Dithienylethene metallodendrimers with high photochromic efficiency
Yuxuan Wang,Qifeng Zhou,Xiaoxiao He,Ying Zhang,Hongwei Tan,
Jianhua Xub,Cuihong Wanga,∗∗,Wei Wanga,Xiping Luoc,Jinquan Chenb,∗∗,Lin Xua,∗∗
a Shanghai Key Laboratory of Green Chemistry and Chemical Processes,School of Chemistry and Molecular Engineering,East China Normal University,Shanghai 200062,China
b State Key Laboratory of Precision Spectroscopy,East China Normal University,Shanghai 200241,China
c Zhejiang Provincial Key Laboratory of Chemical Utilization of Forestry Biomass,Department of Chemistry,Zhejiang A&F University,Hangzhou 311300,China
d College of Chemistry,Beijing Normal University,Beijing 100050,China
Keywords:Photochromic compounds Stimulus response Dendrimers Platinum-acetylide complexes Dithienylethene
ABSTRACT It has been challenging to achieve multi-photochromic systems without affecting the individual photoswitching properties of the constituent units.Herein,we present the design and synthesis of a new family of platinum-acetylide dendrimers containing up to twenty-one photochromic dithienylethene (DTE)units that exhibit both high photochromic efficiency and individual switching properties.Upon irradiation with ultraviolet (UV) and visible (vis) light,the resultant metallodendrimers display high conversion yield and good fatigue resistance.More interestingly,cyclization-cycloreversion kinetics revealed that the photochromic property of each DTE unit in these metallodendrimers is unaffected by its neighbor and the full ring-closure of up to twenty-one DTE units in one single dendrimer has been achieved.
Photochromic compounds are light-sensitive molecules that can undergo isomerization between at least two forms.During the past few decades,photochromic systems have attracted more and more attentions since their physicochemical properties can be finetuned upon being triggered by light or heat [1–10].In particular,an increasing effort has been recently devoted to the development of multiphotochromic molecules that are comprised of two or more photochromic because of their wide applications ranging from molecular sensors,molecular motors,energy and information storage devices,photoactuators,and artificial muscles [11–13].However,in a multiphotochromic system,the steric interaction,interchromophore interaction,excitonic and electronic coupling between adjacent photochromic units usually alter the efficiency and process of photochemical switching,thus leading to the partial photochromic.Therefore,the construction of multi-photochromic systems without affecting the individual photoswitching property of the constituent units remains a great challenge in this field.
Dithienylethene (DTE) represents one of the most popular and extensively studied photoswitches due to its high quantum yield,excellent fatigue resistance and high stability of both isomers,which has been blossoming into an exciting field of research during the past few decades [14–27].Recently,multiphotochromic compounds based on DTE have been reported.Two or more DTE units are connected covalentlyviaorganic spacers (methylene,ethynylene,diyne,phenylene,or silyl bridge,etc.) or organometallic spacers (Pt-acetylide,Ru-acetylide,or Au-acetylide,etc.) in these compounds [28–35].It should be noted that,rapid energy transfer from the ring-open DTE to an adjacent ring-closed one would be favored instead of full photocyclization in the most cases [36].Although great efforts have been dedicated to resolving this problem over the past few years [37],achieving full photocyclization of all DTE units in a single multiphotochromic system without affecting the individual photoswitching property is still a difficult task.
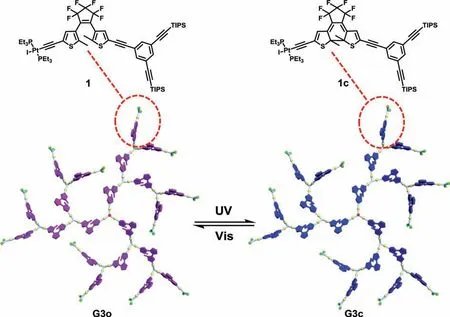
Fig.1.Schematic representation of the structural transformation of DTE dendrimer.The suffix “o” indicates the ring-open form and “c” indicates the ring-closed form.
