Nanostructured materials with localized surface plasmon resonance for photocatalysis
Jun Li,Zizhu Lou,b,∗,Bojun Li
a Institute of Nanophotonics,Jinan University,Guangzhou 511443,China
b State Key Laboratory for Crystal Materials,Shandong University,Ji’nan 250100,China
Keywords:Localized surface plasmon resonance Plasmonic photocatalysis Plasmonic semiconductor Hot electrons Solar energy harvesting
ABSTRACT Localized surface plasmon resonance (LSPR) enhanced photocatalysis has fascinated much interest and considerable efforts have been devoted toward the development of plasmonic photocatalysts.In the past decades,noble metal nanoparticles (Au and Ag) with LSPR feature have found wide applications in solar energy conversion.Numerous metal-based photocatalysts have been proposed including metal/semiconductor heterostructures and plasmonic bimetallic or multimetallic nanostructures.However,high cost and scarce reserve of noble metals largely limit their further practical use,which drives the focus gradually shift to low-cost and abundant nonmetallic nanostructures.Recently,various heavily doped semiconductors (such as WO3-x,MoO3-x,Cu2–xS,TiN) have emerged as potential alternatives to costly noble metals for efficient photocatalysis due to their strong LSPR property in visible-near infrared region.This review starts with a brief introduction to LSPR property and LSPR-enhanced photocatalysis,the following highlights recent advances of plasmonic photocatalysts from noble metal to semiconductorbased plasmonic nanostructures.Their synthesis methods and promising applicability in plasmon-driven photocatalytic reactions such as water splitting,CO2 reduction and pollution decomposition are also summarized in details.This review is expected to give guidelines for exploring more efficient plasmonic systems and provide a perspective on development of plasmonic photocatalysis.
1.Introduction
Evoked by present dilemma of energy shortage and environmental pollution,developing renewable energy is highly urgent.Solar energy as one of “green” energy resources has attracted worldwide attention,but its efficient utilization is a significant challenge.Photocatalysis,an emerging “green technology”,can convert solar energy to chemical fuels to address current global environmental and energy crisis [1–3].Since the discovery of Honda-Fujishima effect on TiO2photoelectrode in 1972 [4],semiconductor photocatalysis has dominated the research area for several decades[5–7].However,intrinsic wide band gaps (>3.1 eV) render the conventional semiconductors (TiO2,ZnO) solely responsive to ultraviolet (UV) light,accounting for only 4% of solar energy.Other visiblelight responsive semiconductors likeα-Fe2O3,are inherited with short charge-carrier diffusion lengths and suffer from poor charge separation efficiency,significantly hampering their applications in photocatalysis [8].In order to enhance the photoactivities of semiconductors,various methods have been proposed,such as doping external elements to tune their band structures for a broad optical response [9,10],constructing suitable heterostructure to retard the charge recombination [11,12],engineering surface architectures for more active sites [13,14]etc.Nevertheless,weak photoresponse and low quantum efficiency are still two major bottlenecks for largescale application of semiconductor photocatalysts.

Fig.1.Schematic illustration of LSPR excitation on metallic NPs.
Along with semiconductor photocatalysis,localized surface plasmon resonance (LSPR) mediated photocatalysis has fascinated much attention in solar energy conversion [15–19].LSPR is one physical phenomenon of free electrons oscillation of with incident photons confined on noble metallic nanoparticles (NPs) surface.Plasmonic metallic NPs (Au,Ag) were initially used to sensitize semiconductors and improve their photoelectric response by constructing noble metal/semiconductor hybrids [20–22].Owing to LSPR effect,plasmonic NPs can transfer solar energy to adjacent semiconductors to drive chemical reactions.Intriguingly,apart from acting as light absorber,plasmonic NPs themselves can also serve as active sites to directly induce catalysis.Plasmon-induced active hot charge carriers,electrons and holes can drive surface catalytic reaction and modulate the reaction path [23–26].LSPR excitation induces strong light harvesting,hot electron generation and localized heating effect,all of which could be beneficial for photochemical reactions [27–30].Although plasmonic noble metals exhibit favorable advantages over traditional semiconductors,high cost and scarce reserve significantly inhibit their practical applications in photocatalysis.Moreover,LSPR of noble metallic NPs mostly localize in visible light region and near-infrared light accounting for more than 50% of solar energy failed to be wellutilized.Note that non-noble metals like Al and Cu also exhibit LSPR properties,which are considered as strong competitors to noble metals in term of their optical response.While both Al and Cu are cost-effective,their wide applicability for photocatalysis is largely hindered by the instability under ambient conditions.For example,Al has an inherent tendency to form oxides upon exposure to air due to high reduction potential (1.66 V).Cu NPs are also prone to oxidation and lead to the formation of Cu2O or CuO.
Developing nonmetallic plasmonic nanostructures that enable broad-spectrum solar harvesting as potential substitutes of noble metals is of significance to sustainable and energy-efficient plasmonic photocatalysis.Recently,various semiconductors with heavy doping (WO3-x,Cu2–xS,MoO3-x,etc.) have emerged as a burgeoning research hotspot due to their strong LSPR absorption in visiblenear infrared (vis-NIR) region [31–38].There has been debate as to whether defect absorption or SPR is responsible for strong absorption of heavily-doped semiconductors.Alivisatos and Manthiram theoretically proved that the intense absorption of WO3-xis arised from the unique property of their outer-dvalence electrons,providing theoretical support for non-metallic LSPR effect[39].Since then,numerous nonmetallic plasmonic nanostructures have springed up as active photocatalysts and opened a new route for the development of photocatalysis [40–45].In this review,we introduce recent advances of plasmonic photocatalysts from noble metal to semiconductor-based plasmonic nanostructures and further discuss their characterization and photocatalytic applications(water splitting,artificial photosynthesis,pollution decompositionetc.) in detail.Finally,we also present an outlook for the development of plasmonic photocatalysts and provide guidelines for optimizing the existing plasmonic systems.
2.Localized surface plasmon resonance
2.1.LSPR property
LSPR is an intriguing phenomenon associated with collective oscillations of free electrons with incident light Fig.1).The extent of interaction between materials and light can be described by a complex dielectric function based on the Drude-Lorentz model as follows (Eqs.1–4):
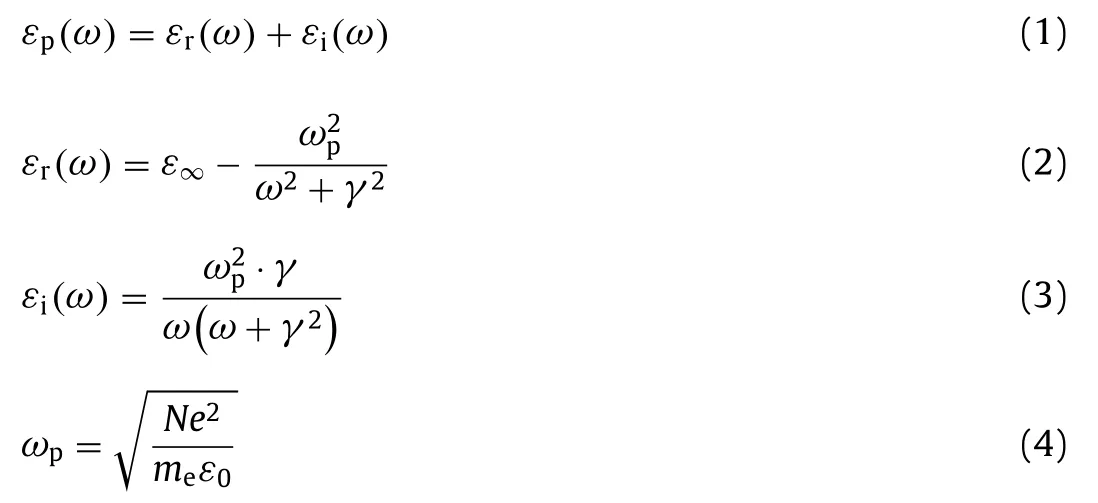
whereεpis dielectric function of materials,εrandεiare real and imaginary parts,respectively.ε∞is the high frequency dielectric constant of materials,ωpis plasma frequency,ωis light frequency,γis the damping constant that represents free carriers scattering,Nis free carriers density,meis the effective mass of free carriers,e is elementary charge andε0is the permittivity of vacuum.εris determined by electric field induced polarization,whereasεibasically corresponds to damping and energy loss upon absorption at NPs surface [46].As light irradiates on metallic NPs (Au,Ag,Cu),the associated electric field induces polarization of electrons density to one surface.At plasmon wavelength,electrons on NPs surface occur resonant oscillation to exhibit a strong LSPR absorption[47–49].
The LSPR wavelength of metallic NPs largely depends on the metal nature,morphology and dielectric constants of the surrounding media (Fig.2).For example,by varying the geometry of Ag NPs from spheres to wires or cubes,both LSPR modes and wavelength are changed [18].Moreover,LSPR wavelength also changes with the surrounding media due to variation in dielectric constant.The LSPR wavelengths of Ag nanocubes were found to red shift as the surrounding refractive index of solvents increased [50].The longitudinal plasmon wavelength of Au nanorods (NRs) exhibits a nearly linear relation with aspect ratio as well as refractive index of surrounding solvents [47,51].By tailoring these parameters,the corresponding photoresponse of metallic NPs can be finely manipulated,which is expected to improve the solar utilization.
