Construction and applications of DNA-based nanomaterials in cancer therapy
Hedong Qi,Yuwei Xu,Pin Hu,Chi Yao,Dayong Yang
Frontiers Science Center for Synthetic Biology,Key Laboratory of Systems Bioengineering (MOE),School of Chemical Engineering and Technology,Tianjin University,Tianjin 300350,China
Keywords:DNA nanomaterials DNA nanotechnology Drug delivery Cancer therapy
ABSTRACT As a biologically active macromolecule,deoxyribonucleic acid (DNA) has the advantages of sequence programmability and structure controllability and can accurately transmit sequence information to specific biological functions.Facing the complex internal microenvironment and heterogeneity in tumor treatment,the construction and applications of DNA-based nanomaterials have become a focus point of research.In particular,the hybridization of DNA molecules with other materials endows DNA-based nanomaterials with multiple functions such as targeting,stimulus responsiveness and regulations of biological activities,making DNA nanostructures great potential in the treatment of major human diseases.In this review,the construction and characteristics of DNA-based nanomaterials are introduced.Then,the functions and applications of DNA-based nanomaterials in the delivery of chemotherapy drugs and gene drugs,stimulus-responsive release and regulation of cell homeostasis are reviewed.Finally,the future development and challenges of DNA-based nanomaterials are prospected.We envision that DNAbased nanomaterials can enrich the nanomaterial system by rational design and synthesis and address the growing demands on biological and biomedical applications in the real world.
1.Introduction
In many types of solid tumors,uncontrolled proliferation of tumor cells and dysfunctional vascular growth will lead to hypoxia and acidification of tumors.In addition,dense extracellular matrix as a natural barrier prevents the effective diffusion of nutrients,therapeutic agents,oxygen,and other molecules [1].As a result,the complex tumor microenvironment (TME) has different physiological characteristics from normal cells/tissues (e.g.,acidic pH,reductive conditions,hypoxia,hydrogen peroxide (H2O2) overexpression).By utilizing the above characteristics,nanomaterials with responsiveness toward different TME stimulation have been designed and applied in specific tumor therapy,improving therapeutic effi-cacy and biological safety [2].
Compared with other nanomaterials,DNA nanomaterials have superior characteristics.DNA molecules have the dual identities of building materials (self-assembly) and therapeutic agents (gene therapy),realizing the fusion of complex structures and diverse functions.The programmability of DNA makes it an ideal building block to construct nanostructures with various sizes and morphologies.The obtained nanostructures can be used as carriers to realize efficient delivery of a variety of drugs [3].Furthermore,the introduction of functional DNA sequences (aptamer,ribozyme,imotif sequence,and G-quadruplex,etc.) endows DNA nanostructures with functions,such as targeting,stimulus responsiveness,and regulations of life activities,and thus promotes the great potential of DNA nanostructures in the treatment of major diseases[4-6].
The construction of DNA-based nanomaterial by combining DNA molecules with other functional materials has opened a new window for developing DNA materials.DNA nanostructures can be used as matrix to hybrid with protein [7],organic molecules [8],liposomes [9],inorganic nanoparticles [10],and other materials.There are two main strategies of hybridization DNA with other materials: 1) covalent bonding between DNA and other materials;2)physical interactions for adsorption or encapsulation of DNA into other materials.Based on the above methods,a variety of DNAbased materials have been constructed and reported,which promoted the development of precision medicine,especially the applications in cancer therapy.
In this review,we focus on the recent advances of DNA-based nanomaterials utilized in cancer therapy (Fig.1).First,we introduce the construction and characteristics of DNA-based nanomaterials.Second,the functions and applications of DNA-based nanomaterials in the delivery of chemotherapy drugs and gene drugs,stimulus-responsive release,and regulation of cell homeostasis are reviewed.Finally,the future development and challenges of DNAbased nanomaterials have prospected.
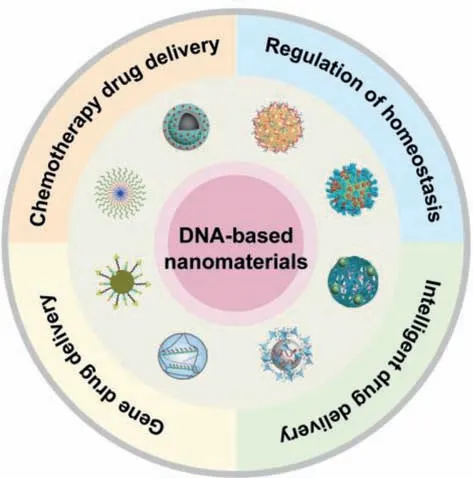
Fig.1.An illustration of four kinds of applications of DNA-based nanomaterials in cancer therapies: chemotherapy drug delivery,gene drug delivery,intelligent drug delivery,and regulation of homeostasis.
2.DNA-based nanomaterials for chemotherapy drugs delivery
As an intelligent and adaptive system,cancer cells can build various defense strategies to resist chemotherapeutic agents,including increasing drug efflux,mutation of drug targets,activating or blocking other signaling pathways,and activating DNA damage repair to suppress cell apoptosis.These defense mechanisms are usually related to the expression changes of resistancerelated proteins and ultimately lead to the failure of chemotherapy [11].Thus,the systemic side effects are caused by the nontargeted uptake of chemotherapeutic drugs (vincristine,paclitaxel,adriamycin,5-fluorouracil,cisplatin,etc.) with the drug resistance of cancer cells caused by a variety of complex mechanisms,are crucial challenges in current cancer therapy.Combining chemotherapy and other emerging therapeutics (such as gene therapy,photodynamic therapy,chemodynamic therapy) provides a critical solution for solving the above challenges in cancer therapy[12,13].