Based on our and others’previous study on platinum-acetyide chemistry [38–41],we envisioned that employing platinumacetylide moieties as bridge to construct multiple DTE systems might be one effective method because the ligand-localized triplet states can be populated and they could inhibit the transfer of excited state energy from ring-open DTE to the ring-closed neighbor.Moreover,platinum-acetylide complexes are thermally robust and stable even upon being exposed to air and moisture [42–51],which can be obtained in high yield by a well-established synthetic methodology.To achieve this goal,dendrimers have evolved to be privileged platforms for the construction of multi-photochromic systems due to their unique highly branched and star-shaped feature [52–56].Herein,we present an efficient and facile approach for synthesis of multiphotochromic organometallic DTE dendrimers by employing the neutral platinum-acetylides as the main scaffold.More impressively,we have achieved full photocyclization of up to twenty-one DTE units in one single dendrimer (Fig.1).This approach could be one promising method for designing new multiphotochromic systems without affecting the individual photoswitching property,thus allowing for high photochromic efficiency.
Organometallic complex 1 (Scheme S1 in Supporting information) was employed as the basic precursor for the divergent dendrimer growth for following two reasons: (i) It contains platinum-acetylide unit that can react with alkyne units as well as two protected alkynes that can be gently exposed for dendrimer growth [57–59];(ii) bis(thien-3-yl) derivatives of perfluorocyclopentene usually exhibit high conversion yields and excellent fatigue-resistant properties.Moreover,the precursor 1 is very stable and soluble in common solvents,which could simplify the subsequent reaction and purification processed during the dendrimer growth [60].The Cu(I)-catalyzed coupling reaction of 1 with 1,3,5-triethynylbenzene produced the first-generation DTE dendrimer G1 at yield of 53% (Scheme S3 in Supporting information).By repeating the iterative deprotection−coupling reactions,the construction of the second-generation and third-generation DTE dendrimers G2 and G3 (Fig.2a) were achieved through a divergent approach (Scheme S3 in Supporting information).All obtained dendrimers Gn are soluble in common solvents such as chloroform,dichloromethane,and THF.The purification of these dendrimers was performed using flash column chromatography and gel permeation chromatography (GPC).
The structures of the resultant organometallic dendrimers were well characterized by using1H and31P NMR spectroscopy (Figs.S4 and S6 in Supporting information and Fig.2b).G1 was selected as a representative to illustrate the structural characteristics of resultant DTE dendrimers.The1H NMR spectrum of G1 shows five signals atδ6.74,6.91,7.27,7.51 and 7.54 with an integral ratio of 3:3:3:3:6.The signals atδ6.74 and 7.27 are assigned to the protons of thiophene heterocycles.The thiophene protons exhibit the upfield shifts resulted from the enhancement of electron density upon covalent linkage with of platinum-acetylide units.The signals atδ7.51 and 7.54 are assigned to the protons inparaandorthopositions of the outer aromatic rings,respectively.Compared with the organometallic complex 1,the appearance of the new peak atδ7.27 attributed to the inner aromatic rings protons indicates the successful coupling of triethynylbenzene with the precursor 1.Moreover,the31P NMR spectrum of G1 shows only one signal atδ11.5,which is consistent to the phosphorus atoms on triethylphosphine ligands.For G2,two sets of signals are observed that are ascribed to different phosphorus atoms of the inner and outer triethylphosphine ligands.31P NMR spectrum of G3 also displays two signals because the signals of two outer triethylphosphine ligands could not be well distinguished.Notably,the sharp NMR signals along with the solubility of these species have ruled out the formation of organometallic polymers.
Mass spectrometric studies of the obtained dendrimers Gn were performed by using matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) (Fig.S10 in Supporting information).For G1,the MALDI-TOF-MS spectrum in reflection mode exhibits a single peak atm/z3999.1,which is attributed to M+with a theoretical monoisotopic mass at 3994.4.The peak of G1 is in good agreement with the theoretical distribution.In the case of G2,the corresponding peak atm/z10,780.5 is observed in the MS spectrum (theoretical average M=10,746 Da).The theoretical exact mass of G3 is 24,249.9 Da that is too large to get a high signal-to-noise ratio peak.Fortunately,the MALDI-TOFMS spectrum of G3 shows a single peak atm/z24,396.2,which supports the formation of discrete DTE dendrimer with the third generation.