The LSPR phenomenon is not restricted to metallic NPs and could also be observed in semiconductors with high free carrier density.Similar to noble metals,LSPR in semiconductors are originated from resonant interaction of free carriers and incident light,as described by the complex dielectric function.However,unlike metals,LSPR in semiconductors can be induced by free electrons or holes depending on the dopant nature,which are labeled as n-type or p-type plasmonic semiconductors,respectively [52].Besides,LSPR wavelength in semiconductors is largely up to free carrier density.Luther et al.investigated the effect of free carrier density on LSPR frequency (wavelength).For NPs size in the range of 2–12 nm,carrier density of 1019–1022cm−3generates LSPR in infrared range while carrier density below 1019cm−3is too low to support one LSPR mode [32].Due to lower carrier density in comparison to noble metals,semiconductors usually exhibit LSPR in broad Vis-NIR region.For example,p-type self-doped copper chalcogenide (Cu2-xX,X=S,Se,Te) nanocrystals (NCs) exhibit LSPR absorption in NIR region generated by free holes oscillation(Figs.3a–c) [53].Control over dopant type and extent can impact the free carrier density,leading to different LSPR energies and spectral shapes within the same host material.Fanget al.reported that for the same doping level,LSPR is at lower energy and has a smaller bandwidth for Ti-doped than Sb-doped In2O3NCs,indicating lower free carrier density due to the formation of deep defect states within Ti:In2O3band gap [54].Similar phenomena have been widely reported across several metal oxides.The plasmonic behavior of Cu2-xS NCs also can be tunedviachanging the composition with different Cu+doping extent.By a versatile postsynthesis reaction,the Cu/S ratio in NCs could be gradually increased from 1.1:1 to 2:1 while preserving their size and morphology.With increasing incorporation of Cu,the LSPR band of covellite Cu2-xS NCs red-shifted and decreased in intensity until it vanished for NCs with Cu2S composition.It is mainly attributed to the decrease of free carrier density in NCs due to the increase in Cu stoichiometry [55].A similar red shift and an increase in full-width at half maximum of LSPR band were also observed with increasing of Cu/S feed ratios,ascribing to the change of free carriers density induced by phase transformation [56].In addition to free carrier density modulation,the shape parameters and crystal structure also greatly influence the LSPR of semiconductor NCs.The Cu2−xX exhibits a diverse crystal phases and morphology,which have a huge impact on their plasmonic response.The djurleite Cu1.94S nanodisks display two LSPR peaks in NIR and Mid-IR regions,while both roxbyite Cu1.8S and covellite CuS have only one peak in NIR region [33](Fig.3d).Cu2–xS nanodisks have two planes labeled as “in-plane” and “out-of-plane”,where each plane corresponds to one LSPR mode.The characteristic LSPR wavelengths vary with the aspect ratio (diametervs.thickness) and carrier density of nanodisks [57].Increasing the aspect ratio induces out-ofplane plasmon to blue-shift and in-plane to red-shift,consistent well with Mie scattering theory (Fig.3e).As only hole carrier density is increased,both out-of-plane and in-plane plasmon are expected to blue-shift according to Drude theory (Fig.3f).These results above indicate that both morphology (size,shape,aspect ratio etc.) and the free-carriers density (related to composition and phase) should be carefully considered to achieve dynamic tailoring for LSPR characteristics of semiconductor NCs.
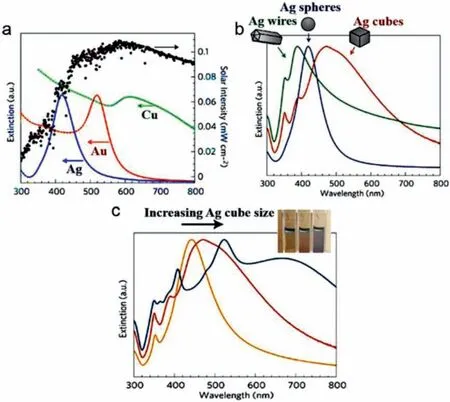
Fig.2.The plasmonic resonant wavelength dependence of metallic NPs on their (a) metal species,morphology of (b) shape and (c) size.Reproduced with permission [18].Copyright 2011,Nature Publishing Group.
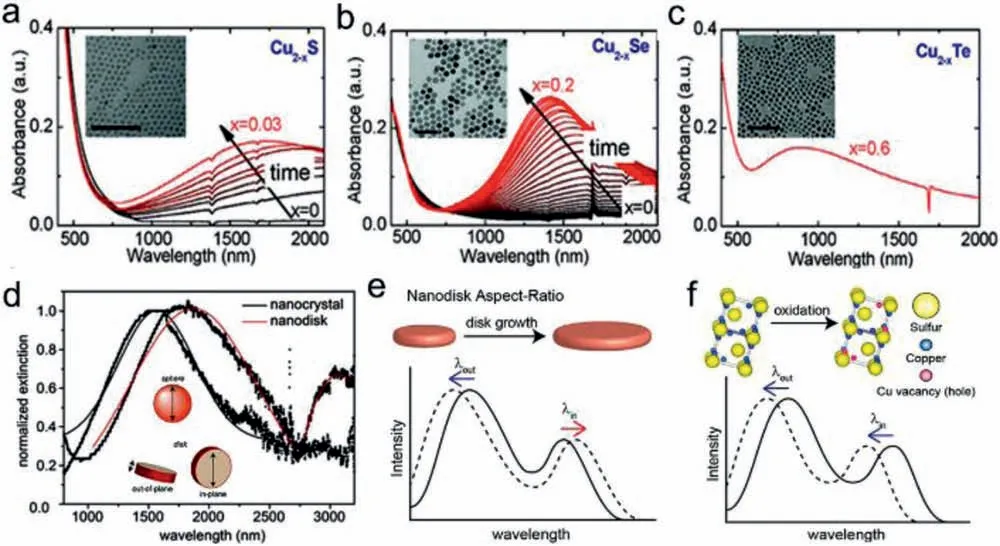
Fig.3.(a–c) Extinction spectra and TEM images of Cu2-xS (x=0–0.03,a),Cu2-xSe (x=0–0.2,b) and Cu2-xTe (x=0.6,c) NCs.Reproduced with permission [53].Copyright 2011,American Chemical Society.(d) Shape-dependent extinction spectra of Cu2–xS NCs with sphere and nanodisks.Reproduced with permission [33].Copyright 2011,American Chemical Society.The LSPR dependence of Cu2–xS nanodisks on (e) aspect ratio and (f) free carrier density.Reproduced with permission [57].Copyright 2012,American Chemical Society.
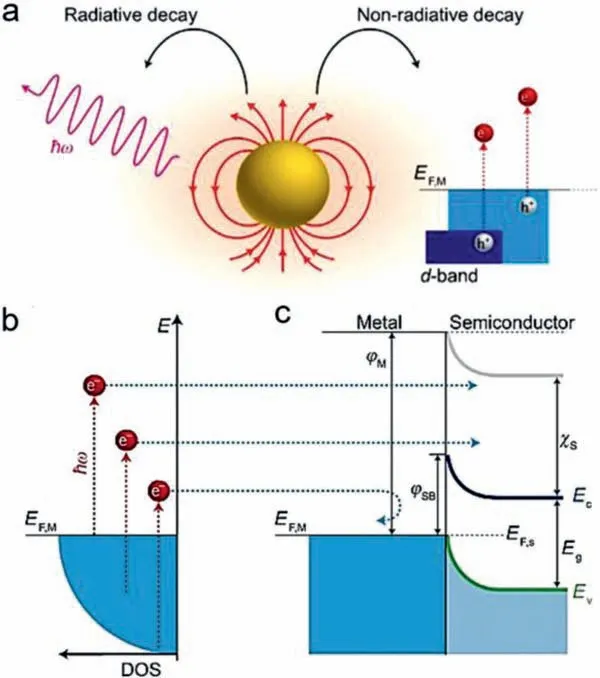
Fig.4.(a) LSPR undergo decay by either radiative photons emission or nonradiative relaxation to generate hot electrons.(b) Hot electrons with a wide distribution range in energy.(c) Hot electrons with large energy can be injected to CB of adjacent semiconductors.Reproduced with permission [58].Copyright 2011,Nature Publishing Group.
2.2.LSPR-enhanced photocatalysis
The LSPR enables significant electric field enhancement on metallic NPs surface,known as near-field enhancement,which is beneficial for efficient light absorption and charge-carrier generation [58–60].This near-field enhancement attenuates exponentially with the distance away from metal NPs surface and spatially limited to nanoscale “hot spots” region around the surface.The excited LSPR undergo decay by either radiative photons emission or nonradiative relaxation (Fig.4),and the latter forms the basis of photocatalytic applications.The hot electrons are generated during the nonradiative decay through intra- or inter-band electrons transition.For Au and Ag NPs,owing to their fairly low-lyingdband below Fermi level,hot electrons generate primarily via intraband transitions within conduction band (CB) and the energies exhibit a wide distribution range of 1–4 eV [18,59,61].Ultrafast measurements have shown that these hot electrons rapidly thermalize in isolated NPs,and the lifetime is extremely short in the range of 1–100 ps [58].However,as metallic NPs are married with semiconductors,hot electrons with sufficient energy can be injected to CB of adjacent semiconductors across interfacial Schottky barrier to impede the decay,greatly altering their dynamics[59,62,63].Similar to conventional electron transfer,hot electron injection is manipulated by the heterostructural interfacial contact and band alignment [25,59,64,65].Therefore,elaborate structural engineering is highly needed for a fast hot electron injection to avoid the decay.For plasmonic semiconductors,LSPR-excited hot carriers (electrons or holes) also exhibit a similar ultrafast dynamic with that of metallic NPs.