2.1.Improve the cellular efficiency of chemotherapy drugs
Chemotherapy is still the primary way of cancer treatment[14,15].Although chemotherapy drugs have a broad-spectrum killing effect,the side effects caused by nontargeting and high toxicity of normal cells seriously affect the body function of patients [16-18].The multiple modes of chemotherapy drugs action on DNA materials can significantly reduce the non-specific release of drugs and reduce the side effects [19].Based on this,Tian and her co-workers proposed using magnesium as an RCA products stabilizer and achieving tumor-targeted DOX delivery(Fig.2A) [20].The RCA template integrated the complementary sequence of Sgc8 aptamer (achieving targeted delivery),drug delivery intercalation sites (loading sufficient DOX),and i-motif complementary sequence (endowing the RCA products responsive to the change of pH conditions).The RCA products were condensed into spheroidal nanoparticles by Mg2+and loading DOX to form Mg-RCA-NanoClew-DOX (Mg-RNC@DOX).Under the acid condition in lysosomes,the i-motif sequence transformed into a quadruplex structure and turned on the hairpin to release DOX.This work demonstrated a prospective strategy to develop controllable nanodrugs for cancer treatment.
In order to improve the efficiency of drug delivery and reduce the side effects of toxic components,it is of great significance to realize the controlled release of therapeutic components.Similarly,the primary aim of the chemotherapeutic drug delivery system is to improve treatment efficiency and complete the precise release of the medicinal drugs [21,22].In precision medicine,excepting balance efficacy and toxicity,it is vital to achieving targeted delivery and controlled release.Therefore,Wu and his co-workers developed a novel DNA-based precision targeted drug delivery strategy that efficiently loaded therapeutic drugs and committed precise delivery to tumor cells,realizing the chemotherapy with low side effects (Fig.2B) [23].The delivery system was a precisionguided missile-like nanostructure (D-PGM) that was composed of a warhead (WH) and guidance/control (GC).WH was a linear structure built with ssDNA to load chemotherapeutics.GC served as a logic gate to realize specific cell subtype recognition.As a result,D-PGM can only be internalized by the cells simultaneously bound with TC01,Sgc4f and Sgc8 sequences,achieving precise tumor chemotherapy.This DNA-based drug delivery system had good biocompatibility and excellent antiserum degradation ability thus can accurately deliver drugs to the target site in a complex biological environment.At present,only a single aptamer is introduced in most of the targeted delivery systems,which leads to potential safety hazards caused by the off-target effect.Moreover,the specific proteins on different cell surfaces are different.According to the various proteins,selecting the appropriate aptamer can release the therapeutic components more accurately and reduce the side effects of treatment,promoting the clinical transformation of DNAbased materials.
2.2.Gene assist in enhancing the effect of chemotherapy
Multi-drug resistance (MDR) is mainly caused by the overexpression of adaptive genes from tumor cells under pressure,which significantly hinders the effectiveness of chemotherapy in cancer research and clinical application [24-26].P-glycoprotein (P-gp) is a typical drug efflux pump with an exceptionally broad specificity on hydrophobic drugs,causing drug efflux and reducing the kill effect toward cancer cells [27,28].Zhang and his co-workers constructed a carrier-free drug delivery system precisely assembled by drug-chemogene conjugates.This strategy combined chemotherapy and gene therapy to treat drug-resistant tumors (Fig.2C) [29].The conjugates were synthesized by linking two paclitaxel molecules with a floxuridine (FdU)-integrated antisense oligonucleotideviaclick chemical reaction.The drug-chemogene conjugates further assembled into spherical nucleic acid-like micelle nanoparticles and achieved efficient drug delivery without using any foreign carrier.This micelle was easily internalized by cancer cells and release chemotherapeutic and gene drugs in the high concentration of glutathione (GSH) environment to promote the apoptosis of cells.Subsequently,the release of fluoride monophosphate (FdUMP) through enzymatic degradation further inhibited the proliferation of cancer cells and achieved synergistic effects,reaching a high tumor inhibition rate of 80%.In addition,with the development of many therapeutic gene targets,gene adjuvant therapy has been promoted[30].For example,silencing the HIF-1αgene can enhance the effect of photodynamic therapy (PDT),silencing the heat shock protein family can weaken the resistance to photothermal therapy(PTT) of cells,and sensitization immunotherapy by silencing PD-L1 related genes.
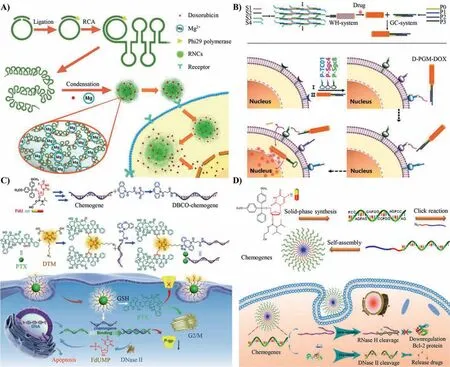
Fig.2.Delivery of chemotherapeutic drugs via DNA-based nanomaterials.(A) i-motif responds to pH releasing DOX.Reproduced with permission [20].Copyright 2018,the American Chemical Society.(B) Specific cell identity and realizing target therapy.Reproduced with permission [23].Copyright 2020,the American Chemical Society.(C) Reverse multi-drug resistance.Reproduced with permission [29].Copyright 2020,Wiley-VCH.(D) Combination of gene therapy and chemotherapy.Reproduced with permission [32].Copyright 2019,American Chemical Society.
