numerous researches have been focused on supported V2O5-WO3/TiO2catalysts,and series of impact parameters such as catalyst synthesis,molecular structures,surface acidity,catalytic active sites,surface reaction intermediates,rate determining-step,and reaction kinetics have been investigated[7,51].However,this type of catalysts needs high reaction temperature (>300°C),which is not suitable of some industrial boilers with lower outlet temperature.
Recently,some other type of catalysts including zeolitesupported Cu and Fe and other transition metal oxides have been developed for NH3-SCR technology to satisfy different application scenarios [1,8–12,52].To enhance the oxidation and SO2-tolerant property of catalyst,a mesoporous MnCeSmTiOxamorphous mixed oxides is constructed by coprecipitation method [11].As shown in Fig.2a,the NO conversion could reach nearly 100% at 140–320 °C and maintain>90% at 400 °C and a gas hourly space velocity of 80,000 h−1.More importantly,the selectivity of N2could be maintained at ≈100% at 100–320 °C and stay at>90% up to 400 °C(Fig.2b).In addition,the influence of adding SO2and water vapor which are inevitable in exhaust and flue gases on the catalytic performance were investigated.As can be seen in Figs.2c and d,slight decrease of NO removal efficiency was observed for MnCeSmTiOxcatalyst,which maintained better activity than that of MnCeTiOx,indicating the excellent SO2/H2O-tolerant.It is found that the NH3-SCR follows Langmuir-Hinshelwood mechanism,and synergistic effect of the Lewis acid sites and oxidation catalyticsites play important roles in the reaction.Sm doping can increase transfer electrons to Mn4+and Ce4+,which facilities the formation of active adsorbed NO2(bidentate nitrate,and bridging nitrate intermediates),enhances the NO conversion at low temperatures and suppresses SO2poisoning.
Using MOFs as the precursor [12],a series of Fe-Doped Mn3O4(FexMn3-xO4) nanoparticles possessing efficient Feoct(octahedral)-OMntet(tetrahedral)site were synthesized and exhibited a NO conversion up to 90% at 180 °C in an ultrahigh gas hourly space velocity(GHSV) of 400,000 h−1.DFT calculations were carried out to obtain insights on how the catalytic activity was affected by Feoct.Interestingly,the formation of oxygen vacancy (Fig.3a) was the ratedetermining step of NO oxidation.With the replacement of Mnoctby Feoct,the required free-energy change for NO-to-NO2oxidation all decreased to some extent,and Feoct-O-Mntetis the most effi-cient active site.It should be note that the charge density between Moctand O were decreased after the replacement of Moctby Feoct,which facilitated the formation of OV (Fig.3b).The enhanced NO oxidation can trigger the “fast SCR” reaction,thus leading to the boosted NH3-SCR performance.
Although NH3-SCR technology can treat high concentration NOxemissions (several hundred ppm,i.e.,500 or 600 ppm) with high conversion efficiency,they are not economically for low concentration NOx(ppb level) purification in atmosphere since this technology can just work under high temperature (>250 °C).More importantly,the NH3-SCR process consumes high-cost and valuable NH3which is usually synthesized by extremely energy dependent Haber–Bosch process.By the way,NH3-SCR is not suitable for many industrial boilers,which have lower outlet temperature and contain impurities including SO2and H2O.In addition,the adsorption and activation of NH3is thought to be the first step in NH3-SCR,therefore,exploiting catalyst that can active NH3and NO simultaneously should be the aim in future to realize NO removal under lower temperature and even mild condition.In a word,NH3-SCR has been successfully applied in industry for almost 50 years,and will be still the dominant technology for high concentration NOxremoval in a long time until novel environmental friendly methods operating under mild conditions can be practically utilized.
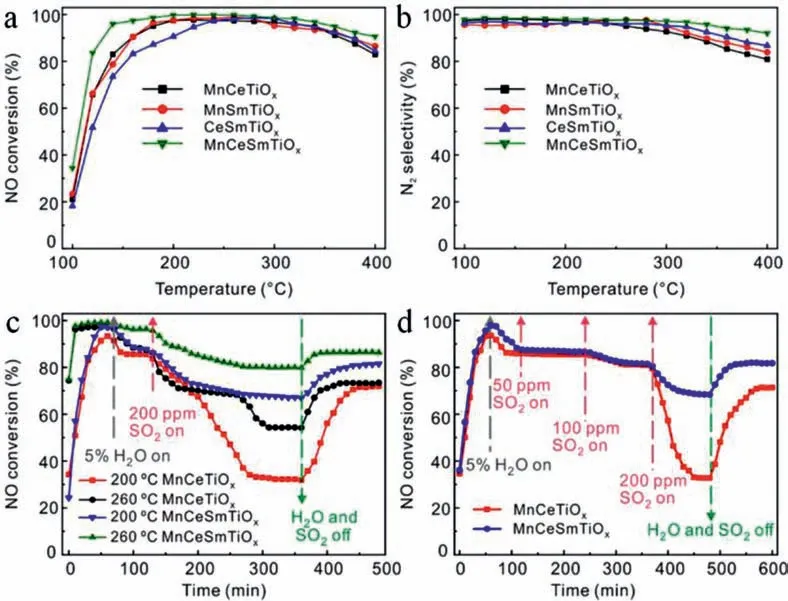
Fig.2.NO conversion (a) and N2 selectivity (b) of MnCeTiOx,MnSmTiOx,CeSmTiOx,and MnCeSmTiOx catalysts.H2O and SO2 tolerance test of MnCeTiOx and MnCeSmTiOx catalysts at different temperatures (c) and introducing SO2 with different concentrations (d) [reaction conditions: v(NO)=v(NH3)=500 ppm,v(O2)=5 vol %,balance Ar,and GHSV=80,000 h-1.Copied with permission [11].Copyright 2020,American Chemical Society.