During plasmonic photocatalytic process,plasmon-induced hot electrons can be transferred to adjacent semiconductor or directly interact with molecules adsorbed on plasmonic NPs surface[24,66–68].The extracted hot electrons can activate specific chemical bonds of reactants and modify the reaction pathway,making a big difference to products selectivity [69].Besides,hot electrons enable accelerated desorption of certain surface-adsorbed species,further promoting the catalytic activity and simultaneously prolong the stability of plasmonic NPs [70].The hot electrons have been demonstrated to play dominant role in various plasmon-enhanced chemical reactions including CO2reduction [71–73],H2evolution[74–76]etc.However,compared to widely studied photochemistry driven by hot electrons,the ones that driven by plasmonic hot holes have received less attention,probably due to much shorter lifetime of hot holes than hot electrons [77].The hot carriers that do not participate in reaction could subsequently dissipate their energy to lattice phononviaelectron-phonon scattering,inducing considerable photothermal effect (heating),which is also favorable for chemical conversion since both reaction rates and routes are closely related to temperature [78–81].The photothermal heating is an effective driving force to accelerate the reaction by promoting the adsorption-desorption of surface adsorbent and shifting chemical equilibria toward higher products yield [82,83].In plasmonic photocatalysis,by structural design of plasmonic catalysts,hot carrier driven reactant activation and photothermal effect could coexist and synergistically make positive contributions to enhance plasmonic catalytic efficiency [84–87].
The electric near-field enhancement,plasmonic hot carriers and thermal effect are representative features of LSPR,which are mostly investigated in plasmonic photocatalysis and offer guidelines for developing efficient photocatalysts.To date,based on these plasmonic effects,various plasmonic nanostructures have been reported as active photocatalysts and herein is primarily divided into two big categories of noble metal-based and nonmetallic plasmonic photocatalysts according to the components.The synthesis,LSPR property and photocatalytic applications of these photocatalysts are discussed in details below.
3.Noble metal based plasmonic photocatalysts
3.1.Metal-semiconductor composites as plasmonic photocatalysts
For conventional semiconductor photocatalysts,narrow photoresponse range and low quantum efficiency are two major challenges for their practical applications.The controlled integration of semiconductor with plasmonic noble metals (Au,Ag) has been considered as an effective route to improve the situation.In 2008,Awazu group deposited TiO2on Ag/SiO2core-shell structures and initially proposed the concept of “plasmonic photocatalysis”.The enhanced electric field on Ag NPs surface was pioneered to afford better photocatalytic performance for decomposition of methylene blue (MB) [20].The same year,Huang group proposed a stable plasmonic photocatalyst of Ag@AgCl with strong absorption in visible region for efficient degradation of organic dyes [21].Compared with spherical NPs with only one LSPR mode,anisotropic metallic NPs with multipolar LSPR modes could achieve broader vis-NIR light harvesting.Liuet al.prepared Au NRs loaded TiO2phototcatalysts and found that both transversal and longitudinal plasma of Au NRs (with tunable adsorption from 630 nm to 810 nm) could induce photocatalytic 2-propanol oxidation [88].The adjustable light adsorption caused by tunability of Au NRs aspect ratio is benefit to developing photocatalyst with specific light harvesting.Moreover,Au NRs and metal NPs (Au,Ag or Pt) co-decorated CdS nanowires(NWs) ensemble with tunable and progressive harvesting of vis-NIR light (λ >570 nm) has also been reported for distinctly boosting photoreduction of 4-nitroaniline and water splitting to H2[89].The results show that finely progressive control over a series of factors,including interfacial interaction,morphology optimization and cocatalyst addition in metal-semiconductor (Au NRs-CdS) heterostructures,can lead to tunable and improved performance for vis-NIR driven plasmonic photocatalysis.
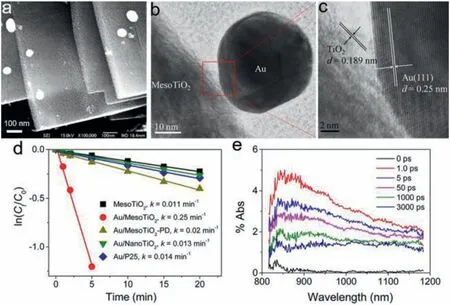
Fig.5.(a) SEM images and (b,c) HRTEM images of Au/MesoTiO2.(d) Kinetic linear fitting curves for photocatalytic MB degradation over different samples under 460–700 nm light irradiation.(e) Time-resolved DRS observed after 530 nm laser flash photolysis of Au/MesoTiO2.Reproduced with permission [90].Copyright 2013,American Chemical Society.
In plasmonic metal-semiconductor photocatalyst,plasmoninduced hot electrons can be injected into CB of nearby semiconductors across interfacial Schottky barriers,dominantly driving the photocatalytic redox reaction.Bianet al.successfully deposited Au NPs on TiO2mesocrystals by a simple impregnation method (Fig.5) [90].The diffuse reflectance spectroscopy (DRS)measurements demonstrate that LSPR-induced hot electrons are injected from Au NPs to TiO2mesocrystals,directionally migrate from basal surfaces to the edges of plate-like TiO2nanocrystal.Such anisotropic electron flow significantly retarded the charge recombination of hot carriers in Au NPs,thereby improving visiblelight driven photocatalytic organic-pollutant degradation.
Plasmon-induced hot electron injection in metal-semiconductor system provides a promising avenue to improve the efficiency of photocatalysis.To this end,the metal-semiconductor interface is of significance and should be elaborately engineered to facilitate rapid hot electron injection that is competitive with ultrafast hot electron relaxation.Apart from surface decoration,core-shell geometry is favorable in view of its maximized contact and optimal configuration for efficient charge transfer.Yadong Li group produced core-shell structures of Au@MS (M=Cd,Zn) with controllable shapes and unique atomically organized interfacesviaaqueous cation exchange-facilitated nonepitaxial growth.Beneficial from interfacial- and structural-optimization,Au@MS enables improved hot electron injection with an estimated quantum yield of∼48% for boosting plasmon-enhanced photocatalytic H2evolution[74].Recently,various Au NR@ZnO core-shell photocatalysts with tunable shell thickness were also successfully prepared to achieve solar-driven CO2conversion to CH4,further demonstrating the redox reactivity of plasmonic hot electrons.With ZnO shell acting as electron acceptor,the hot electrons prolonged the lifetime,which are more conducive to being captured by absorbed molecules to participate in the reaction.Both of Au core and ZnO shell are coexcited by solar light,synergistically contributing to significant enhancement of CO2photoreduction [91].
The coupling manners between metal and semiconductor are also crucial for photocatalytic performance of plasmonic metalsemiconductor photocatalysts.Instead of depositing metallic NPs onto semiconductor,anisotropic growth of semiconductor onto Au NPs afford better interfacial contact between them.Besides,anisotropic metallic NPs with high-curvature sites such as nanocubes corners and nanocones tips,are found to be locations for maximum electric field distribution compared with spherical NPs [92].Because of the lightning rod effect and decreased radiative damping,anisotropic metallic NPs with high-curvature sites concentrate the incident energy and largely facilitate plasmonic hot electrons generation and transfer,which play significant roles for activating surface chemical reaction [93].Therefore,controlled selective overgrowth of semiconductors at high-curvature sites of metallic NPs is valid strategy to obtain active plasmonic photocatalysts.Au triangular nanoprisms (Au TNPs) with unique anisotropic structures and multipolar LSPR modes,offer diverse sites of corner,edge and surface for coupling with semiconductors to construct various plasmonic photocatalysts.Lou et al.constructed anisotropic Ag2S-edged Au TNPs by controlling preferential overgrowth (Fig.6).Under vis-NIR light irradiation,Ag2S-edged Au TNPs achieve maximum H2generation rate (796 μmol h−1g−1),almost four times higher than those of Ag2S-covered Au TNPs (216 μmol h−1g−1)and pure Au TNPs (none).Single-particle photoluminescence (PL)measurements have been performed and an obvious PL quenching in Ag2S-edged Au TNPs demonstrates SPR-induced hot electron transfer from Au-TNPs to Ag2S for boosting H2generation[75].Plasmonic hot electrons have been demonstrated to play a vital role in photocatalysis.However,only a few hot electrons with high energy can cross the as-formed Schottky barrier between metal and semiconductor for photocatalysis.The photocatalytic efficiency of plasmonic metal-semiconductor heterostructure is still challenged by slow charge mobility,inefficient hot electrons utilization and lack of active sites for redox reactions.
3.2.Bimetallic/multimetallic composites as plasmonic photocatalysts
Due to fast electrons transfer between metallic parts,bimetallic/multimetallic NPs are expected to show unique performance in photocatalysis.Various bimetallic NPs with enhanced charge separation have been developed,including Au-Pt,Au-Pd,Au-Cu,etc.[94–97].In order to achieve efficient light utilization and high catalytic activity simultaneously,the intimate integration of plasmonic and catalytic metals is strongly desirable [98].Majima group developed anisotropic Pt/Pd-tipped Au NRs,which simultaneously act as light absorber and catalytic active site to boost H2evolution from water splitting [94]and formic acid dehydrogenation [95],respectively (Fig.7).Both PL quenching and light scattering from single Pt/Pd-tipped Au NRs confirmed hot electron transfer from excited Au to Pt/Pd.To further promote generation and transfer of hot electrons,Tanget al.reported freestanding heterosuperstructures of Au NRs@Pd (Au@Pd SSs),where ordered Pd nanoarrays are precisely grown on AuNRs surfacesviaseed-mediated approach.With help of strong antenna effect in ordered Pd nanoarrays,plasmonic AuNRs engender abundant hot electrons to promote molecular oxygen activation;while vertical organization of Pd on Au NRs with small contact not only exposes rich active sites for reactants but also prolongs hot electron lifetime,largely enhancing C−C coupling reaction [99].