2.3.Combination of gene and chemotherapy drugs
The development of drug delivery systems,especially combined drug delivery systems,promoted personalized precision medicine.However,the construction of integrated drug delivery systems still exist challenges,mainly including: 1) designing of biocompatible nanocarriers that meet the high loading rate of nucleic acid agents and chemical drugs at the same time;2) achieving the synergic effect between nucleic acids and drugs [31];3) controlling the release order of nucleic acids and drugs to realize the gene-guided chemotherapy process and inhibiting the anti-apoptotic pathway of cancer cells.Zhang and his co-workers synthesized a nanoscale drug delivery system with chemogene chains to enhanced therapeutic efficiency (Fig.2D) [32].The chemogene drug was synthesized with artificial bases containing antisense oligonucleotide(ASO) that conjugated with azide-terminated diblock copolymer poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG-b-PCL).This copolymer self-assembled into spherical nucleic acid-like hybrid nanomaterials by hydrophilic and hydrophobic interaction.This chemogene drug down-regulated the expression of anti-apoptotic sequence and restored the drug-sensitive of tumors,providing a solution to multi-drug resistance.In addition,other functional nucleic acid sequences can also be integrated into the spherical nucleic acid to regulate gene expression and achieve a broad treatment range.
3.DNA-based nucleic acid reagents delivery system
Functional nucleic acids have a promising application prospect in the treatment of cancer.However,several physiological barriers in the organism weaken the therapeutic effect of naked nucleic acids.In order to deliver nucleic acid reagents into cancer cells efficiently and safely,nucleic acids are usually encapsulated in carriers,which can be expeditiously taken by specific target cells[33-35].It is critical to enhancing the targeting ability of carriers while maximizing the anticancer effect and minimizing toxicity or side effects on healthy tissues [36].A carrier with a high loading efficiency and can protect nucleic acid from degradation is ideal for nucleic acid drug delivery.In addition,using the rationally designed carrier can significantly improve the delivery and transfection efficiency.
3.1.Improve the cellular efficiency of nucleic acid reagents
As catalytic nucleic acid sequences,DNAzyme has been recognized as a promising therapeutic agent for selective regulation of gene expression.Unlike RNA (siRNA/miRNA) and ASO,DNAzyme does not need the assistant of intracellular nuclease to complete the process of gene silencing.Instead,it can achieve the cleavage of the target mRNA substrate through catalyzed hydrolysis and then realize mRNA cleavage and gene silencing.Although DNAzyme can significantly down-regulate oncogenes,the limitedin vivodelivery efficiency and catalytic cleavage efficacy seriously hinders the broad application of DNAzyme in disease treatment [37-39].Liu and her co-workers developed carrier-free DNA hybrid nanomaterials to achieve the combination of gene therapy and PDT(Fig.3A) [40].The nanomaterial was composed of aggregationinduced emission (AIE) photosensitizer (PS) (TBD-Br),singlet oxygen (1O2) generation actuator,therapeutic DNAzyme,and its corresponding cofactors of Zn2+.Under laser irradiation,AIE-PS transformed intracellular oxygen (O2) into1O2and destroyed the structure of lysosomes,promoting the escape of nanomaterial from lysosomes.The disintegrated nanomaterial released DNAzyme and Zn2+cofactor to achieve efficient cleavage of EGR-1 mRNA.The damaged mRNA cannot be translated to synthesize EGR-1 protein,realizing the regulation of suppressor gene.
Meanwhile,1O2produced by PDT improved the curative effect and promoted the apoptosis of cancer cells.Likewise,Liu and her co-workers used polydopamine-Mn2+nanoparticle (Mn-PDA)to realize the combination of gene therapy and photothermal therapy (PTT),improving the apoptosis of cancer cells (Fig.3B) [41].The nanoparticle responded to the GSH to release Mn2+as cofactors of DNAzyme and induced the gene silencing process.Upon laser irradiation,PDA converted the light energy into heat energy,realizing the rise of local temperature and inactivation of various intracellular proteins to promote cancer cell apoptosis.Moreover,the PDA induced into the system was used for multi-mode imaging to detect and locate tumors.In addition,nucleic acid molecules are polyanions,which are not easy to internalize by cells,and are easily removed by metabolic tissues or organs in the body.Therefore,it is imperative to balance the stability of therapeutic nucleic acids in the circulation of the body and the specific dissolution of specific sites.
3.2.Improve the stability of nucleic acid reagents
Naked RNA is extremely unstable in blood circulation [42].The half-life of unprotected RNA is usually only a few minutes,which greatly limits the application of RNA for therapeutics [43].Moreover,due to its tiny size,siRNA is hard to be internalized by cells and easily gets cleared by kidney [44].Yang and his co-workers constructed a DNA polymer nanocarrier based on hybridization chain reaction (HCR) to achieve efficient loading and controlled release of siRNA (Fig.3C) [45].First,DNA was covalently coupled with phenylboronic acid by radical polymerization to prepare polymer nanoframeworks containing initiator DNA.Then,the ATP aptamer,which was hybridized with siRNA,was modified on the end of the hairpin chain.While interfaced with initiator-DNA,the interaction between the hairpins initiated and formed the long doublestranded DNA (dsDNA) chain.Through HCR cascade reaction,the polymer nanoframework efficiently loaded siRNA.In response to the high concentration of adenosine triphosphate (ATP) in tumor cells,siRNA was released and then silence the target mRNA.The polymer nanoframeworks improved the stability of siRNA and realized the spatiotemporally controllable assembly and disassembly of nucleic acid drugs.Besides,Yang and his co-worker developed a mesoporous silica-based miRNA delivery system to treat myocardial infarction injury (Fig.3D) [46].The positive charged mesoporous silica was first synthesized and then loaded miRNAviaelectrostatic interaction.The mesoporous silica was able to load sufficient miRNA and provided excellent protection of miRNA.The stem cell membrane was coated to help the materials escape from the immunologic system and provide antibodies bound to cardiomyocytes for targeting delivery.After the materials were internalized into cardiomyocytes,the materials decomposed,and the loaded miRNA got released,subsequently inhibiting the apoptosis of cardiomyocytes.This CM-miR-MSN behaved like the natural exosomes released from cells,significantly repaired the myocardial infarction injury.The construction of nanomaterials suitable for cell internalization with the integration of multi-functional therapeutic units and a combination of the RNA sequences can promote personalized therapy based on RNA.