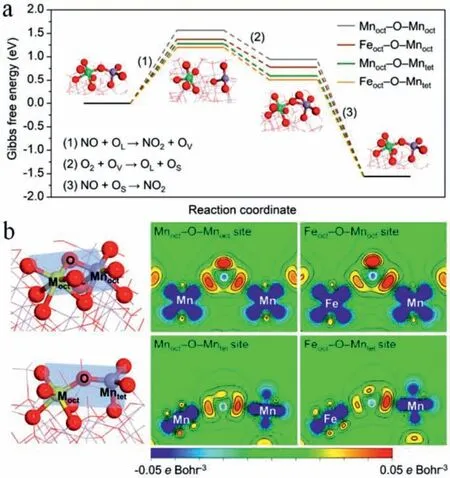
Fig.3.(a) Gibbs free energy (400 K) diagram over NO-to-NO2 oxidation based on Mars and van Krevelen (MvK) mechanism and (b) charge density difference contour plots for Mnoct-O-Mnoct,Feoct-O-Mnoct,Mnoct-O-Mntet and Feoct-O-Mntet sites.Copied with permission [12].Copyright 2020,American Chemical Society.
3.2.Electrocatalytic NORR
Different form the above NH3-SCR process,it was reported that that NO could be reduced to NH3instead of N2or other inorganic compounds over Pt (100) or Co-N4single site based on DFT results[41,42],and a similar conversion pathway was proposed as Fig.4[41].This finding is promising in terms of coupling NO removal and NH3synthesis,which convert exhausted gas to NH3directly.In fact,Longet al.found that Cu exhibit higher activity for NORR than NRR,as well as superior NH3selectivity relative to N2and H2production,and the designed Cu foam electrode achieved a recordhigh NH3production rate of 517.1 μmol cm−2h−1with a Faradaic efficiencies (FE) of 93.5% at −0.9 Vvs.RHE accompanied a significantly enhanced current density (Figs.5a-c) [43].NH3is always the dominant product at different tested potentials with high FE (Figs.5d and e).Moreover,the turnover frequency (TOF) increases from 2630 s−1to 7840 s−1with the potential varying from 0.3 V to–1.2 V (Fig.5f).Additionally,associative Heyrovsky distal-O pathway(AHDO) NORR pathway (Eqs.9-13) was proposed based on DFT result.
To avoid direct use of highly concentrated NO gas,a rationally designed electrolyte containing the EDTA-Fe2+metal complex (EFeMC) which is known for its rapid NO capture ability was adopted to selectively produce NH3by NORR [44].Fig.6a gave the H-type electrolysis cell that was used for NORR.The electrolyte is Ar gas-purged or NO gas saturated 50 mmol/L EFeMC in a naturalpH phosphate buffer solution (PBS),noted as PBS-MC50-Ar and PBS-MC50-NOsrespectively.In addition,a nanostructured Ag electrode was selected because of its ability to sufficiently suppress the HER,while Pt foil and glassy carbon (GC) were also used as cathode materials for control experiments.
The quite distinguished LSV features in Fig.6b indicated that HER was spontaneous and dominant reaction on Pt,while NO@EFeMC species was involved in the electrochemical reaction on Ag and GC.As a result,nearly ∼100% FE for NH3production in potential ranges of −0.15 V to −0.29 VRHEand −0.29 V to−0.34 VRHEfor Ag and GC electrode were observed respectively,and the economic analysis is carried out using itemized cost estimation,indicating its market competitive ($0.03 k/Wh).
Although the above pioneering reports present a new scenario for reusing the exhaust NO gas,the real NO reduction mechanism and more advanced materials with higher efficiency should be further explored.In addition,electrocatalytic converting NO into value-added NH3can be operated under mild condition which is energy saving.However,high flow rate of pure NO gas (i.e.,5–30 mL/min) are required due to the low solubility of NO in water.By the way,the above-mentioned electrocatalyst can directly active NO molecule,which can provide scientists with new material designing direction in other NO removal fields like photocatalysis and thermocatalysis,where NO is usually considered inactivated.The electrocatalytic NO reduction is promising in consideration of the high value product of NH3and the controlled selectivity by tunable bias voltage.
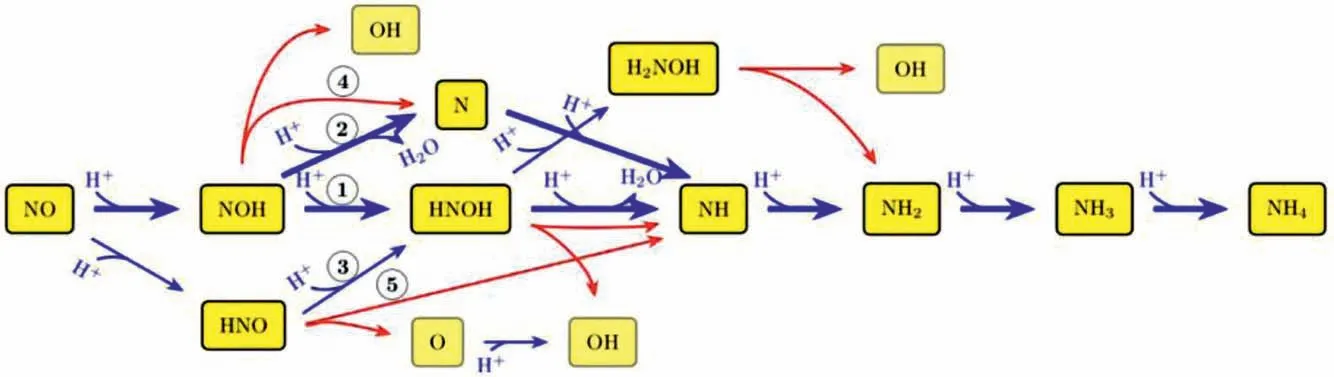
Fig.4.Most probable pathways found in kinetic Monte Carlo (kMC) simulations are indicated by numbers within circles.Blue arrows refer to electrochemical steps and red arrows to chemical steps.Thrick blue arrows mark the most probable pathways.To simplify the notation,(H++e−) is expressed as H+.Copied with permission [41].Copyright 2017,American Chemical Society.