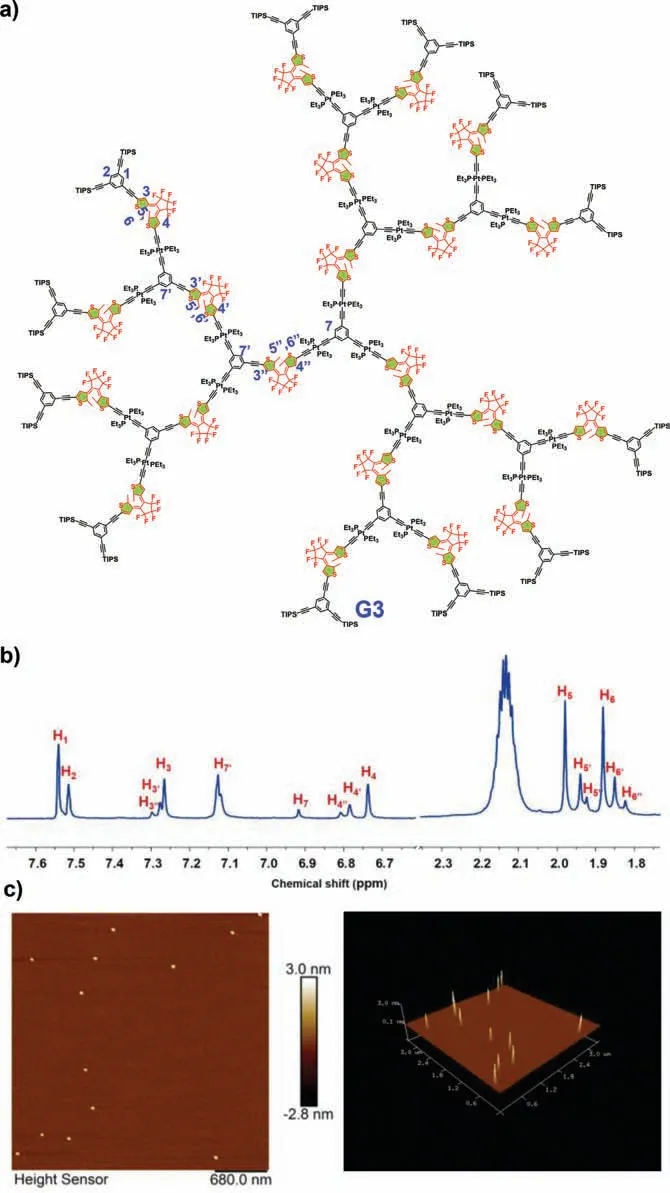
Fig.2.Characterization of DTE dendrimer G3.(a) Structure of G3.(b) Partial 1H NMR spectrum (CD2Cl2,500 MHz,r.t.) of G3.(c) Two-dimensional (left) and three-dimensional (right) AFM images of G3.
Two-dimensional diffusion-ordered NMR spectroscopy (2DDOSY) was performed to obtain the further structural information of the resultant DTE dendrimers in solution (Fig.S9 in Supporting information).A decrease in the measured weight averaged diffusion coefficients (D) (8.13× 10–9for G1;6.03× 10–9for G2;3.31× 10–9for G3) is observed,indicating an increase in the hydrodynamic diameter with the growth of dendrimer generation.By studying the direct atomic force microscopy (AFM) images on a surface,the structural parameters of the DTE dendrimers could be obtained.As shown in Fig.S11 (Supporting information),a consistent set of small particles are observed in AFM images of Gn,which are attributed to the adsorbed DTE dendrimers species,respectively.The detailed cross-sectional measurements on a large number of isolated features reveal the gradually increased average heights with each generation of dendrimers (ca.1.0 nm for G1;ca.1.9 nm for G2;ca.3.1 nm for G3 (Fig.2c)).
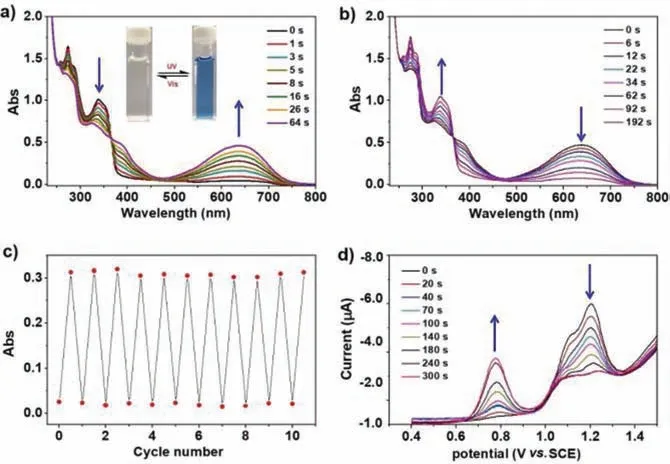
Fig.3.Characterization of structural transformation of DTE dendrimer G3.(a and b) Absorption spectral changes of G3 (9.5× 10–8 mol/L in CH2Cl2) upon (a) UV(365 nm) irradiation and (b) visible-light (> 530 nm) irradiation.The inset photographic image in (a) shows the color changes of G3 upon alternating UV and visiblelight irradiation.(c) Fatigue resistance of G3 upon alternating UV (365 nm) and visible-light (> 530 nm) irradiation.(d) Changes in the differential pulse voltammograms of G1 (2× 10–5 mol/L in CH2Cl2) irradiation at 365 nm.For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.