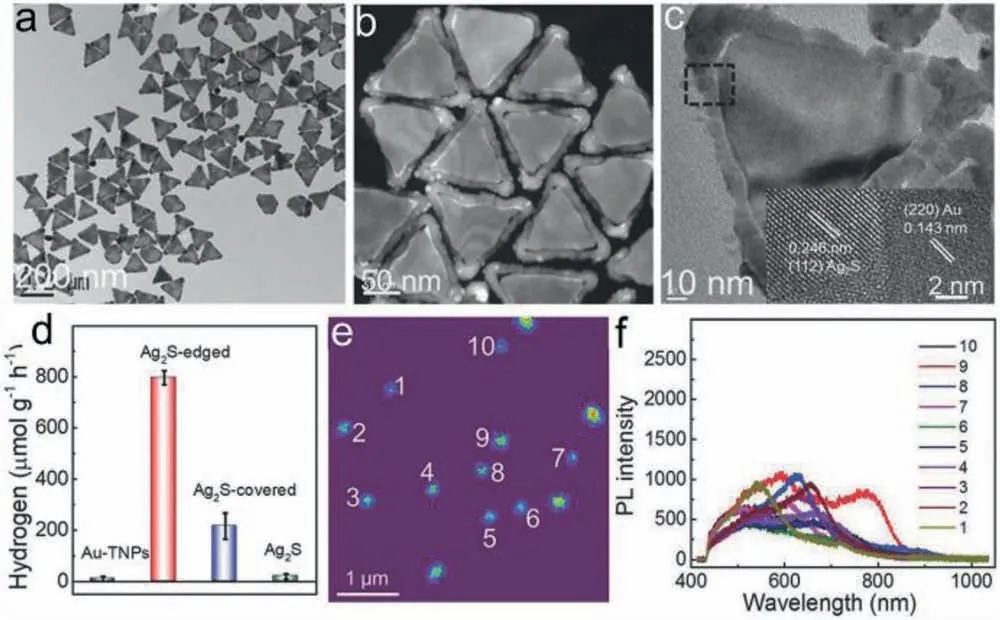
Fig.6.(a) TEM,(b) High-angle annular dark-field scanning TEM and (c) HRTEM images of Ag2S-edged Au TNPs.(d) Average H2 generation rates over Ag2S-covered,Ag2Sedged,pure Au TNPs and Ag2S photocatalysts under Vis-NIR light irradiation.(e,f) Single-particle PL image and spectra of Ag2S-edged Au TNPs.Reproduced with permission[75].Copyright 2018,Wiley-VCH.
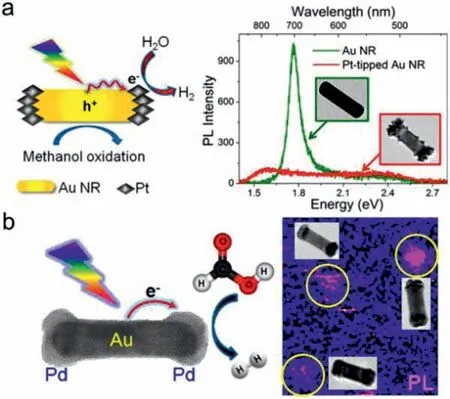
Fig.7.Single-particle PL study of (a) Pt-tipped Au NRs for plasmon-enhanced water splitting and (b) Pd-tipped Au NRs for plasmon-enhanced HCOOH dehydrogenation.Reproduced with permission [94,95].Copyright 2014,American Chemical Society.
Apart from 1D NRs,2D Au TNPs are also promising components for bimetallic plasmonic photocatalysis,which have multiple SPR modes including in-plane dipole SPR (DSPR),multipole SPR (MSPR)and out-of-plane SPR in visible-NIR region.Louet al.synthesized three types of anisotropic Pt-covered,Pt-edged,and Pt-tipped Au TNPs by seed and subsequently controlling overgrowth,as shown in Figs.8a–f.With intense electric field and more interface contact,Pt-edged Au TNPs facilitates hot electron transfer,leading to superior photocatalytic activity for H2generation than the other two.By single-particle PL spectra,in-plane DSPR mode of Au TNPs was demonstrated as the dominant channel for hot electrons transfer to edged Pt [100].Byin situetching of single Au TNP,2D Au obtuse TNP (O-TNP) and nanodisk were obtained to construct various anisotropic Pt-Au heterostructures as plasmonic photocatalysts[101].The Pt-edged Au O-TNP with larger tip area and electric field enhancement is beneficial for hot electron transfer and charge separation,resulting in higher efficiency in photocatalytic H2generation (Figs.8g–i).To improve charge separation,reduced graphene oxide (rGO) with high conductivity was introduced to facilitate hot electrons transfer from plasmonic to catalytic metals.Ternary 2D Au-TNP/rGO/Pt nanoframe (NF) as plasmonic photocatalysts are preferable for boosting H2generation under vis-NIR light.The hot electrons generated on Au TNP are quickly transferred to rGO and further collected by loaded Pt NF cocatalyst,leading to efficient charge separation for high photocatalytic activity [102].Recently,Huanget al.reported multimetallic heterostructure of Pd-dotted Ag@Au hexagonal nanoplates (HNPs),where plasmonic core-shell Ag@Au structure act as an ideal light absorber and Pd nanodots provide more catalytic active sites [103].The heterostructure exhibits an excellent catalytic activity for formic acid (HCOOH) dehydrogenation even at 0 °C with turnover frequency of 1062 h−1,due to plasmon-induced hot electron transfer from Ag@Au HNPs to Pd dots.
By analyzing the relaxation path,most hot carriers may undergo further thermalizationviaelectron-phonon scattering and lead to photothermal heating,which is also an effective driving force for accelerating surface reaction.However,the two plasmonic effects of hot-electrons and photothermal conversion are usually entangled,making it hard to quantify their individual contribution to chemical reactions.To distinctly differentiate these two effects,Huang and co-workers reported a bar-shaped core-shell Au@Pd system toward Vis-NIR light-driven catalytic styrene hydrogenation,since that hot electrons have been considered as detrimental to hydrogenation [104].Intriguingly,the Pd shell thickness could serve as a knob to maneuver the processes of hot-electron transfer and photothermal conversion,building a platform for unraveling their roles in catalytic reactions.As Pd shell thickness was tuned to 14 atomic layers precisely,photothermal heating effi-ciency was maximized while the side effect of plasmonic hot electrons was suppressed.Consequently,owing to plasmonic thermal effect,Au@Pd achieved high conversion yield of 76% in photocatalytic styrene hydrogenation to ethylbenzene,comparable to that achieved via thermally driven process at 80 °C.For most chemical reactions,both hot electrons and photothermal effect make positive contributions,synergistically enhance plasmonic catalytic effi-ciency.
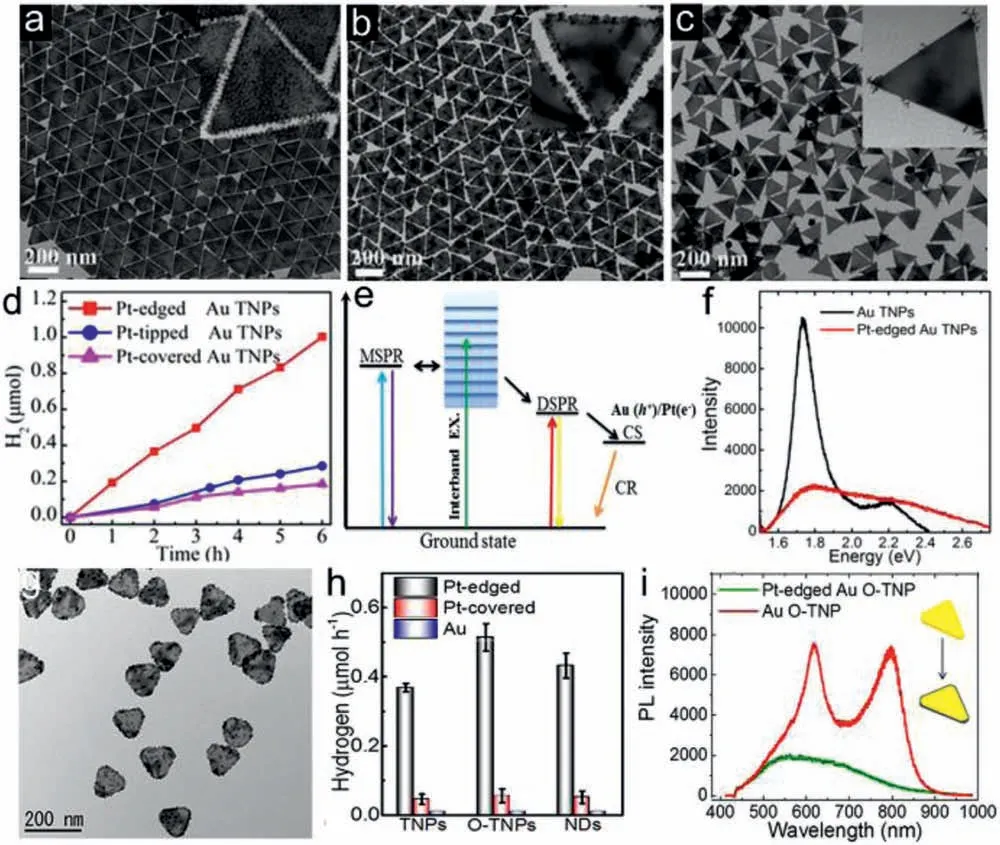
Fig.8.TEM images of (a) Pt-covered,(b) Pt-edged and (c) Pt-tipped Au TNPs.(d) H2 evolution over different photocatalysts.(e) Schematic diagram of plasmon radiative decay in Au TNPs.(f) Single-particle PL spectra.Reproduced with permission [100].Copyright 2016,American Chemical Society.(g) TEM image,(h) H2 generation rates and(i) Single-particle PL spectra of Pt-edged Au O-TNPs.Reproduced with permission [101].Copyright 2017,American Chemical Society.
There are several distinct features of hot carrier contribution to chemical reaction that can be used to experimentally differentiate from photothermal effect including: (1) Linear dependence of reaction rate on light intensity due to the fact that hot carrier generation rate increases linearly with photon flux [105],(2) a higher kinetic isotope effect or modified products selectivity in chemical reaction compared with that driven by thermal energy at same reaction temperature [106],(3) similar tendency of apparent quantum efficiency (AQE) with LSPR absorption spectrum of plasmonic NPs [73].A clear separation of these two effects facilitates the rational design of plasmonic photocatalysts for efficient photochemical applications and solar energy utilization.