Fig.5.Experiments of NORR and NRR over different catalysts and theoretical microkinetic simulation.Reaction rate (a) and Faradaic efficiency (b) for NORR on a Pt foil,Cu Foil,and Cu foam for 600 s,and NRR over Cu foam for 2 h at -0.9 V vs. RHE.(c) Linear Sweep Voltammetry (LSV) for Pt foil,Cu foil and Cu foam in NO-saturated 0.25 mol/L Li2SO4 Cu foam in Ar or N2-saturated 0.25 mol/L Li2SO4 acquired with a scan rate of 10 mV/s at 25 °C.Reaction rate (d) and Faradaic efficiency (e) of NORR on Cu foam at varying potentials.(f) The measured ammonia production rates over the Cu foam plotted as theoretical TOF at different potentials.Copied with permission [43].Copyright 2020,John Wiley &Sons,Inc.
3.3.Photocatalytic NORR
Compared to thermo- and electro-catalysis,environmentally friendly semiconductor-based photo-catalysis using solar energy is attractive.S.Royet al.found that N2and N2O could be detected when NO was passed over Pd2+doped TiO2(Ti0.99Pd0.01O1.99) catalyst under UV light irradiation [39].
In general,45% dissociation of NO was observed in the first run with decreased efficiency in the following runs,and the NO conversion efficiency could be maintained at ∼80% when mixture of CO and NO gas was adopted.In addition,the creation of oxide ion vacancy in Ti0.99Pd0.01O1.99was primary requirement for NO reduction,and the presence of CO seemly inhibited partial conversion of NO to NO2which would block the oxide ion vacancy sites.To inhibit the formation of NO2,Fe3+doped TiO2(Fe-TiO2,Fig.7) with oxygen vacancies which was stabilized by Fe3+were also reported to act as catalytic centers that capture the oxygen end of the NO molecules and further dissociate NO into N2and O2(4.5% conversion efficiency),and the photoinduced reduction of Fe3+to Fe2+almost completely suppressed the formation of NO2and thus enhanced the selectivity of the reaction for N2formation [40].
3.4.Oxidation of NO by strong oxidants
Haoet al.evaluated the macrokinetics of NO oxidation by vaporized H2O2association with ultraviolet light,and found the method was more effective than conventional UV-H2O2reaction system [48].In addition,advanced oxidation process based on UVH2O2was reported to simultaneously remove NO and SO2from coal-fired flue gas with high efficiency.
3.5.Photocatalytic oxidation of NO
Although rare literatures are reported for photocatalytic NO reduction,numerous works are focused on photocatalytic NO oxidation (Table 1) [2–4,6,17–19,21–25,28–31,34–38,53–106].To clarify the collaboration of different type of ROS in photocatalytic NO oxidation,Dinget al.designed Bi self-doped Bi2MoO6to modulate the ROS generation [49].Based on DFT calculation,band-position and series of photoactivities with different scavengers analyses,they found that•O2−could improve the oxidation of NO to NO2as well as•OH and h+mainly contributed to the further oxidation of NO2to NO3−.Generally,ultrathin 2D materials possess unique electron transfer property.In addition,their band-gap and redox potential can be modulated by foreign atom doping.For example,Wanget al.[50]designed ultrathin (001) facet exposed Bi2MoO6nanosheets with homogeneous C-doping (UC-BMO) by a hydrothermal process to enhance the photoinduced carries separation through internal electric field and negatively shift the CB of UC-BMO for the activation of molecular oxygen without any attenuation of light absorption simultaneously.Band-gap analysis based on DRS,VB-XPS and Mott–Schottky plot for different examples are shown in Figs.8a-c.As revealed in Fig.8d,the VB and CB of B-BMO is considered not enough for both water (H2O/•OH,2.38 Vvs.NHE,pH=0) and oxygen (O2/•O2−,−0.33 Vvs.NHE,pH=0) activation,while,the obtained CB position (−0.46 Vvs.NHE,pH=0) of UCBMO is negatively enough to transfer photogenerated electrons to oxygen and then activate molecular oxygen.For photocatalytic nitric oxide removal tests,UC-BMO exhibited 4.3 times higher than that of bulk BMO without obvious increasing of NO2production rate.Finally,the whole process of NO removal can be summarized as the following three steps (Fig.9) based on the results of the radical scavenger studies.