4.DNA-based intelligent responsive drug delivery system
Besides the genetic information,the structural and functional information is also encoded in the DNA chains.Therefore,the different sequences endow DNA strands with different functional properties,promoting the development of functional materials.Especially,responsive DNA modules can be introduced to endow hybrid materials with specific stimuli-responsive and precise recognition ability [47].By utilizing the programmability of sequence,controllable assembly of DNA nanomaterials can be realized;moreover,through introduction of functional sequences with responsive stimuli factors (such as pH,light,oligonucleotides,small molecules and metal ions),intelligent drug delivery system can be constructed,which achieved accurate drug release and avoided potential toxic and side effects [48].
4.1.pH responsive system
Single stranded DNA is difficult to be uptaken by cells,and it is easy to be degraded by nucleasein vivo.Therefore,it is necessary to construct suitable carriers to improve the uptake effi-ciency and stability of nucleic acid reagents.ZIF-8 can achieve tumor-targeting accumulation through enhanced permeability and retention effect (EPR effect),effectively protect the loaded nucleic acid drug from degradation,and improve cell uptake effi-ciency [49,50].The acidic tumor microenvironment induces the collapse of imidazole framework,promoting the release of encapsulated drugs and Zn2+[51].Wang and his co-workers utilized ZIF-8 to encapsulate chlorin e6 (Ce6) labeled DNAzyme to construct a nanoplatform for gene/photodynamic combined therapy(Fig.4A) [52].Under laser irradiation,Ce6 mediated the transformation of O2to1O2.Thus,ZIF-8 provided loading sites for therapeutic Ce6-DNAzyme and supplied cofactors for DNAzyme gene therapy.These Ce6-DNAzyme@ZIF-8 nanoplatforms enhanced the cellular uptake of therapeutic Ce6-DNAzyme,and exhibited pHmediated drug release process.The therapeutic strategy of simultaneously delivering DNAzyme and photosensitizer to the tumor site can effectively inhibit the expression of EGR-1 protein and promote PDT to produce1O2-mediated apoptosis,achieving the combined gene/photodynamic therapy.In addition to the pH-responsive drug release of inorganic materials,the allosteric structure of i-motif and DNA triplex can also complete the drug release.The design of functional cascade intelligent materials has a considerable therapeutic effect.For example,the metal ions released by the degradable inorganic components can be used as the media of Fenton chemical reaction and as the cofactors of DNAzyme;Through combined gene therapy,multiple lethal genes were silenced simultaneously to enhance cell apoptosis.
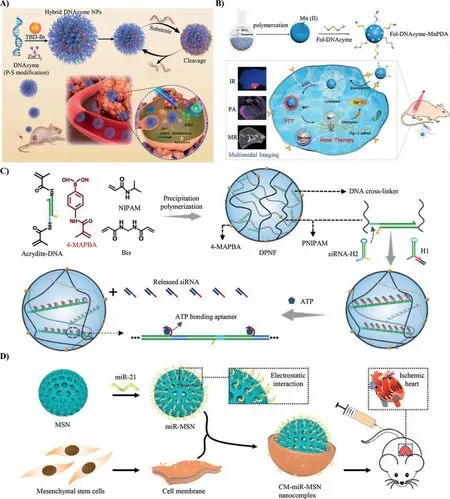
Fig.3.Delivery of nucleic acid reagents via DNA-based nanomaterials.(A) Carrier-free DNA hybrid nanomaterials the cellular efficiency of DNAzyme.Reproduced with permission [40].Copyright 2021,the American Chemical Society.(B) Polydopamine-Mn2+ nanoparticle (Mn-PDA) improves the cellular efficiency of DNAzyme.Reproduced with permission [41].Copyright 2018,the American Chemical Society.(C) DNA polymer nanocarrier improves the stability of siRNA.Reproduced with permission [45].Copyright 2021,Nature Publishing Group.(D) CM-miR-MSN improves the stability of miRNA.Reproduced with permission [46].Copyright 2020,Elsevier Ltd.
4.2.ROS responsive system
The nucleic acid agent delivery systems that are “too stable”in physiological environments always show insufficient release of drugs [53,54].Thus it is imperative to construct carriers that can respond to specific pathophysiological factors [55].Shi and his coworkers developed a new kind of nanomaterials (3I-NM@siRNA)that could release siRNA in response to reactive oxygen species(ROS) through “triple interaction” (Fig.4B) [56].Compared with the conventional materials only stabilized by electrostatic interactions,3I-NM@siRNA showed higher stability during the circulation of blood,which was beneficial to the cancer therapy progress.Furthermore,when the 3I-NM@siRNA was enriched in the tumor region stimulated by ROS,the hydrophobic interactions in the material would first be destroyed.Then the charge transformation promoted by the carboxyl group would interfere with the electrostatic and hydrogen bond interactions.This self-destruct process realized the effective release of siRNA.In addition,the modification of 3INM@siRNA surface by peptide-2 peptide was conducive for the materials to penetrate the blood-brain barrier and improved the targeting efficiency to gliomas.This work demonstrated that controlling multiple interactions to balance the relative protection of gene drugs without affecting the release efficiency at target cells had a broad prospect in cancer therapy.
