Selective probes targeting c-MYC Pu22 G-quadruplex and their application in live mice imaging
Zhuo Yu,Wenbo Hung,Liqio Shi,Shoyong Ke,∗,Shengzhen Xu
a National Biopesticide Engineering Research Centre,Hubei Biopesticide Engineering Research Centre,Hubei Academy of Agricultural Sciences,Wuhan 430064,China
b College of Science,Huazhong Agricultural University,Wuhan 430070,China
Keywords:Benthiazole Conjugated system Chemosensor G-quadruplex DNA In vivo imaging
ABSTRACT Several probes containing benzothiazole-guided conjugated systems (BGCS) were designed and synthesized,and two molecules (BGCS5 and BGCS6) of which were discovered as selective probes targeting c-MYC Pu22 G-quadruplex DNA.The fluorescence intensity of BGCS5 and BGCS6 in the presence of c-MYC Pu22 far exceeds that of the typical G4 probe TO1.Especially,the fluorescence of BGCS6 increased almost 193-fold in the presence of c-MYC Pu22 G4 compared to that alone in aqueous buffer condition with almost no fluorescence and 10–30 folds than those in the presence of other DNAs,which will be useful tools for disease detection in mammals.
Nucleic acids containing four strands of continuous guanine tend to form an irregular four-stranded structure called Gquadruplex (G4).G4s motifs occur in the promoters of lots of functionally important oncogenes,such asc-MYC,Bcl-2,c-Kit[1–6].In recent years,G4s has attracted wide attention because of its potential biological functionin vivo[7,8]and its application in the detection of other biomolecules without labeling with fluorescent or luminescent probes [9–12].The effective detection of G4 structure in living cells will promote the development of telomere targeted anticancer therapy and four-chain specific drugs.The studies of the interaction between G4 DNA and small molecules that bind and stabilize G4s is not only conducive to a better understanding of molecular recognition,but also of great significance for cancer diagnosis and the development of anticancer drugs.Meanwhile,the highly selective fluorescence probes are helpful and indispensable methods to visualize the various biological processes [13–16].
There are many small molecules have been demonstrated to have the properties of binding to G4s and producing fluorescence[17–37].These compounds do not produce background fluorescence signal when they are not bound to the analyte,but they emit very significant fluorescence when combined with G4s.Therefore,in the practice of scientific research,if the G4 fluorescence probe with high affinity,high selectivity and high fluorescence intensity can be developed,which can not only enable researchers to better understand the pathogenic mechanism of cancer at the molecular level,but also help to the research and development and improvement of anticancer drugs.Especially,benzothiazole dye Thioflavin T (ThT) has been reported as a typical fluorescent probe of G4[17–20].It has been widely used in selective staining and identification of amyloid fibers [21–23]in vivoandin vitro.Mohanty and his colleagues confirmed that ThT can induce human telomere sequence 22AG to form parallel G4 and antiparallel G4 in Tris-Buffer and water,respectively.These two cases produce greater fluorescence enhancement than double-stranded or single-stranded DNA[17].Thiazole orange (TO1) is also an asymmetric cyanine cationic dye with excellent affinity to G4 [24,25].When it is used as a fluorescence probe and the target substance is not detected,its fluorescence is turned off;it has almost no background interference in fluorescence detection,so it is a very popular G4s fluorescent dye,which is widely used in the detection of DNA/RNA with G4 structure.Although TO1 has excellent fluorescence properties,the dye cannot specifically recognize the specific sequence of G4s,so its application value will be greatly reduced.
In addition,benzothiazolyl group containing nitrogen and sulfur heteroatoms has unique fluorescence properties,which is often regarded as a fluorophore in the discovery of novel probes.It plays an important role in the output of fluorescence signals and the provision of binding sites.Using benzothiazole groups as mother nuclei,the design and synthesis of fluorescent probes with better selectivity [38–45]has gradually become a research hotspot in the field of fluorescent probes.Benzothiazole group was first paid attention to because of its important applications in biopharmaceutical [46,47]and medical diagnosis [48].Due to the existence of large planar and large delocalizedπbonds,benzothiazoles have high fluorescence quantum yields.
So,based on the aforementioned and the structure of TO1,we designed a series of benzothiazole-guided conjugated systems(BGCS) as shown in Fig.S1 (Supporting information),and hope to discover novel probes with specific recognition for G4 DNA.Through our efforts,it is found that the analogue probe BGCS5 and its derivative BGCS6 have a very strong specific recognition effect onc-MYCPu22 G-quadruplex sequence.When the probe binds toc-MYCPu22,it can produce strong red fluorescence.The excitation and emission wavelengths of the compound for confocal microscope imaging are 559 nm and 603 nm,respectively.In addition,the synthesis and structural modification of these benzothiazoleguided conjugated scaffolds are convenient.Because of its good water solubility and great fluorescence intensity,the compound has great application potential in cells and organisms.