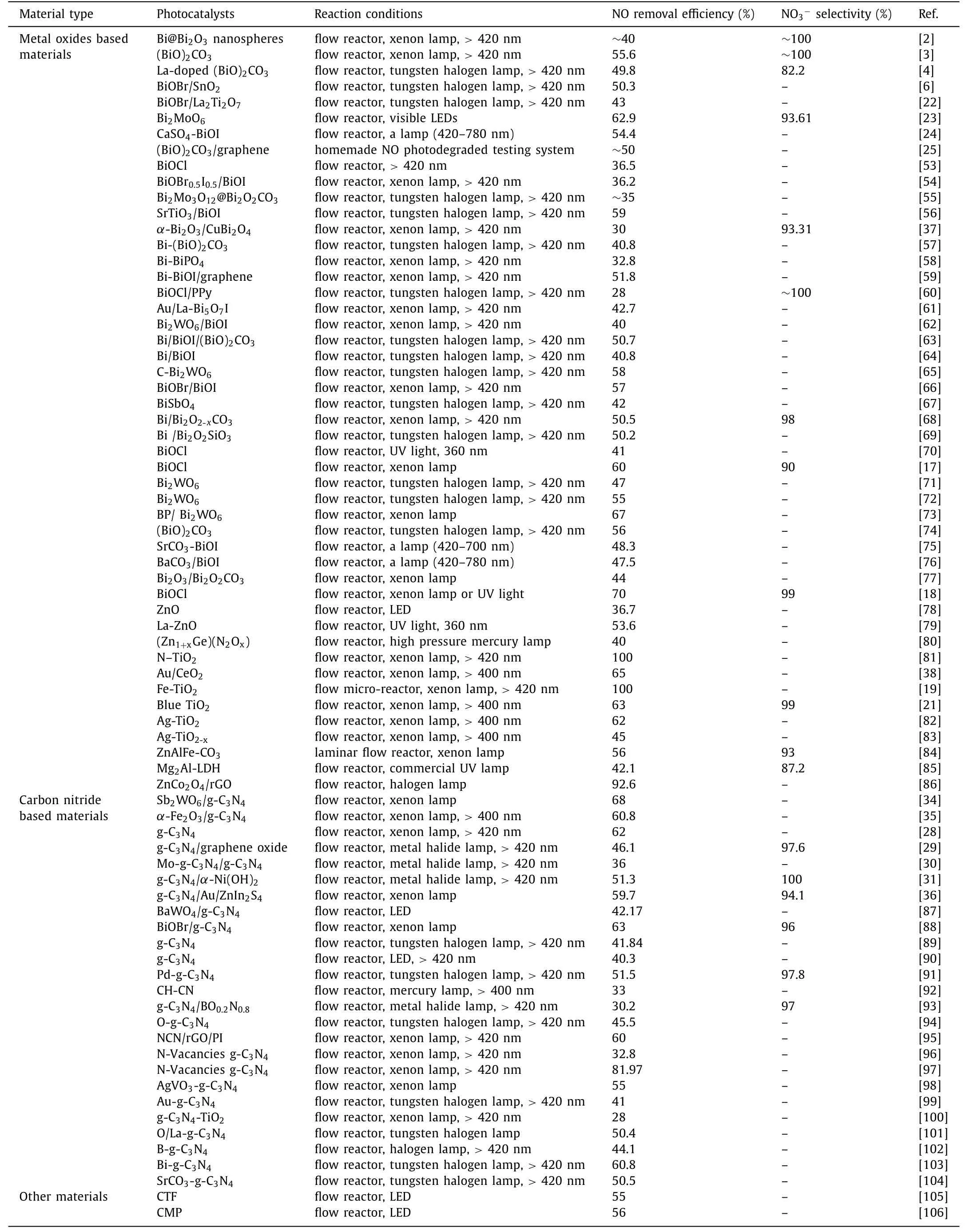
Table 1 Partial recent progress in photocatalytic NO oxidation.

Fig.6.Electrochemical NORR in PBS-MC50-NOs and PBS-MC50-Ar electrolytes on different cathode materials: platinum (Pt),glassy carbon (GC),and silver (Ag).(a) Schematic diagram showing NO capture by FFeMC present in the electrolyte and its electrochemical reduction to ammonia.(b) Linear sweep voltammograms (LSVs) for Pt,GC,and Ag electrodes for the electrochemical NORR in PBS-MC50-NOs and PBS-MC50-Ar electrolytes.(c) Faradic efficiencies and current densities for the formation of NH4+ in the potential range of −0.55 V to 0 V.Values were averaged,and error bars indicate the standard deviation (n=3 replicates).Copied with permission [44].Copyright 2020,American Chemical Society.
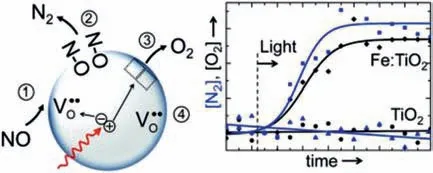
Fig.7.Photocatalytic conversion of NO to N2 and O2 over Fe-doped TiO2.The sample was irradiated with UV light,and the target pollutant was 100 ppm NO in He.Copied with permission [40].Copyright 2012,American Chemical Society.
Except for energy band engineering,construction of OVs which can facilitate the absorption and activation of O2for ROS generation and then enhance the oxidation of NO is an essential strategy.Wanget al.firstly investigated the impact of bromide ions (Br−) on OVs construction on different facets of Bi2MoO6,and found that Br−can boost and stabilize the OVs on (001) facets of Bi2MoO6,while hardly affect that on (010) facets [23].As a result,BMO-001-Br exhibited dramatic enhancement in NO removal efficiency than that of BMO-001 (Fig.10a),while no change was observed for BMO-010-Br.In addition,the effect of Br−concentration and repeatability of BMO-001-Br were evaluated (Figs.10b and c) as well as the detection of NO3−and NO2byproduct (Figs.10d and e).More interestingly,near-infrared light (λ=730 nm) induced photocatalytic NO removal was observed over BMO-001-Br (Fig.10f),indicating the light absorption region was extended simultaneously.This work provides new insight into understanding the exogenous ions on construction and stabilization of OVs and the roles of OVs in photocatalytic NO removal.
Although surface oxygen vacancies can normally enhance photocatalytic activity,they suffer from instability and deactivation in continuous photocatalytic procedure.Taken Bi metal nanoparticle decorated Bi2O2CO3nanosheets with oxygen vacancy (Bi@OV-BOC)as model,Donget al.found that O2and H2O molecules would not fill into oxygen vacancies because they can be activated by Bi metal nanoparticle.In addition,the synergistic effect of Bi metal nanoparticles and oxygen vacancies could promote the completely oxidation of NO to NO3−instead of NO2.As depicted in Fig.11a,Bi@OV-BOC exhibited the highest NO removal efficiency because of the co-effect of Bi metal nanoparticles and oxygen vacancies.To certify the influence of water on oxygen vacancies,water was used as dispersion solvent instead of ethanol,which resulted in the significant decrease in NO removal ratio for OV-BOC (Fig.11b).This phenomenon is rationalized by the fact that H2O filled into oxygen vacancies of OV-BOC (Fig.11c).However,Bi@OV-BOC always keeps the same color and stable photocatalytic activity whatever the dispersion solvent is (Fig.11c),indicating that Bi metal nanoparticles prevent H2O molecules from filling into oxygen vacancies,and then the stability of Bi@OV-BOC was enhanced (Figs.11d-f).