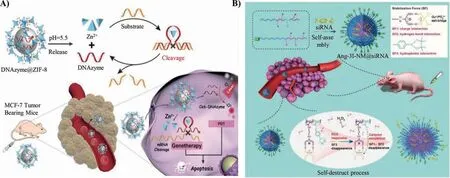
Fig.4.DNA-based nanomaterials achieve drug release by pH and ROS response.(A) The Ce6-DNAzyme is released by pH.Reproduced with permission [52].Copyright 2019,Wiley Publishing Group.(B) The 3I-NM@siRNA is released by ROS.Reproduced with permission [56].Copyright 2018,Wiley Publishing Group.
4.3.Mental ions responsive systems
The metal ions responsive systems of G-quadruplex endow the materials with controllable drug release and self-assembly in living cells.Willner and his co-works developed a DNA-modified metal-organic framework (MOF) that responded to specific stimuli(Fig.5A) [57].MOF was first synthesized,and the amine functionalities were then modified into carboxylic acid functionalities to interact with functional DNA sequences.The modification of MOF with G-quadruplex served as a K+responsive switch.The switch stayed OFF in the presence of 25 mmol/L K+,and the 18-crown-6-ether induced the switch to turn on to realize the release of loaded rhodamine 6G.What is more,the combination of the Gquadruplex and Hemin exerted the function of enzyme-like catalysis and served as horseradish peroxidase.This work demonstrated that the functional DNA could be used to achieve the controllable release.
In addition to the gating unit,the conformation transition of G-quadruplex can also be used as a crosslinker to promote polymer self-assembly.Basu and his co-works proposed a method mediated by G-quadruplex to construct hyperpolymerin situ(Fig.5B)[58].Hydroxyapatite-DNA (HA-DNA) conjugates were composed of HA and G-quadruplex.The formation of hyperpolymer showed the dependence of K+,indicating that G-quadruplex as a linker can induce the entanglement of polymer chains to form macroscopic gels.The HA and G-quadruplex were nontoxic molecules themselves,but the macroscopic gels exerted too much pressure beyond the endurance of the cells.Therefore,the cells were killed by the destruction of the stability of cell membrane and metabolism.The response process of the G-quadruplex had a great potential in precise cancer therapy and regulation of cellular activities.
4.4.Biomolecular responsive systems
Surgical inflammation caused by trauma may cause tumor recurrence or accelerating the development of local residual tumors and increase the risk of tumor invasion and metastasis[59,60].To effectively deal with postoperative tumor inflammation,Gu and his co-workers used DNA nanococoon (DNC) to achieve the controlled release of anti-PD1 antibody (aPD1) and cytosinephosphate-guanine (CpG) sequences (Fig.6A) [61].The aPD1 and CpG sequences were integrated into DNC.Restriction enzyme was encapsulated in the inflammation responsive disintegrated monostearate triglyceride (TGMS) to form a polymer cage and then adsorb on the DNC surface through electrostatic interaction.After the polymer cage degraded in the inflammatory site,the restriction enzyme specifically cleaved DNC to promote DNC fragmentation.In other words,when the DNC was injected into the tumor inflammation sitein situ,TGMS polymer cages got decomposed under the action of inflammation-related proteases.The encapsulated enzyme was then released and transformed DNC into CpG and aPD1 sequences.This responsive DNC cleavage technique ensured the sustained release of immunomodulators,enhanced T cell activity at tumor inflammation sites,and destroyed tumor tolerance,inducing persistent immune responses.In this combined therapy,the tumor recurrence was significantly inhibited.
The stability of nucleic acid was improved by conjugation with synthetic polymers.By design and selection of polymers,the internalization of nucleic acid materials by cells can be promoted.In addition,due to the stable construction of polymer scaffold,the nucleic acid-polymer conjugates should also be rationally designed to meet the requirements of being decomposed intracellularly [62].In this regard,Zhang and his co-workers constructed a cross-linked nucleic acid nanomaterial to deliver therapeutic siRNA (Fig.6B)[63].First,a skeleton structure enriched with therapeutic siRNA complementary chains (DNA-grafted polycaprolactone,DNA-g-PCL)was formed based on click chemical reaction.Then the skeleton was cross-linked by siRNA linker (siRNA-L) through the principle of base complementary pairing to form nanoscale materials.DNAg-PCL brush had abundant cross-linking sites that loaded a large amount of siRNA.The nanomaterials effectively protected siRNA from the degradation of RNase H,ensuring good physiological stability.The enhanced cell uptake efficiency increased the concentration of siRNA,resulting in excellent therapeutic effects.Due to the programmability of bases,the sequence combined with therapeutic nucleic acid achieved specific responsive function,making the DNA-based nanomaterials a universal platform to enhance the therapeutic effect.