The general synthetic routes of compounds BGCS1–6 were outlined in Scheme 1,and details for the experimental section can be found in Supporting information.
Scheme 1.Synthetic route for compounds BGCS1–6.Reagents and conditions: (a) Toluene,reflux,overnight;(b) cat.KOH,EtOH,reflux for 4–8 h;(c) 1-methylquinolin-1-ium iodide,cat.KOH,EtOH,reflux.
The recognition abilities of BGCS1–6 were fully investigated by monitoring the spectra changes of fluorescence in the presence of different equivalents of DNA (Table S1 in Supporting information) separately.First of all,through the analyses of UV–vis absorption spectra for all obtained molecules,we can find that the chemical sensor BGCS5 has a characteristic peak at about 590 nm(Fig.1A).Only when 1.0 equiv.c-MYCPu22 was introduced into the buffer (10 mmol/L K2HPO4/KH2PO4pH 7.0,100 mmol/L KCl)of BGCS5 (4 μmol/L),a distinct increment of fluorescence was observed (Fig.1B).These results indicate that BGCS5 can distinguishc-MYCPu22 from many other DNA species.Subsequently,similar experiments were also carried out for sensor BGCS6,and the same spectral changes were found (Figs.1C and D).However,the other molecules BGCS1–4 almost no recognition properties at the same test condition (Figs.S2-S5 in Supporting information).
Meanwhile,as a typical G4 fluorescent probe,TO1 has a remarkable fluorescence enhancement effect on most G4 sequences.In this test result (Figs.S6 and S7 in Supporting information),there are five tested sequences with fluorescence intensity above 2500,but TO1 has a poor fluorescence enhancement effect onc-MYCPu22 sequence,with fluorescence intensity below 1000.In the comparison of histograms,the fluorescence intensity of BGCS5 and BGCS6 in the presence ofc-MYCPu22 is close to or exceeds 20,000 (Fig.S7B),which far exceeds that of TO1.Based on the above results,we believe that the fluorescent probes BGCS5 and BGCS6 have excellent recognition ability forc-MYCPu22 sequence,and the fluorescence enhancement effect greatly exceeds that of traditional G4 probes.In summary,BGCS5 and BGCS6 have a highly selective detection ofc-MYCPu22 based on the present test results.
Furthermore,the fluorescence properties of the sensor were explored to establish if the changes in fluorescence could be observable through the ’naked eye’under UV light radiation.It was observed that BGCS5 showed selectivity toward the various DNAs and only the fluorescence color changes under UV light radiation were observed by ’naked eye’experiments induced byc-MYCPu22 sequence,and the fluorescence color of the solution also quickly changed from colorless to bright red.However,no detectable color responses were observed when adding other analytes such as VEGF,c-Kit1,TBA,TTA,c-Kit2,CGG12,19AT,dT30.It can also be seen from Fig.2A that as the number of equivalents ofc-MYCPu22 increases,the color changes were observed in naked-eyes in which the solution changed from pale red to shinyred.Subsequently,similar phenomenon was also observed for compound BGCS6 (Fig.2B).
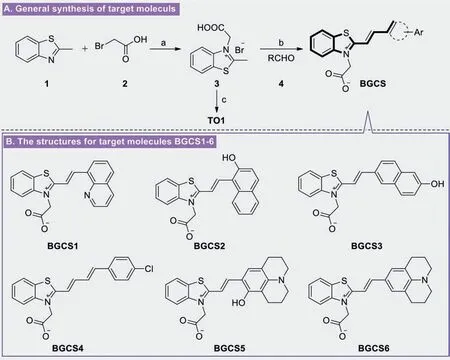
In order to investigate the specific concentration for selective DNA and its associated fluorescence changes,the chemosensors BGCS5 and BGCS6 was titrated by successive increment of number of equivalents forc-MYCPu22 and monitored the fluorescence spectra.From fluorescence titration spectra depicted in Fig.3,the sensors BGCS5 and BGCS6 showed an obvious peak at 617 nm,the fluorescence bands gradually increased with the addition of increasing equivalents ofc-MYCPu22.No other analytical substrates showed similar changes in maximum excitation wavelength.Moreover,the fluorescence titration experiment also indicated that 1.625 μmol/L and 1.128 μmol/Lc-MYCPu22 Gquadruplex forming DNA oligonucleotide was able to activate fluorescence emission of BGCS5 and BGCS6,respectively.