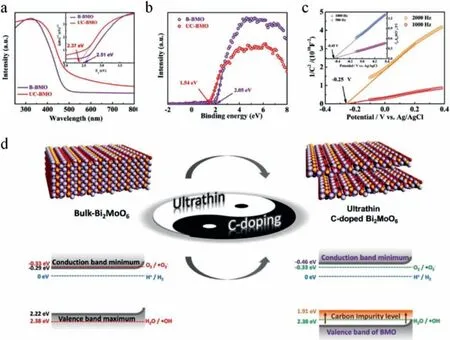
Fig.8.(a) UV–vis absorption spectra,the corresponding Tauc plots (inset),and (b) valence-band XPS (VB-XPS) spectrum of the as-prepared samples.(c) The Mott–Schottky plots of B-BMO (inset) and UC-BMO in 0.5 mol/L Na2SO4 aqueous solution.(d) Schematic illustration of the bandgap engineering strategy and corresponding energy band diagram.Copied with permission [50].Copyright 2017,John Wiley &Sons,Inc.
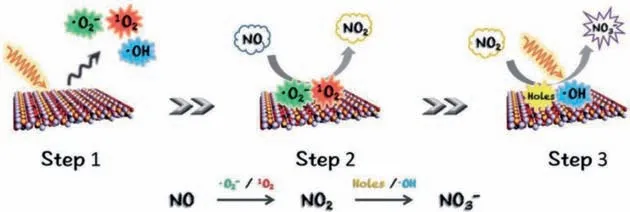
Fig.9.The supposed steps of photocatalytic NO removal on UC-BMO.Copied with permission [50].Copyright 2017,John Wiley &Sons,Inc.
As mentioned above,the function of•O2−in the photocatalytic oxidation process of NO (NO+•O2−→NO3−or NO+•O2−→NO2)is debatable.To clarify this issue,Liet al.investigated the influences of specific geometric structures of•O2−on the reaction selectivity [18].Taken BiOCl(001) with surface OV as model,they found that terminal end-on•O2−favored the formation of NO2and the side-on•O2−could oxide NO to NO3−based on DFT and experimental data.As can be seen in Fig.12a,NO was oxidized to peroxynitrite (OONO-,a transient precursor toward the NO2formation)when NO approached the terminal end-on•O2−,and then release of gaseous NO2was favorable (pathway I).In contrast,direct oxidation of NO into NO3−could be operated without any barrier(ΔE=−3.10 eV) over side-on bridging•O2−(pathway II).In addition,charge density difference calculation was adopted to trace the interfacial charge transfer and uncover the remarkably promoted thermodynamics of NO oxidation by side-on bridging•O2−(Figs.12b-d).
Photocatalytic tests indicated that defect-free BiOCl was ineffi-cient for NO removal under visible light irradiation (Fig.13a).As for BiOCl-OV,up to 70% NO removal ratio was observed within 15 min and only 4 ppb NO2was detected which was expected,indicating the NO3−production selectivity exceeding 99%.In addition,Photocatalytic NO removal rates were linearly related with•O2−in different concentrations (Fig.13b).Based on reactive species trapping experiment,photocatalytic NO oxidation to nitrate was considered directly related to•O2−generated via BiOCl intraband excitation and the generation of holes (O−) and•OH could be avoided (Fig.13c).Besides,the influence of microstructure (nanospheres or nanosheets) on the long-term stability for NO removal was investigated (Fig.13d).
Different form the above single electron-trapped center (VO’) in BiOCl (001),an archetypal F center (V”O”) that can localize two electrons over BiOCl (010) was designed to selectively oxide NO to nitrate with an interfacial charging-decharging process,as shown in the following Eqs.36–40 [17]:
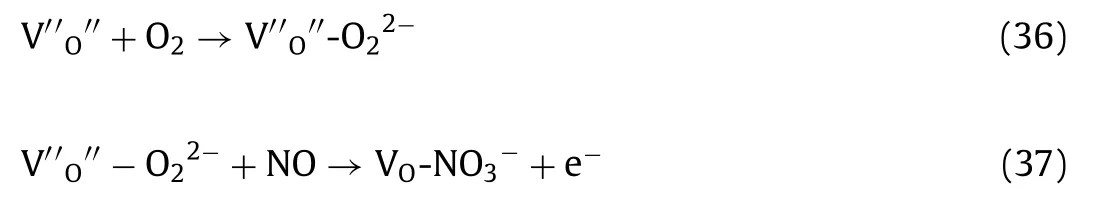
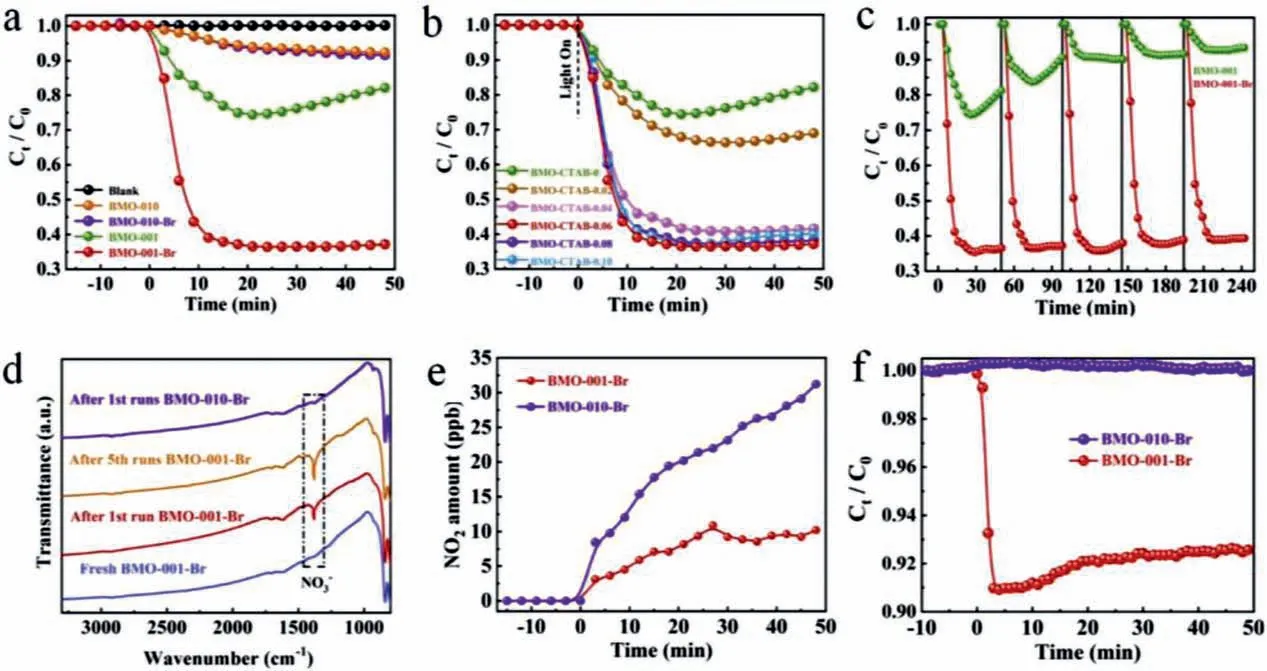
Fig.10.Photocatalytic activities of as-prepared catalysts toward removal of NO under visible light irradiation (a),Photocatalytic activities of as-prepared Bi2MoO6 with different concentration of CTAB toward removal of NO under visible light irradiation (b).Cyclic NO removal tests with BMO-001 and BMO-001-Br under visible light irradiation(c).FTIR spectra of as-prepared Bi2MoO6 before and after irradiation (d).NO2 generated by BMO-001-Br and BMO-010-Br during the photocatalysis (e) and the Photocatalytic activities of BMO-001-Br and BMO-010-Br toward removal of NO under near-infrared single wavelength LED light (λ=730 nm) (f).Copied with permission [23].Copyright 2020,Elsevier.