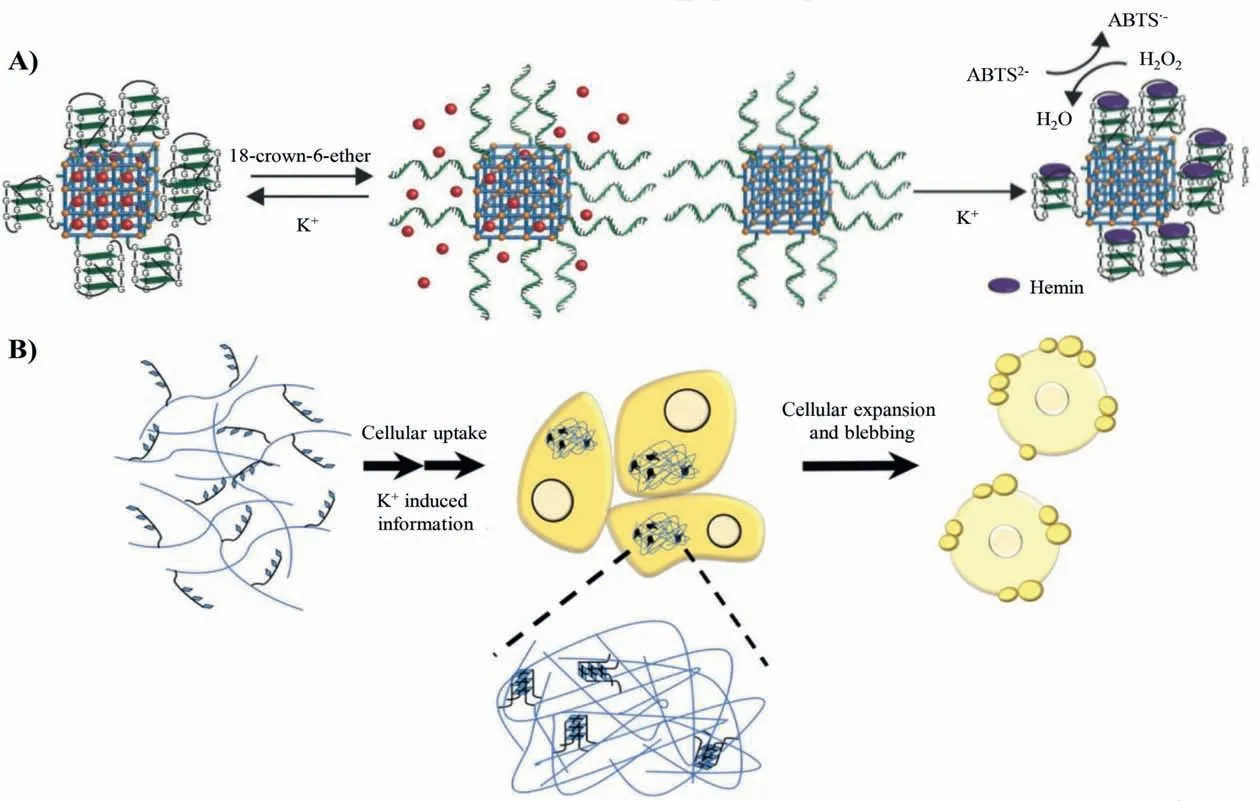
Fig.5.DNA-based nanomaterials achieve drug release and self-assembly.(A) G-quadruplex as gating unit in response to K+ to release rhodamine 6 G.Reproduced with permission [57].Copyright 2016,Wiley Publishing Group.(B) G-quadruplex as crossing-linker to promote self-assembly of the materials.Reproduced with permission [58].Copyright 2018,the American Chemical Society.
Drug delivery systems can enhance the therapeutic effect by retaining the molecular biological activity and improving drug utilization.In order to further enhance the biological specificity and therapeutic efficacy,drug delivery system with active targeting and stimuli-responsive ability has been developed.These systems can be explicitly accumulated in tumor sites and release drugs under specific conditions [64].On this matter,Gu and his co-workers designed a nanocarrier composed of three different functional components: doxorubicin (DOX),cross-linked shell formed by protamine and hyaluronic acid (HA),and DNA scaffold (composed of ATP aptamer and complementary single-stranded DNA) (Fig.6C)[65].DOX was inserted into the GC base pair.The drug carrier was composed of ATP aptamer and the corresponding blocking single chain enriched with GC base pairs,which provided sufficient loading sites for DOX.After the ATP aptamer specifically binding to ATP molecule,the drug carrier transformed from complementary double-strand to allosteric self-folding of ATP aptamer,promoting the release of DOX and the single blocking strand from the double strand.Negative charge materials were not easily to be internalized by cells.By adjusting the protamine ratio,the negative charge of the DNA scaffold was neutralized,and the nanocarrier became positively charged.Anionic HA,active ligands,and receptors (such as CD44 and RHAMM) were coated on the outer layer of the complex to enhance the targeting of the nanocarriers and formed a protective shell.The HA shell of the nanocarrier degraded by the HAase in tumor cells and the therapeutic components were released to show anti-tumor effect.As a bioactive molecule,ATP had a high content in tumor cells and tissues;thus,it is of great significance for precision medicine to encapsulate drugs by ATP aptamers to achieve controllable drug release.
Primer exchange reaction is when strand displacing polymerase can interface with a hairpin and continuously synthesize ssDNA is triggered to a short DNA primer [66].This amplification process can realize the detection of trace amounts of intracellular mRNA and enhance the effect of cancer therapy.Chu and her co-workers developed a DNA-based machine activated by tumorrelated biomarkers (Fig.6D) [67].The DNA-based machine employed nanoscale ZIF-8 to encapsulate chain replacement polymerase and probe.First,the machine would activate the replacement process by opening the hairpin with survivin mRNA.Then the primer combined with the open hairpin and exposed the site of KEP enzyme to achieve branch migration and continuously released the Bcl-2 ASO.The released Bcl-2 ASO realized imaging of survivin mRNA and gene therapy.Thus,the DNA machine could amplify the intracellular mRNA and respond to polymerase by designing the nucleotide sequence and hairpin of the DNA machine.Therefore,it could be widely used in the early diagnosis and treatment of tumors.