Fig.1.(A,C) Change in UV–vis spectra of BGCS5 and BGCS6 (4 μmol/L) in the buffer (10 mmol/L K2HPO4/KH2PO4,pH 7.0,100 mmol/L KCl),respectively.(B,D) Fluorescence spectra of BGCS5 and BGCS6 (4 μmol/L) (λex=590 nm) with addition of various analytes (4 μmol/L) in the buffer (10 mmol/L K2HPO4/KH2PO4,pH 7.0,100 mmol/L KCl),respectively.

Fig.2.Visualization of BGCS5 and BGCS6 mixed with DNA oligonucleotides under UV light (365 nm).
In addition,the recognition test was investigated to explain the possible binding mechanism between the sensors (BGCS5 and BGCS6) andc-MYCPu22,it can be seen that the fluorescence bond at 617 nm was enhanced first and weaker later with the increasing concentration ofc-MYCPu22.The Job-plots (Fig.S8 in Supporting information) indicate a stable 1:1 stoichiometry ratio was formed in the binding complex of the sensor (BGCS5 and BGCS6)andc-MYCPu22 in the buffer (10 mmol/L K2HPO4/KH2PO4pH 7.0,100 mmol/L KCl),and the association constants betweenc-MYCPu22 and BGCS5 or BGCS6 is 3.550 × 105or 2.506 × 105L/mol,respectively (Fig.S9 in Supporting information).The highly selective recognition for these two molecules may be due to their special structure combined with the large polar unit at one end and the hydrophobic moiety with large steric hindrance at the other end,which can stack with target DNA during the recognition process,and forms a lock-key pairing with the grooves in the G-quadruplex plane.In addition,the special tricycle julolidine scaffold in these two molecules may be more suitable to formπ-πstacking,and lead to more conducive to fluorescence.
To investigate the feasibility of BGCS5 and BGCS6 as a fluorescence probe,theirin vitrocytotoxic effects on human melanoma (A875) and human hepatocellular liver carcinoma(HepG2) cell lines were evaluated using the standard MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay[49–51]with 5-fluorouracil (5-FU) as a positive control.The results in Table S2 (Supporting information) indicated that these two probes exhibited very weak cytotoxic effect on the tested cancer cell lines (IC50>98 μmol/L),which demonstrated the absence of toxicity of these molecules and might be used as candidates for selective recognition G4sin vivo.
Based on the aforementioned results,the possible application ofin vivoimaging of the sensor was investigated.The compound BGCS5 was dissolved in buffer and injected into mice through tail vein for fluorescence imaging,and the procedures were approved by the Ethics Committee of Hubei Biopesticide Engineering Research Center.The imaging results (Fig.4) clearly show that in addition to the residual fluorescence in the tail due to intravenous injection,the bright fluorescence mainly appears in the abdominal area of mice,that is,the visceral areas such as liver,stomach,kidney,spleen and so on,which indicates that the genes containingc-MYCPu22 sequence may mainly exist in animal viscera.
Fig.3.(A,C) The change of emission peak of BGCS5 and BGCS6 at 617 nm depending on the concentrations of c-MYC Pu22.(B,D) Changes in fluorescence spectra for the chemosensor BGCS5 and BGCS6 (0.8 μmol/L) in the buffer (10 mmol/L K2HPO4/KH2PO4,pH 7.0,100 mmol/L KCl) with sequential addition of c-MYC Pu22 from 0 to 57 μmol/L,respectively.
Fig.4.Mice fluorescence imaging using ∼100 mW/cm2 590 nm laser excitation and 650LP filter (50 ms exposure time).
As special nucleic acid structure,G4s have attracted wide attention in different fields.However,it has been a great challenge to find novel ligands or fluorescent probes that can specifically recognize the G4 structure of a specific sequence.In this study,we found that the fluorescence probes BGCS5 and BGCS6 exhibited significant selectivity and strong luminescence response toc-MYCPu22 over other G4 structure.BGCS5 and BGCS6 showed good biocompatibility to tumor cells (IC50>98 μmol/L),and the animal imaging experiments indicate that these two probes have great application prospectsin vivo.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
The authors thank Prof.Xuhong Qian (School of Chemistry and Molecular Engineering,East China Normal University) for his helpful advice and encouragement.This work was financially supported by the Innovation and Application of Key Technologies of Quality-improving and Efficiency-increasing of Fengtou Ginger Industry (No.2020–620–002–06) and Natural Science Foundation of Hubei Province (No.2020CFB717),and the authors also gratefully acknowledge the partial support from the Program for Leading Talents of Hubei Academy of Agricultural Sciences (No.L2018031) and the Youth Science Foundation of Hubei Academy of Agricultural Sciences (No.2021NKYJJ17) and Hubei Agricultural Science Innovation centre (No.2019–620–000–001–27).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.087.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