Fig.11.Photocatalytic NO removal activity of the samples dried in ethanol (a);Photocatalytic activity for NO removal of the samples dried in water (b);The color of the sample dried in ethanol and water,respectively (c);Photocatalytic cycling tests for Bi@OV-BOC (d) and OV-BOC(e);Color change of the samples dried in ethanol before and after reaction (f).Copied with permission [57].Copyright 2020,Elsevier.
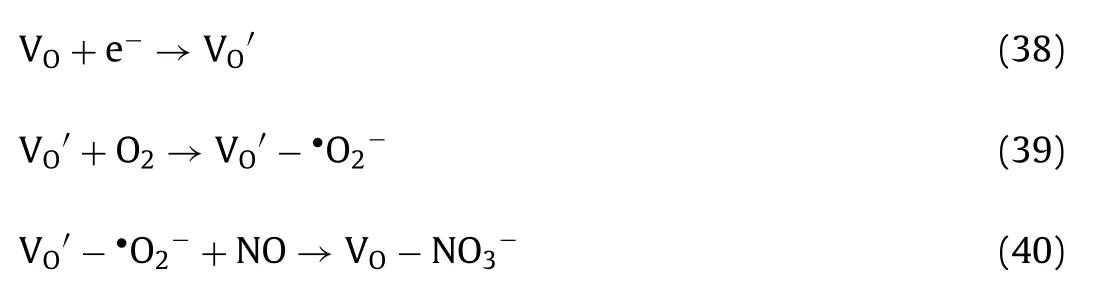
It was found that direct oxidation of NO to NO3−was an exothermic process with a small energy barrier without forming any intermediates (Fig.14a).In addition,V′′O′′ was transformed into VO’through electron back-donation along with O22−-catalyzed NO oxidation (Figs.14b-f).Fig.15a showed the continuous flow reactor for photocatalytic NO oxidation tests.Control experiment exhibited that almost no activity was observed for all of the catalysts tested.However,60% NO removal efficiency was detected for BOC-010-V”O” (Fig.15b),which was 1.8 times than that of BOC-001-VO’.Meanwhile,the generation of NO2was suppressed to ∼10% (Fig.15c).Finally,interfacial charging-discharging strategy accounts for oxidation of NO into nitrate with both high efficiency and selectivity was shown in Figs.15d and e.

Fig.12.(a) Free energy change against the reaction coordinate for the oxidation of NO by •O2−on BiOCl (001) surface in different geometries.(b) Geometric transition from peroxynitrite to nitrate.TS represents transition state.Charge density difference and O2 partial DOS of the BiOCl (001) surface adsorbed with (c) O2 and (d) nitrate.The yellow and blue isosurfaces with an isovalue of 0.005 au represent charge accumulation and depletion in the space.The vertical dashed line in the DOS shows the VBM.Copied with permission [18].Copyright 2018,American Chemical Society.

Fig.13.(a) Photocatalytic NO removal over the as-prepared BiOCl under visible light.(b) Influence of generated •O2−on the NO oxidation kinetic constants and the NO2 concentration.(c) Schematic illustration of photocatalytic NO removal on BiOCl-OV.(d) Transient photocatalytic NO removal on BiOCl-OV.Copied with permission [18].Copyright 2018,American Chemical Society.
Except for the above-mentioned bandgap engineering and OV construction,fabrication of heterojunction based on different semiconductors with quick internal charge transfer is an efficient strategy to enhance the photoactivity of catalysts.Huet al.synthesized a Z-scheme heterojunction (Fig.16) of 2D/2D BP/MBWO nanosheets based on black phosphorus (BP) and monolayer Bi2WO6,and the optimal 12% BP/MBWO catalyst exhibited a NO removal ratio as high as 67%,which was much higher than that of MBWO (only 26%) [73].The unexpected improvement for the photoactivity was attributed to efficient charge transfer and broad light harvesting range.