5.DNA-based regulation of cell homeostasis system
5.1.GSH depletion to promote H2O2 accumulation
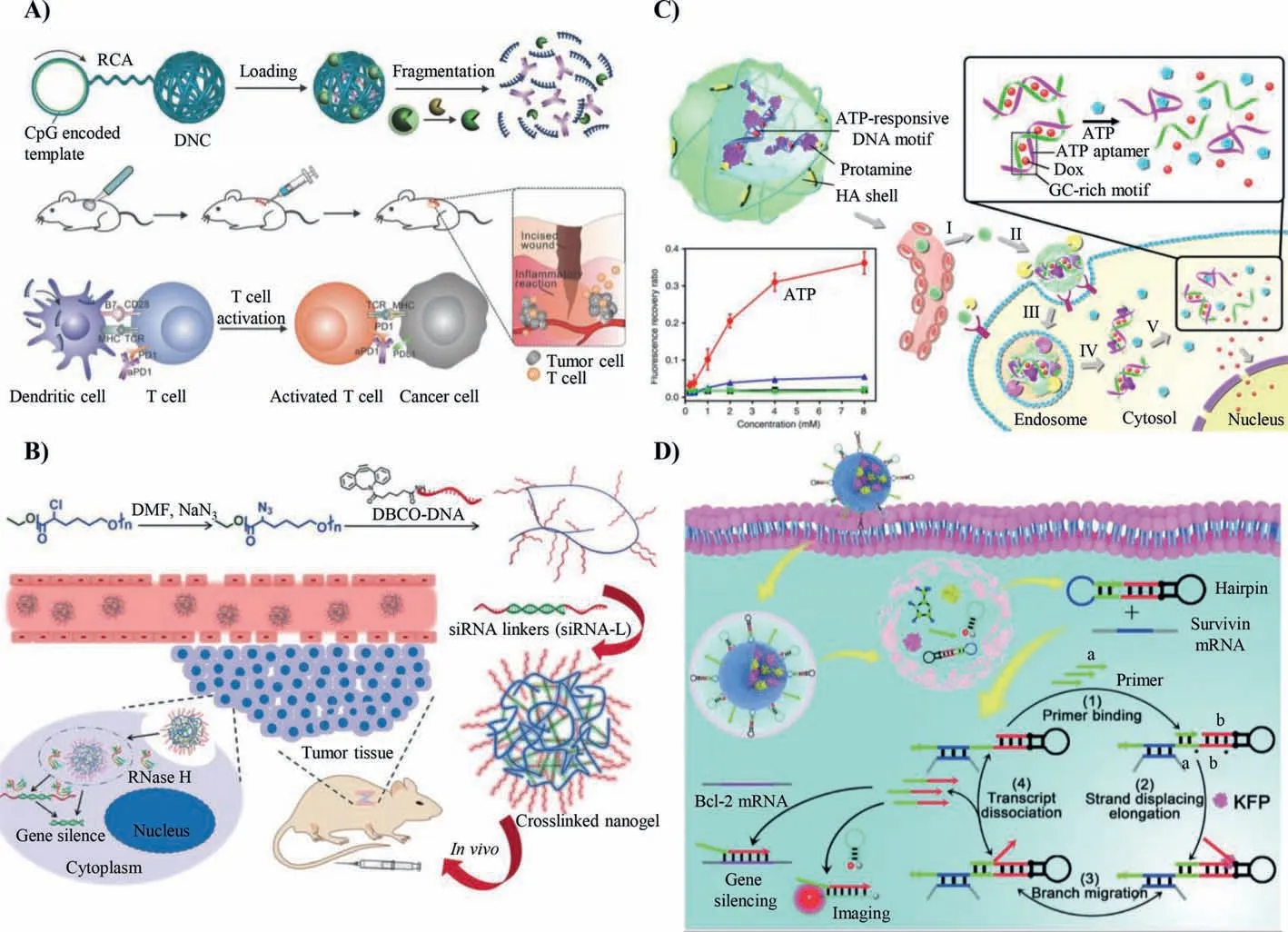
Fig.6.DNA-based nanomaterials achieve biomolecular responsiveness.(A) Inflammation-related protease responsive promotes CpG and aPD1 release.Reproduced with permission [61].Copyright 2016,Wiley Publishing Group.(B) RNase H responsive promotes siRNA release.Reproduced with permission [63].Copyright 2018,Wiley Publishing Group.(C) ATP responsive promotes DOX release (mM: mmol/L).Reproduced with permission [65].Copyright 2014,Nature Publishing Group.(D) Primer exchange reaction.Reproduced with permission [67].Copyright 2020,Royal Society of Chemistry.