4.Nonmetallic materials as plasmonic photocatalysts
Recently,various nonmetallic nanomaterials have emerged as potential LSPR hosts and can be classified based on the origin of their LSPR properties.The ones that would be discussed in this review are heavily doped metal oxides (MoO3-x,WO3-x,NiO),metal chalcogenides (Cu2-xS,Cu2-xSe),and other semiconductors(TiN,Bi2WO6,BP)etc.We will highlight the development of these plasmonic semiconductors regarding on their synthesis methods and their promising applicability in plasmon enhanced photoredox catalysis such as H2evolution,CO2reduction,nitrogen reduction,pollution decomposition.
4.1.Plasmonic metal oxides
Plasmonic metal oxides with rich oxygen vacancies (OVs) have drawn tremendous interest in photocatalysis owing to their abundant reserve,facile synthetic method and good electrochemical stability.The OVs,basically a type of intrinsic defects,can serve as electron donor sites to modulate optical properties of a material [107].Introduction of rich OVs is an effective strategy to increase free electrons density in semiconductors,inducing strong LSPR properties.In addition,OVs also facilitate charge transfer between semiconductor and adsorbed intermediates,thereby leading to enhanced photoredox reaction.Mostly reported metal oxides with OVs-induced LSPR are WO3-xand MoO3-x,which have been widely used for plasmonic photocatalysis.
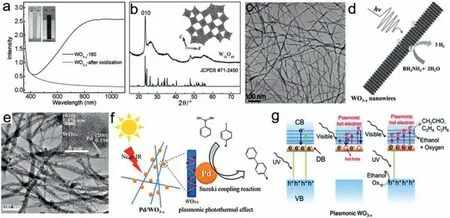
Fig.9.(a) UV–Vis absorption spectra,(b) XRD patterns,(c) TEM image and (d) schematic photocatalytic H2 evolution from ammonia borane over WO3−x NWs.Reproduced with permission [112].Copyright 2015,WILEY-VCH.(e) TEM image and (f) schematic photocatalytic Suzuki coupling reactions over Pd/WO3−x NWs.Reproduced with permission [113].Copyright 2015,Elsevier B.V.(g) Photoelectron and hot electron generation in self Z-scheme heterostructure of WO3−x under UV-Vis irradiation.Reproduced with permission [114].Copyright 2019,the Royal Society of Chemistry.
4.1.1.Tungsten trioxide
Tungsten trioxide (WO3-x),an electrochromic material with suitable band gap of 2.6 eV,exhibits great potential in photocatalytic solar conversion.By removing oxygen atoms,disorder structure is formed in WO3lattice with large amounts of oxygen vacancies.The low valence state of W5+are generated,forming different non-stoichiometric oxides such as W20O58,W18O49and W24O68[108].With more introduction of OVs,the properties of WO3-xare changed from semiconductor to metal,exhibiting strong LSPR in vis-NIR region.Various methods have been reported to synthesize WO3-xwith abundant OVs for enhanced photocatalysis.For instance,by annealing pristine WO3under H2atmosphere or vacuum at different high temperatures,WO3-xsamples with controlled OVs has been prepared successfully,displaying an improved photocurrent density [109,110].Wanget al.tailored the amount and distribution of OVs on surface or bulk by tuning H2concentration during thermal treatment [111].The amounts of bulk OVs on WO3monotonically rise with increasing of H2concentration,while that of surface OVs presents a volcano-type variation tendency.The WO3sample thermal-treated in 20% H2(WO3-H2O) contains the largest amount of surface OVs,which play more decisive role to achieve higher charge-carriers separation efficiency and photocatalytic O2evolution from water splitting.
One mostly used method to synthesize WO3-xis simple surfactant-free solvothermal treatment.Louet al.synthesized WO3-xNWs via one-step solvothermal treatment using WCl6or W(CO)6as precursors and absolute ethanol as solvents.Owing to rich OVs on surface,WO3-xNWs exhibit strong LSPR absorption in vis-NIR region and greatly promote H2generation from ammonia borane and Suzuki coupling reactions (Figs.9a–f) [112,113].Louet al.found that under UV–vis irradiation,the unique electronic band structure of plasmonic WO3−xcan act as a self Zscheme heterostructure (Fig.9g).UV-excited photoelectrons are injected into conduction band of WO3−x,stabilizing free electron density and accelerating plasmonic hot electron generation for photocatalytic ethanol dehydrogenation to aldehyde [114].Moreover,both morphology and crystalline phase of WO3-xwere affected by species/concentration of precursors and solvents,temperature and time in solvothermal reaction [115].Interestingly,NIR light excited plasmonic photothermal effect can make a big difference to the products selectivity.Under UV-vis-NIR irradiation,the synthesized WO3−xNWs with abundant OVs selectively promote ethanol dehydration,yielding a remarkable ethylene generation rate of 16.9 mmol g−1h−1.Besides,plasmonic WO3−xwith thin amorphous surface was obtained using simple hydrochloric acid-assisted solvothermal method and also exhibits superior photocatalytic performance on CO2reduction reaction [116].Intermediates detection indicates that the adjacent OVs around W4+on amorphous surface of WO3-xact as active sites for C−C coupling,leading to high-selective ethylene generation.
4.1.2.Molybdenum trioxide
Similar to WO3-x,Molybdenum trioxide (MoO3-x) is another reported metal oxide with OV-induced LSPR properties for solardriven photocatalysis.Yamashitaet al.firstly reported that plasmonic MoO3-xnanosheets display an enhanced H2production from ammonia borane under visible light [40](Figs.10a–c).To date,plasmonic MoO3−xhas been successfully synthesizedvialiquid exfoliation approach,solvothermal method,soft template synthesis method,CO2assisted approach [117–121]etc.For example,Liuet al.reported to obtain amorphous plasmonic MoO3−xnanosheets by MoS2oxidation and subsequent supercritical CO2-treatment[117].Etmanet al.report the synthesis of MoO3−xnanosheetsviaa liquid exfoliation approach to achieve efficient photocatalytic dye degradation [118].Liet al.synthesized MoO3−xnanobelts by a simple solvothermal method,which are utilized for photocatalytic N2fixation [119].The surface OVs of MoO3−xwas found to chemisorb N2molecule and reduce its activation energy,playing a critical role in photocatalytic N2reduction to NH3(Figs.10d–f).The obtained MoO3-xviasolvothermal method also exhibit high activity for photo-thermal synergistic CO2reduction under UV–vis-NIR irradiation.OVs-induced LSPR in MoO3-xenables intense absorption in NIR region,leading to a strong thermal effect.Besides,OVs also improves CO2adsorption on the defective surface,decreases the barrier of CO2hydrogenation and carrier recombination during catalytic CO2conversion [121].
Apart from WO3-xand MoO3-x,Li group recently synthesized a new plasmonic semiconductor Bi2O3-xwith rich OVs by oxidizing commercial bismuth powder in atmosphere at 453.15 K (Figs.10g–i).The OVs induce LSPR in Bi2O3-xacross the wavelength range of 600–1400 nm and enhance CO2molecules adsorption,which enable efficient photocatalytic CO2reduction to CO (100% selectivity)under low-intensity NIR irradiation.Similar to noble metals,the photocatalytic CO generation rate over Bi2O3-xshows a nearly linear dependence on light intensity and temperature,which suggests that catalytic performance originates from the synergetic effect between light irradiation and heating [122].
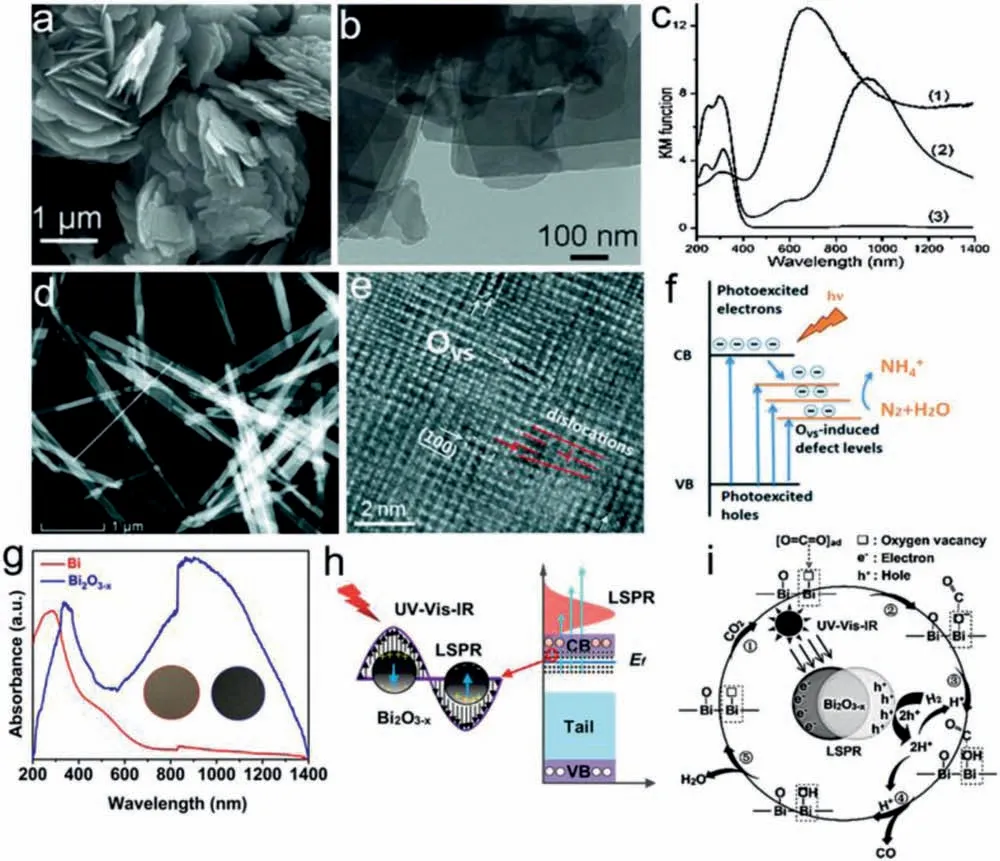
Fig.10.(a) SEM image,(b) TEM image and (c) UV-vis-NIR DRS of MoO3-x nanosheets.Reproduced with permission [40].Copyright 2014,Wiley-VCH.(d) HAADF STEM image and (e) Atomic scale HAADF image showing ordered OVs in MoO3−x nanobelts.(f) Electron transfer process during OVs-mediated N2 fixation.Reproduced with permission[119].Copyright 2019,the Royal Society of Chemistry.(g) Absorption spectra,(h) Schematic illustration of LSPR excitation and (i) possible pathway for photocatalytic CO2 reduction over Bi2O3−x.Reproduced with permission [122].Copyright 2020,Wiley-VCH.