Recently,some metal-free organic semiconductors like g-C3N4,CMPs (conjugated microporous polymers),MOFs and COFs are hot materials in this field due to their unique visible light absorption property or pore structure.For example,three CMPs (Fig.17)consisting of alternating electron-rich and electron-deficient units with ethynyl linker and changeable optical bandgaps were synthesized by Xianget al.,for which the structure-activity relationship was deeply investigated,and the highest NO removal efficiency reached to 56% [106].Zhanget al.found that the H atoms ofα-Ni(OH)2improve the adsorption and activation of O2and oxygen atoms to promote NO and NO2oxidized to nitrate,and the fabricated g-C3N4/α-Ni(OH)2showed enhanced NO removal ratio of 51.3% without any toxic NO2generation (Fig.18) [31].
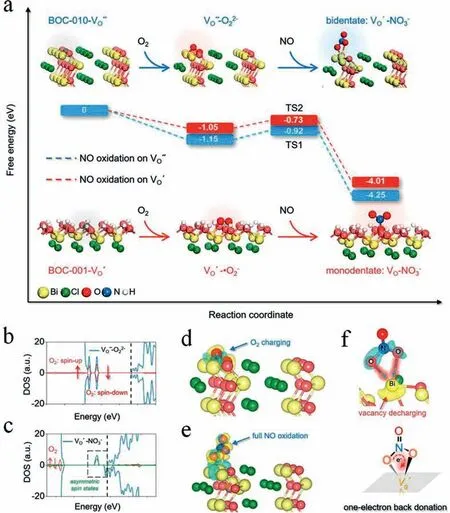
Fig.14.(a) Free energy change during O22−- and •O2−-mediated NO oxidation on the VO of BOC-010 and BOC-001,respectively.TS represents the transition state.DOS of (b) O22−-adsorbed (c) NO3−-adsorbed BOC-010 with VO.Vertical dashed line in the DOS shows the CB minimum.Charge density difference of (d) O22−-adsorbed (e)NO3−-adsorbed BOC-010 with VO.(f) Regional charge density difference between O22−and VO on BOC-010.The yellow and blue isosurfaces with an isovalue of 0.005 au represent spatial charge accumulation and depletion,respectively.Copied with permission [17].Copyright 2019,American Chemical Society.

Fig.15.(a) Reaction chamber for the continuous indoor NO removal.(b) Photocatalytic NO removal over BiOCl under simulated solar light and (c) the accompanying NO2 generation.(d) Schematic illustration of NO removal on BOC-010-VO′′ and (e) the corresponding electronic excitation process.Copied with permission [17].Copyright 2019,American Chemical Society.
Generally speaking,gaseous NO is inactivated due to the preferential adsorption of O2rather than NO in most reported literatures.And the total NO removal efficiency could be further improved if NO can be activated simultaneously.To realize this aim,an Au/CeO2photocatalyst with abundant OVs was designed by Shanget al,in which O2and NO were adsorbed and activated into•O2−and NO+on OV and Au nanoparticle respectively [38].As indicated in Fig.19,a∗OONO structure was formed with overcoming the 0.11 eV barrier when O2and NO were activated on the interfaces of Au/CeO2.And then∗OONO precursor transformed to nitrate with releasing 1.10 eV energy spontaneously.The DFT results suggested that photocatalytic NO removal on Au/CeO2surfaces occursviaLangmuir-Hinshelwood (L-H) mechanism,which was different from that of CeO2catalyst undergoing an Eley-Rideal (ER) mechanism,where inactivated NO was attacked by active•O2−.In addition,Donget al.reported that nitrogen defects were introduced into the framework of g-C3N4by heating g-C3N4powder in the hydrogen atmosphere,and nitrogen atoms were partially removed with hydrogen.The N defects sites can promote the formation of NO+reaction intermediate,and then accelerated the photocatalytic NO oxidation reaction [89],as shown in Fig.20.

Fig.16.Photocatalytic mechanism of NO removal and water splitting by BP/MBWO heterojunction under visible-light irradiation (the various redox potentials versus RHE).Copied with permission [73].Copyright 2019,John Wiley &Sons,Inc.
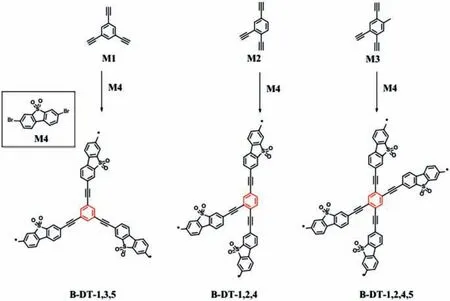
Fig.17.Structures of monomers and synthesis of CMP photocatalysts by Sonogashira cross-coupling polycondensation.Copied with permission [106].Copyright 2018,Elsevier.
In a word,NO removal by environmentally friendly photocatalytic technology is promising in consideration of direct utilization of clean solar energy and numerous efforts have been made in the past decades [107–111].Especially,low concentration of NO(∼500–600 ppb) can be treated by this method under mild conditions with high efficiency.However,direct converting NO into valuable NH3is still blank,and production of undesirable NO2is usually inevitable.In addition,single-atom catalysts,which have realized their value in series of energy and environment issues,are ignored in the development trail of this field.
4.Conclusions and perspectives
In summary,selective catalytic oxidation or reduction of nitrogen oxides is vital to the global environment and human health.In this review,the recent main achievements in the field of nitrogen oxides removal are discussed and summarized,including thermocatalysis,electro-catalysis and photo-catalysis.In spite of significant progress have been achieved,there are still issues requiring complete solving: (1) The standard evaluation and reaction system should be established,especially reactors,energy source and detection equipment.(2) Exploring more efficient catalyst for NO removal with high selectivity.(3) Establishing novel and highly efficient methods to convert NO into value-added products such as NH3other than harmless N2or NO3−.(4) The mechanism investigation of NO removal pathway and structure-activity relationship.(5) Designing practical instruments to purify NO in urban atmosphere.For NH3-SCR,novel strategy or catalyst should be explored to carry out NH3-SCR under lower temperature or even ambient condition.For many cases,the lower outlet temperature of industrial boilers as well as impurities including SO2and H2O will decrease the catalytic activities of NH3-SCR catalysts.In addition,these catalysts are easy to be poisoned by SO2present in boiler flue gas.Therefore,the SO2-tolerance should be taken into consideration preferentially.By the way,the investigation on adsorption and activation of NO on NH3-SCR catalyst is urgent.