Under normal physiological conditions,the concentration of ROS is controlled at a low level by multiple organelles.The dynamic generation and elimination of ROS maintain the conventional redox balance and prevent the ROS level from reaching the cell death threshold [68,69].The abnormal metabolism of tumor cells and the redox adaptive response triggered by carcinogenic signals can increase ROS levels.In order to maintain an effective redox balance and avoid the oxidative damage caused by excessive ROS,tumor cells activate their inherent antioxidant defense system to improve their antioxidant capacity.This improved antioxidant capacity endows the cancer cells with new equilibrium homeostasis.Cells maintain a high generation and elimination rate of ROS and keep their content below the toxicity threshold [69].The mechanism of endogenous redox adaptation in tumor cells includes the activation of redox-sensitive transcription factors and the expression of ROS scavenging molecules.The level of ROS in tumor cells is much higher than that in normal cells.Therefore,tumor cells are more dependent on endogenous antioxidant defense systems than normal cells and are more vulnerable to the enhancement of oxidative stress [70,71].Based on this,Bu and his coworkers constructed a nanoplatform that could increase the production of H2O2and achieve the depletion of GSH,exerting the tumor therapeutic efficacy by breaking the dynamic balance of oxidation and reduction in hypoxic tumor (Fig.7A) [72].They used cysteine ferrous phosphotungstate (FeCysPW) as the core and ZIF-82 as the shell to load catalase DNAzyme (CAT Dz) and formed a FeCysPW@ZIF-82@CAT Dz nanoplatform.When the nanoplatform was internalized by tumor cells,the zeolite imidazole framework 82 (ZIF-82) shell loaded with catalase CAT Dz was degraded to produce Zn2+and electrophilic organic ligands.Zn2+could provide the corresponding cofactor for CAT Dz mediated gene silencing process.Electrophilic organic ligands could realize the GSH depletion process,reducing the ROS clearance by downregulating the content of reducing substance (such as GSH),breaking the balance of redox homeostasis in cells,diminishing the cell defense mechanism,and amplifying the pernicious effect of hydroxyl radical (•OH).Meanwhile,the FeCysPW would promote chemodynamic therapy to transform H2O2into high cytotoxic•OH and kill cells.Removal of intracellular reducing substances to enhance chemodynamic therapy could effectively improve the therapeutic effect of hypoxia tumors.
5.2.Enzymatic transformation to promote the production of H2O2
The effect of chemodynamic therapy is also easily limited by low intracellular H2O2content.GOx can catalyze the conversion of glucose into H2O2in the tumor to enhance the effect of chemodynamic therapy [73].Huang and his co-workers developed a biodegradable O2self-supplying nanoplatform (GMCD),which enhanced the effect of PDT in tumor treatment by activating cascade catalytic reaction (Fig.7B) [74].CAT in GMCD effectively promoted the decomposition of endogenous H2O2into O2,attenuating the hypoxia environment in tumor site and supplied the O2consumption for GOx catalysis,thus accelerating the decomposition of glucose in tumor.In addition,the produced O2also enhanced the1O2production of DVDMS under light irradiation to enhance the effi-cacy of PDT.Meanwhile,the Fenton reaction mediated by Mn2+transformed the H2O2produced by GOx into the highly toxic•OH,which amplified the oxidative damage of ROS to cancer cells and enhanced the therapeutic effect.Therefore,the above-mentioned GMCD,which enhanced the PDT effect through cascade reaction,realized O2self-supply,and TME activation degradation process significantly inhibited tumor growth and achieved dual-imaging.
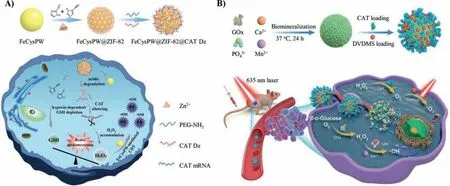
Fig.7.DNA-based nanomaterials achieve GSH depletion and H2O2 production.(A) Regulation of redox balance.Reproduced with permission [72].Copyright 2020,Wiley Publishing Group.(B) Improving the content of H2O2.Reproduced with permission [74].Copyright 2021,Wiley Publishing Group.
Because of the self-regulation function of cells,destroying the homeostasis balance of cells can enhance the efficacy of chemodynamic therapy.The types of manner mainly containing: 1) prolonging the life of the product (consumption of reducing substances);2) increasing the substrate content of the reaction (introduction of GOx,silencing MCT4 protein).By carefully designing the regulation process of redox steady-state can promote the development of this non-invasive treatment.
5.Conclusion and future perspectives
In this review,we summarized the progress in the construction of DNA-based nanomaterials and their applications in cancer therapy.DNA-based nanomaterials can be endowed with stimulus responsiveness by introducing functional nucleic acid sequences,thus expanding their application scope.On the other hand,the modifiability of DNA enhances the chemical stability and functional properties of DNA.From the perspective of application,DNA-based nanomaterials with inherent biocompatibility and degradability have been utilized in the delivery of chemotherapy drugs and gene drugs,stimulus-responsive release,and regulation of cell homeostasis.Despite many achievements to further improve the performance of DNA-based nanomaterials,there are still some challenges that remain: 1) Facing the complex internal microenvironment,it is still required to comprehensively consider and solve that how to improve the uptake efficiency and bioavailability of drugsin vivo,from the aspects including molecular design,micro-nano structure construction,and material-biological interaction.2) To meet the requirements of broader application,DNA-based nanomaterials need to transform from microscopic phenomena in test tubes to the real world.However,the high cost of DNA-based nanomaterials hinders their development.It is significant to develop new synthesis methods and build precise amplification instruments,reducing the synthesis cost of DNA materials.3) Although abundant DNA-based nanomaterials have good biocompatibilityin vivoandin vitro,the current assessment methods are relatively primary,far from enough for the evaluation of material safety.We believe that DNA-based nanomaterials will become toolboxes to help us explore the molecular mechanisms,cellular metabolism levels,and pathways of major human diseases and serve a wide range of fields such as tumor therapy,biological tracing,genomics,artificial intelligence.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (Nos.21621004 and 22174097),Tianjin Natural Science Foundation (Basic Research Plan,Nos.18JCJQJC47600 and 19JCQNJC02200).
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
- C–F bond functionalizations of trifluoromethyl groups via radical intermediates