4.1.3.Other metal oxides
Nickel oxide (NiO) with superior electrochemical stability has been widely studied in photoelectrocatalysis.Nevertheless,as a ptype semiconductor with intrinsic hole doping and carrier selftrapping of amorphous nature,NiO NCs fail to act as individual photocatalyst to offer electrons for reduction reaction.Linet al.reported 2D amorphous NiO nanostructure as a plasmonic photocatalyst for solar H2evolution without any cocatalysts [123].They prepared 2D plasmonic amorphous NiO nanoflakes (2DPA) by laser ablation of bulk crystalline NiO powders in methanol solution.The 2D architecture can suppress the self-trapping induced carrier recombination and introduce LSPR property in NiO by increasing the electron doping.The solar H2evolution rate over 2DPA photocatalyst was improved by a factor of 19.4 owing to plasmon-mediated charge releasing.Another metal oxide indium oxide (In2O3) has also attracted interest for tunable LSPR that occurs in near- to mid-NIR region by aliovalent ion doping.Two plasmonic systems of Ti and Sb doped In2O3NCs have been reported,which allow for a significant expansion and tunability of plasmon band [54].By a self-assembly technique,In2O3was encapsulated into bundled TiO2NW arrays,displaying broadband LSPR absorption of 84% in range of 400–2500 nm.This plasmonic photocatalyst achieved an improved catalytic activity for methyl orange degradation arising from LSPR effect of the In2O3membrane,which extends light response and excites hot carriers [124].
4.1.4.Metal oxides heterostructures
The OVs are easily removed in aqueous solution,leading to unstable LSPR effect and decreased catalytic efficiency.In addition,excited hot electrons on the surface can react with the adsorbates and the associated free electron density decrease,which further weaken the LSPR effect and hot electron generation.To solve this problem,Louet al.proposed the concept of electron injection to maintain free carrier density and stabilize LSPR effect of OVsdoped semiconductors by constructing heterostructures with other semiconductors [76].Heterostructural CdS/WO3−xNWs were synthesized byin-situsolvothermal growth of WO3−xon CdS NWs,which exhibit superior activity than WO3−xand CdS alone for photocatalytic H2generation.Single-particle PL study demonstrated that photogenerated electrons in CdS NWs are injected into conduction band of WO3−x,which stabilizes its LSPR effect and boosts continuous hot electrons generation for efficient photocatalysis(Figs.11a–c).Plasmonic heterostructure TiO2-mesocrystals/WO3−xNWs (TiO2-MCs/WO3−xNWs) were also constructedviaa simple solvothermal procedure,and used for photocatalytic CO2reduction [125].The obtained heterostructure exhibits much higher activity and selectivity (16.3 μmol g−1h−1,83%) than TiO2-MCs(3.5 μmol g−1h−1,42%) and WO3−xNWs (8.0 μmol g−1h−1,64%)for CH4generation (Figs.11d–f).The PL study demonstrates the photoelectron injection from TiO2to WO3−xboosting hot electron generation,which plays a great role in highly selective CH4generation from CO2reduction.For efficient photoelectron injection,Liet al.recently proposed to construct atom-sharing heterostructure of MoS2/MoO3–xwith good interfacial contact via light-inducedin situpartial oxidation of MoS2nanosheets [126].The OVs induce intense LSPR of MoO3–xand promote photoelectron injection from MoS2into MoO3–x,leading to stable LSPR and continuous hot electron generation for enhanced photocatalysis.An enhanced CO generation rate of 32.4 μmol g−1h−1with high selectivity of 94.1% was achieved over the heterostructure under UV–vis-NIR irradiation (Figs.11g–i).Wei group synthesized flexible MoS2@MoO3core-shell nanowires with tunable plasmon resonance using a twostep method,realizing broadband light absorption and improved interfacial contact for better carrier transport [127].The plasmonic MoS2@MoO3exhibits good stability and flexibility in photocatalytic water splitting and yields an optimized H2evolution rate of 841.4 μmol h−1g−1.
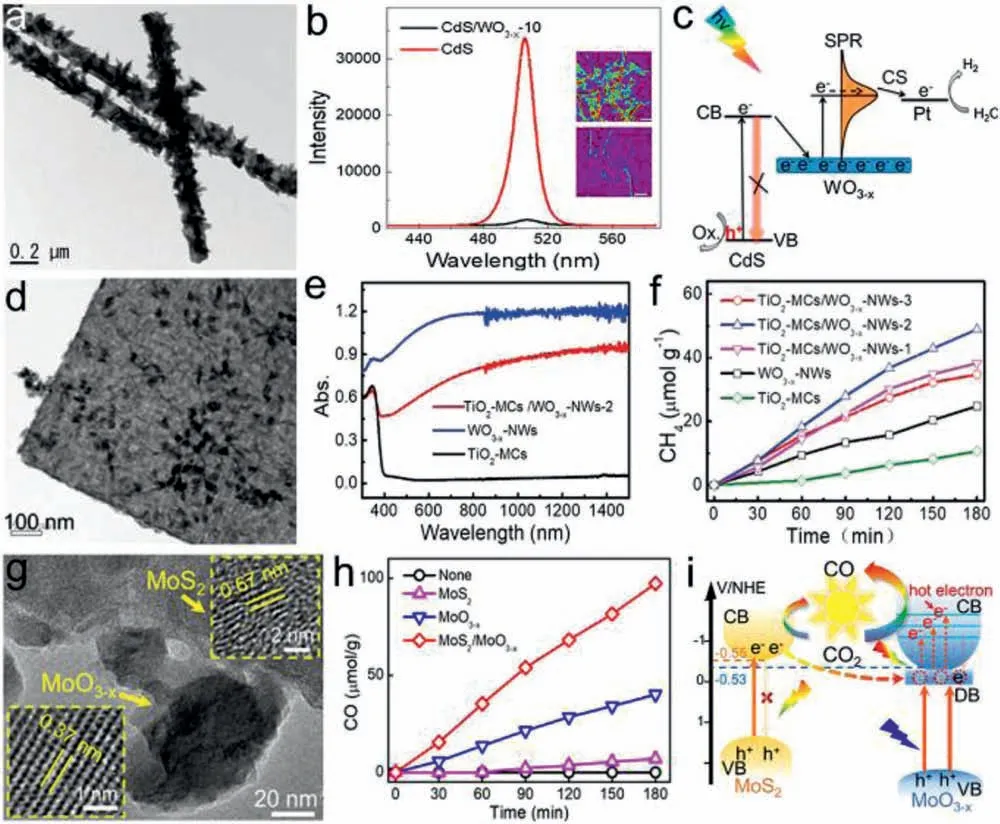
Fig.11.(a) TEM image of CdS/WO3−x.(b) Single-particle PL spectra and (c) schematic diagram showing the electrons injection from CdS to WO3−x.Reproduced with permission [76].Copyright 2017,Elsevier B.V.(d) STEM image of TiO2-MCs/WO3−x NWs.(e) Absorption spectra and (f) CH4 evolution over the as-prepared photocatalysts.Reproduced with permission [125].Copyright 2019,Wiley-VCH.(g) TEM images,(h) CO evolution and (i) excitation and electron transfer over MoS2/MoO3-x heterostructure.Reproduced with permission [127].Copyright 2021,American Chemical Society.