Fig.18.NO remove ratio (a) and NO2 fraction (b) over different samples;NO remove ratio (c) and NO2 fraction (d) of recycle experiment.Copied with permission [31].Copyright 2020,Elsevier.
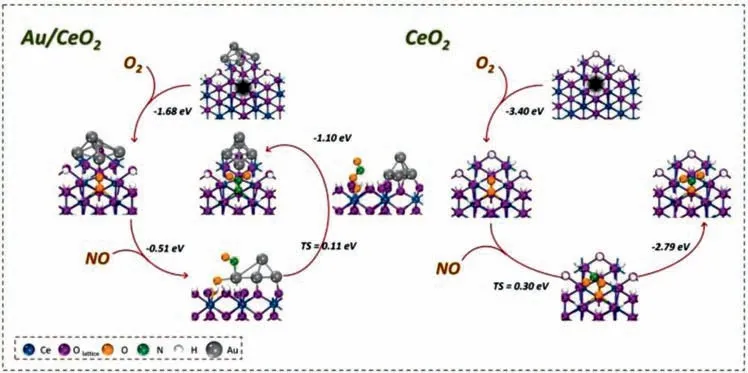
Fig.19.DFT calculations for NO oxidation on the Au/CeO2 and CeO2 with (100) surface exposed.Copied with permission [38].Copyright 2020,Elsevier.
Although numerous reports are focused on semiconductor based photocatalytic NO removal,the selectivity of nitrogen oxides removal for most photocatalyst are prominent problem and the formation of NO2is unavoidable.Additionally,the reported catalytic mechanisms for photocatalytic NO removal are various,which need further investigation.For example,OV is generally considered to play significant role in O2adsorption and activation,however,the controllable and quantitative fabrication of OV on semiconductors as well as systematic investigation of OV on NO removal efficiency ruling out other factors has not been clearly investigated.Moreover,the biggest issue in this field is insufficient practical application study,that is,the evaluation of the purification of the present photocatalyst on urban atmosphere is urgent.For emerging electro-catalytic NH3production from NO reduction,the collection of efficient catalyst and clarifying relative mechanism is of great significance.
In addition,this field is also filled with opportunities.Herein,some constructive opinions on NO removal are proposed.Firstly,coupling of solar energy,thermal energy and electrical energy is scarce in nitrogen oxides removal,in which environmentally friendly photothermo- and photoelectro-catalysis are promising,which have been proven to be extremely efficient strategy in other fields.Very recently,some groundbreaking works on gas-phase photoelectron-catalytic oxidation of NO (∼550 ppb) are reported using TiO2-based photocatalyst with high efficiency [112,113],and the typical experimental setup are displayed in Fig.21.This novel technology can overcome the disadvantage of photocatalysis,which suffers from rapid recombination of photogenerated electron holes.Secondly,nitrogen oxides are usually converted into uneconomic N2or NO3−in most reports.Direct electrochemical ammonia synthesis from nitric oxide has been realized very recently,which should be given priority in the future considering the value of NH3.Thirdly,exploring single atom catalyst suitable for NO removal is urgent,which is one of the hottest materials in the current state.Fourthly,coupling removal of NO and NH3through photocatalytic technology may enhance the purification of pollutants in atmosphere under mild conditions.Finally,the investigation of solving practical environmental and ecological problems,such as urban atmosphere,using the aforementioned technologies especially solar energy driven photo-catalysis and photoelectro-catalysis should be the prior choice.
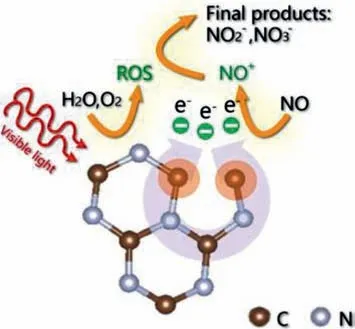
Fig.20.Illustration of photocatalytic NO removal on N defects sites containing g-C3N4.Copied with permission [89].Copyright 2020,Elsevier.
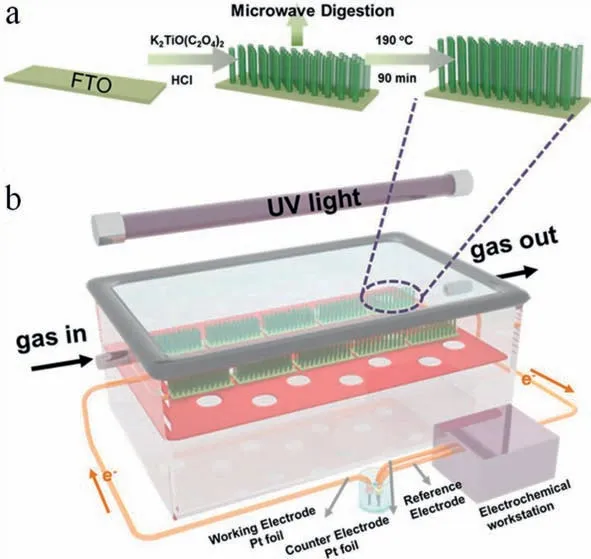
Fig.21.Schematic illustrations of the fabrication route for TiO2-NRs/FTO photoanodes (a) and the gas-phase PEC oxidation reactor (b).Copied with permission [112].Copyright 2020,American Chemical Society.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Nos.21703075,51872107,52073110,51902121),Natural Science Foundation of Hubei Province (No.2020CFB694) and Fundamental Research Funds for the Central Universities (No.2662020LXPY005).
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
- C–F bond functionalizations of trifluoromethyl groups via radical intermediates