Similar to metals,plasmon-induced hot electrons are expected to play great roles on promoting catalytic reaction.By constructing semiconductor heterostructures,LSPR effect can be stabilizedviaphotogenerated carrier injection maintained free carrier concentration.However,currently reported semiconductor heterostructures still suffer from fast recombination of hot carriers,which hinders commercial application of plasmonic catalyst.Besides,single plasmonic semiconductor fail to meet the required potential to driven reduction reaction.A valid strategy to overcome these restrictions is to integrate plasmonic catalysts with appropriate active semiconductors for constructing a new generation of plasmonic heterostructures.Zhanget al.constructed a plasmonic Zscheme photocatalyst by solvothermally integrating 1D plasmonic W18O49nanograsses onto exfoliated 2D graphitic carbon nitride(g-C3N4) nanosheets [128].The plasmon-excited hot electrons of W18O49nanograsses can be injected into neighboring g-C3N4that possesses abundant active sites and strong redox capacity,boosting long-lived hot electron generation for improved photocatalytic protons reduction.Almost a full-spectrum-driven H2evolution effi-ciently was achieved over W18O49/g-C3N4heterostructure through the synergistic effect between Z-scheme charge-carriers separation and plasmon induced hot electrons injection (Figs.12a and b).They also fabricated W18O49/TiO2branched heterostructure via solvothermal growth of plasmonic W18O49NWs branches onto TiO2nanofiber backbones [129].Using ultrafast transient absorption spectroscopy combined with FDTD simulations,plasmonic hot electrons were demonstrated to transfer from W18O49branches to TiO2backbones within a very short timescale of 200 fs,much faster than the relaxation process (7–9 ps).Such an ultrafast transfer effectively improves the generation and separation of plasmonic hot electrons,thereby leading to an enhanced IR-driven catalytic activity for H2generation from ammonia borane (Figs.12c and d).By pulsed laser deposition and plasma sputtering reaction deposition,plasmonic Z-scheme core-shell W18O49/g-C3N4nanocone arrays were also successfully prepared to achieve more efficient plasmon-excited hot electron injection,spatial carriers separation and carrier lifetime extension [130].Besides,Z-scheme heterostructure photocatalyst of W18O49/CdS was also synthesized byin-situanchoring 0D W18O49quantum dots on the surface of 1D CdS NRs [131].Both bulk and surface photo-induced carriers are separated efficiently,achieving an improved photocatalytic H2evolution performance (Figs.12e–g).A Z-scheme BiO2-x/Bi2O2.75heterostructure photocatalysts with rich OVs was preparedviaa simple low-temperature hydrothermal method [132].The Zscheme interfacial heterojunction boosts the separation and migration of photoinduced charge carrier as well as improves the redox ability.Consequently,the as-prepared BiO2-x/Bi2O2.75exhibits an enhanced photocatalytic activity in RhB degradation compared to pure BiO2-x,ascribing to the synergistic effects of OVs-induced LSPR and Z-scheme heterogeneous interface.In addition to band alignment,steering an electron flow is of significance for developing efficient plasmonic catalysts.Wenet al.prepared plasmonic catalyst by coating ZIF-8 (zeolitic imidazolate frameworks) on plasmonic MoO3-xsurface and subsequent depositing Pd NPs on ZIF-8 (Pd/MoO3-x@ZIF-8) [133].Plasmon-induced hot electrons in MoO3-xare injected into ZIF-8 and further transferred to Pd active sites through a Schottky junction,which greatly accelerating plasmon-induced electron transfer from MoO3-xto Pd active sites.The heterostructure formed effectively retards the recombination of hot electron-hole pairs in MoO3-x,leading to a higher catalytic activities for nitroaromatics hydrogenation.
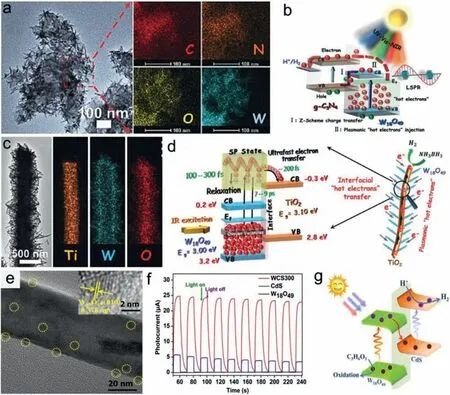
Fig.12.(a) TEM and elemental mapping images,(b) energy band and photoinduced charge-carriers transfer process of W18O49/g-C3N4 heterostructure.Reproduced with permission [128].Copyright 2017,Wiley-VCH.(c) TEM and corresponding elemental mapping images,(d) schematic kinetics process of plasmon hot electrons for H2 generation from NH3BH3 over W18O49/TiO2 branched heterostructure.Reproduced with permission [129].Copyright 2018,Wiley-VCH.(e) TEM images of W18O49/CdS heterostructure and corresponding HRTEM image of (010) plane of W18O49 component (inset).(f) Transient photocurrent response and (g) Illustration of H2 evolution mechanism over W18O49/CdS NRs.Reproduced with permission [131].Copyright 2021,Elsevier B.V.
4.2.Plasmonic metal chalcogenides
Metal chalcogenides with narrow bandgaps are one of potential candidates for photocatalytic applications.Similar to metal oxides,plasmonic property in chalcogenides also can be induced by introduction of vacancies.However,contrary to metal oxides,LSPR property in chalcogenides arises due to collective oscillation of cation vacancy-induced free holes in valence band (VB) rather than free electrons.Metal chalcogenides can be obtained by binding ligand-coordinated chalcogenide species to metal precursors.By changing metal-chalcogen ratios,tunable stoichiometric ratios and cation vacancies are achieved [52].An emerging class of copper chalcogenide materials for plasmonic photocatalysis will be discussed in this section.
Self-doped copper chalcogenide (Cu2-xX: X=S,Se,Te) have drawn great attention due to their LSPR absorption in NIR region,which is generated by the oscillation of Cu vacancy induced free holes.Various approaches have been reported to synthesize Cu2−xX with tunable crystal phase,stoichiometry and morphology,including solvothermal,hot-injection,wet chemical and templated method [134–138].Shaoet al.prepared a range of vacancydoped Cu2−xS NCs (Cu1.2S,Cu1.4S,Cu1.75S and Cu1.94S) with size of ∼10 nm through a hot injection method [138].By varying the injection volume of sulfur powder-oleic acid,the doping level of samples were manipulated to finely tune the LSPR wavelength.The obtained Cu1.94S NCs with the highest LSPR energy exhibited superior photocatalytic activity in dye degradation due to Cu vacancies induced high density of free holes.Ganet al.prepared Cu2−xSe NCsviaa hot injection method and observed plasmon-driven chemical reaction of 4-nitrobenzenethiol dimerization on Cu2−xSe surfaces with considerable yields [136].
The Cu2−xX-based heterostructures have attracted extensive attention because they can inhibit the photogenerated carriers recombination for improved photocatalysis.Plasmonic Cu2-xS nanodots were successfully deposited on two-dimensional (2D) g-C3N4nanosheets by one-step hydrothermal growth method [139].With efficient charge separation and strong light absorption,the 0D/2D plasmonic Cu2-xS/g-C3N4nanosheets achieved 100% degradation rate in 20 min for typical antibiotic pollutant,showing excellent catalytic degradation effect under UV–vis-NIR broad spectrum.Lianet al.synthesized CdS/Cu7S4heterostructured NCs by a seeded growth reaction of disk-shaped Cu7S4NCs and a subsequent partial cation exchange with Cd2+[140].The CdS/Cu7S4NCs achieved efficient photocatalytic H2evolution driven by nearto shortwave-IR light (up to 2500 nm) irradiation.The apparent quantum yield reached 3.8% at 1100 nm,which exceeds most IRlight energy conversion systems reported.Spectroscopic results revealed the plasmon-induced hot electron injection and long-lived charge separation (>273 μs) at p−n heterojunction of CdS/Cu7S4NCs,which contributes to efficient IR light to hydrogen conversion (Figs.13a–d).Moreover,a hollow sandwich-layered octahedral structure of Cu2−xS/CdS/Bi2S3with p−n−p type tandem heterojunctions was constructed via continuous growth deposition method [141].This unique structure provides large surface area,rich reaction sites and improved separation and transfer of photogenerated carriers.Under Vis-NIR irradiation,Cu2−xS/CdS/Bi2S3as photocatalyst displays a high H2production rate (8012 μmol h−1g−1),and 2,4-dichlorophenol is almost degraded completely in 150 min.Though Cu2−xS primarily produce hot holes,the electrons of hot electron−hole pairs appear to be more active to drive chemical reactions in Cu2−xS-based photocatalysts above,possibly due to the shorter lifetime of holes compared with electrons.From the kinetic perspective,it is difficult to achieve sufficient collection of hot holes for photocatalysis.Hot holes transfer has been proposed as a possible mechanism for contributing to photocatalytic activity by constructing heterostructured nanocrystals (HNCs) composed of plasmonic Cu2−xS and other semiconductors acting as acceptor.Lianet al.elucidate LSPR-induced hot holes transfer in CdS/CuS HNCs using time-resolved infrared (TR-IR) spectroscopy(Figs.13e–h) [142].The spectroscopic results provide an insight into a novel multi-step holes transfer mechanism named plasmoninduced transit carrier transfer (PITCT),in which the excited hot holes were not directly injected into CdS phase,but transferred stepwise through the carrier trapping state.Schematic decay processes of hot holes generated in CuS and CdS/CuS HNCs are shown in Fig.13h.For single CuS,the generated hot holes decayedviaphonon–hole scattering (1),hole trapping to the shallow state (2)or deep state (3),followed by relaxation to intrinsic hole state.For CdS/CuS HNCs,the holes in deep state transferred to VB of CdS(4,PITCT) and then the holes in CdS moved to trapping state,displaying structureless absorption in vis-region and recombination to the initial state.The PITCT of CdS/CuS HNCs realizes high quantum yield of 19% and longlived charge separation (9.2 μs),contributing to a superior oxidation catalytic activity for MB dye than those of CuS or CdS NCs alone under NIR irradiation.
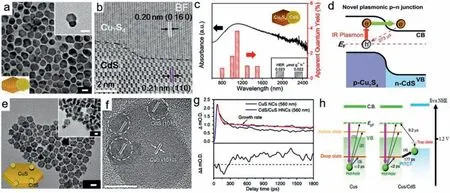
Fig.13.(a) TEM image,(b) bright-field scanning TEM image and (c) absorption spectrum and AQY for H2 evolution reaction over CdS/Cu7S4 HNCs.(d) Hot-electron injection of plasmonic p–n CdS/Cu7S4 HNCs upon IR light excitation.Reproduced with permission [140].Copyright 2018,American Chemical Society.(e) TEM image and (f) HRTEM image of CdS/CuS HNCs.Scale bars: 10 nm.(g) Decay profiles of CuS and CdS/CuS HNCs at 560 nm,in which the rising part corresponds to the trapped holes of CdS in CdS/CuS HNCs.(h) Schematic illustration of LSPR-induced holes transfer [142].Copyright 2018,Nature Publishing Group.
4.3.Other plasmonic semiconductors
In addition to oxides and sulfides,transition metal nitrides(TMNs) is also a type of material with a significant prospect for extensive photocatalytic applications due to their distinct physical,electronic properties and metal-like properties.Recently,Chenget al.systematically discussed and summarized different roles of TMNs materials in photocatalytic systems including semiconductor active components,co-catalysts,LSPR components,etc[143].To date,TiN and WN have been applied for photocatalysis through LSPR effect,which is verified by both theoretical and experimental results.For example,plasmonic nanohybrid of plasmonic TiN nanocubes decorated with Pt NCs has been reported to efficiently drive H2evolution from NH3BH3dehydrogenation.The apparent quantum yield reaches 120% under resonant light at 700 nm driven by hot electrons only.Under solar irradiation,the activity of TiN−Pt nanohybrids is enhanced by one order owing to the synergistic effect of plasmonic hot electrons and photothermal heating [84].Heterostructures comprised of plasmonic TiN and other semiconductors have been proved to exhibit higher photocatalytic activity due to hot electron injection.Boltassevaet al.synthesized core–shell TiN@TiO2NPs with broad LSPR in red-NIR region.Under 700 nm fs-pulsed laser illumination,TiN@TiO2NPs effectively converts ground-state oxygen into singlet oxygen,driven primarily by hot electrons transferred from plasmonic TiN core to TiO2shell.Analytical calculations also reveal the unique advantages of TiN@TiO2heterostructures in hot-electron driven photocatalysis [144].Another TMN of tungsten nitride (WN) with strong LSPR in NIR region has also been employed as photocatalyst for overall water splitting operated at red-light up to 765 nm [145].Huanget al.proved the NIR-driven photocatalytic performance of plasmonic cubic-phase WN in effective reactive oxygen species activation by both density functional theory (DFT) calculation and experimental observation [146].
Apart from widely reported semiconductors above,several novel semiconductors with LSPR in vis-NIR region also have been developed as plasmonic photocatalysts.Luet al.successfully constructed plasmonic Bi2WO6with strong LSPR around 500–1500 nm region by electron doping [147].Two types of OVs on W-O-W (V1)and Bi-O-Bi (V2) sites are precisely controlled by chemical methods,obtaining Bi2WO6-V1 with LSPR and Bi2WO6-V2 with defect absorption across Vis-NIR region separately.The DFT calculations indicate that V1 induced energy states have a small energy range of 0.25 eV and are close to conduction band,which facilitates photoelectron transfer and trapping for a long lifetime,leading to LSPR in Bi2WO6.A 93% PL quenching on Bi2WO6-V1 was observed by single-particle PL microscopy,demonstrating the photoelectron trapping on V1 sites.Plasmonic Bi2WO6-V1 boosts high-selective CH4generation with a rate of 9.95 μmol g−1h−1from photocatalytic CO2reduction,which is 26-fold higher than 0.37 μmol g−1h−1of Bi2WO6-V2 under UV–vis irradiation (Figs.14a–d).Both DFT-simulation andin situFourier transform infrared spectra on Bi2WO6surface prove that V1 sites facilitate CH4generation.The results imply the possibility of electron accumulations on the state to generate high carrier density and LSPR property.Introducing a doping electronic state to trap electrons is an effective strategy to modulate LSPR effect in semiconductors.Moreover,boron phosphide (BP) containing abundant III-V elements with strong covalent bonding exhibits indirect band gap of ∼2.0 eV,excellent charge mobility and thermal stability (>1000 °C),is potential to be a promising photocatalytic material [148,149].Recently,Tianet al.reported a metal-free plasmonic core-shell BP@g-C3N4photocatalyst with LSPR effect derived from free electrons collective oscillation on BP surface induced by abundant P-vacancies formed under high temperature [150].The BP@C3N4catalyst exhibits excellent solar energy utilization efficiency in UV–vis-NIR region and shows a remarkable photocatalytic activity for water splitting even under 730 nm illumination.The H2production rate can reach up to 31.5 μmol g−1h−1under visible light irradiation,which is 112.6 and 34.3 times higher than that of C3N4and Pt/C3N4,respectively.The BP LSPR can facilitate generation and separation of charge carriers near the surface of C3N4,and plasmon-induced hot electrons transfer to CB of C3N4for significantly improved water reduction reaction (Figs.14e–h).
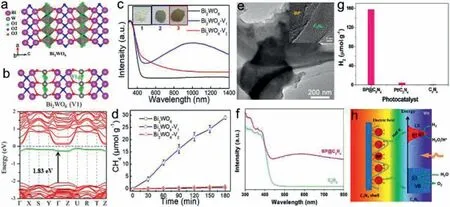
Fig.14.(a) Crystal structure and (b) DFT-calculated band structure of Bi2WO6 with OVs on W-O-W sites.(c) UV-vis-NIR DRS with color images and (d) CH4 generation over different Bi2WO6 samples.Reproduced with permission [147].Copyright 2021,American Chemical Society.(e) TEM (HRTEM) images and (f) UV-vis absorption of BP@C3N4.(g) Photocatalytic H2 generation and (h) proposed mechanism over metal-free plasmonic BP@C3N4 composite.Reproduced with permission [148].Copyright 2021,Elsevier B.V.
5.Conclusions and future prospects
Plasmonic photocatalysis has emerged as a promising technology to address the energy and environmental crisis.Owing to intriguing optical-electrical features such as intense light absorption,improved charge carrier separation,hot carriers generation and photothermal effect,plasmonic photocatalysts have effectively made their way to solar energy conversion applications including water splitting,CO2reduction,pollution decompositionetc.Although considerable progress has been made in plasmonic metalbased photocatalysts,high-cost and poor absorption in NIR region inhibited their large-scale practical applications,and also inspired the exploration of alternate plasmonic materials.Recently,heavily-doped semiconductors like nonstoichiometric oxides,sulfides,etc.have gained significant attention for the construction of LSPR with abundant oxygen/cation vacancies induced large free carrier density.By tuning the geometry and free carrier density,these semiconductors enable broadband solar energy harvesting across UV-vis-NIR range,highly desired for efficient photocatalysis.Even though recent efforts have been devoted to the development of novel plasmonic semiconductors for plasmon-driven photoredox reactions,there remain challenges accompanying them such as low utilization of hot carriers,obscure mechanism of hot carrier-driven reactions and unclear plasmon-related photochemical and physical process,etc.To realize full potential of plasmonic materials for development of efficient photocatalytic systems,prospective studies are still highly necessary towards the following regards.
5.1.Exploration of novel nonmetallic plasmonic materials
Heavily doped semiconductors exhibit great potential in photocatalysis due to their low cost,abundant reserve and strong LSPR in vis-NIR region.However,single plasmonic semiconductor still suffer from the low utilization of hot carriers,especially for hot hole in p-type semiconductors.Construction of asymmetric heterostructures of plasmonic and catalytic semiconductors with suitable potential position is an effective approach,where hot carrier transfer boosts long-lived hot carrier generation and separation for efficient photocatalysis.Besides,Millironet al.discussed the presence of depletion layer near semiconductors surface that have a dramatically decreased free carrier density,largely damping LSPR effect.Therefore,developing plasmonic semiconductors with thin depletion layers is highly desirable,which also facilitates hot carriers separation for photocatalysis.Since the efficiency of plasmonic photocatalysts depends on geometry,configuration of plasmonic nanostructures should be specifically engineered with consideration of many design trade-offs.For example,2D ordered plasmonic semiconductors or porous structure with distinct optical features,ease decoration and more active sites,might be a promising candidate for plasmonic photocatalysis.Generally,high-efficiency plasmonic photocatalysts possess features including stable LSPR effect,efficient charge carrier separation,suitable potential position and abundant active sites.
5.2.Uncover the mechanism of hot carrier-driven reactions
Plasmonic hot carriers have been demonstrated to play critical role in photocatalysis,but the underlying reaction mechanism still needs to be systematically studied.In contrast to progress of n-type plasmonic photocatalysts based on plasmon-induced hot electron transfer,there are few reports on hot holes transfer process regardless of its importance.The unclear behavior of hot holes remained an obstacle for the comprehensive understanding of hot carrier-drive reactions.An in-depth understanding of photochemical-physical process of hot carrier generation,transfer and recombination would clarify the role of hot carriers contributing the photocatalytic activity,and also beneficial to developing efficient plasmonic photocatalysts for high selectivity of anticipated products with zero side reactions.Recently,advanced experimental techniques including time-resolved infrared spectroscopy,singleparticle PL spectroscopy,etc.have been employed to study the relaxation and transfer processes of hot carriers.However,there are still much processes left in fuzziness,and more techniques,especially the ones with high temporal-spatial resolutions,need to be introduced in research of plasmonic photocatalysis.
5.3.Insights into plasmon-related physicochemical process
Despite numerous efforts have been devoted to LSPR-driven catalytic reactions,there is still big room for the investigation of new processes,especially plasmonic photo-thermocatalysis.An insight into plasmon-related physicochemical processes should refer the knowledge from both plasmonic photocatalysis and photothermocatalysis to give a comprehensive description of the catalytic reactions.In situcharacterization and temperature monitoring of plasmonic photocatalyst experimentally would be helpful to establish an in-depth understanding of detailed plasmon-related physicochemical process.Moreover,modeling and simulated studies could also be conducted to give insights into the underlying photocatalytic reaction mechanisms,such as thermal transfer,electronic transitions and interfacial charge transfer behaviors,which are expected to offer guideline for optimization of plasmonic photocatalysts.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.11904133,51872125),Guangdong Natural Science Funds for Distinguished Young Scholar (No.2018B030306004)and GDUPS (2018),the Fundamental Research Funds for the Central Universities (No.21619322) and Regional Joint Foundation in Guangdong Province (No.2019A1515110210).
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
- C–F bond functionalizations of trifluoromethyl groups via radical intermediates