The light-driven switching of these dendrimers was firstly studied by1H and31P NMR spectroscopy.Again,G1 was selected as a representative to illustrate such light-driving switching process.When the solution of G1 in CH2Cl2(1.2× 10–4mol/L) was irradiated with UV light at 365 nm,the1H NMR spectral signals atδ6.78 for H4and 7.31 for H3are gradually weakened and finally disappeared,where two new signals atδ6.07 for H4and 6.50 for H3are increasingly enhanced due to the photocyclization reaction G1o →G1c.The loss of aromaticity of the thiophene heterocycles in the photocyclization reaction leads to this characteristic upfield.The signals of the -CH3proton of G1o are observed atδ2.02 (H5)and 1.91 (H6).Upon irradiation of G1o under UV light at 365 nm,the two signals are gradually attenuated and finally vanished with the occurrence of two new CH3signals atδ2.19 (H5) and 2.18(H6).The similar results were observed in the case of G2 and G3.It should be noted that,in the more complex case of G3,the signals atδ7.27,7.28 and 7.30 that are assigned to the protons of three layered thiophene heterocycles shift upfield to the signals atδ6.46 and 6.45,which are assigned to the ring-closed inner two and outer DTE units with the integral ratio 3:4 ((3+6):12).The photochromic reaction G1o →G1c has been also supported by31P NMR spectroscopy.When a CH2Cl2solution of G1o is irradiated at 365 nm,the phosphorus signal atδ11.51 decreases gradually and finally vanishes,whereas a new broad phosphorous signal is observed atδ11.90.It reveals that G1o is converted to G1c quickly upon irradiation at 365 nm and the single ring-closed intermediate did not appear.At the photostationary state (PSS),the integral ratio of phosphorous signals between G1o and G1c suggest the presence ofca.95% of G1c and 5% of G1o.The transformation behavior induced by light irradiation of Gn was further investigated by UV–vis techniques (Figs.3a and b).The absorption spectrum of G3 (in CH2Cl2at 298 K) exhibits an intense band in the near-UV region (ca.330 nm) with the weaker transition at higher energy(ca.275 nm).Upon irradiation at 365 nm,the colourless solution of G3 quickly turns to blue (insert in Fig.3a).Notably,two new absorption bands at 390 and 637 nm are observed in the visible region along with a well-defined isosbestic point at 374 nm arising from the corresponding ring-closed photostationary state (PSS)of G3viathe typical photocyclization.During light irradiation,the UV–vis changes are simple and clean with a well-defined isosbestic point,which provides evidence for the existence of only two lightabsorbing components.This means that each photochromic unit is isolated from the others [33,37].According to the previous study on DTE-based systems,the low energy absorption is attributed to theπ-conjugation delocalizing throughout the DTE ligands due to the formation of ring-closed form,then leading to the decrease of HOMO-LUMO gap.Similarly,UV irradiation of G1 or G2 at 365 nm in CH2Cl2results in the ring-closed PSS of G1 or G2,respectively,accompanied by a color change from colourless to blue along with the formation of two intense absorption bands at 390 and 637 nm.Moreover,the photoisomerization between the ring-open and ringclosed forms of the dithienylethene moieties could be repeated more than ten times (Fig.3c).The dendrimer G1 exhibits an irreversible Pt-based oxidation wave at 1.21 V together with an irreversible DTE-centered wave at 1.11 V (Fig.3d).Upon irradiation of G1 at 365 nm to the PSS,the oxidation potentials of both Ptand DTE-centered waves are distinctly less anodic because of the enhancedπ-electron density with photocyclization at both DTE units.Particularly,the presence of two successive DTE-based oxidation waves at 0.78 V and 1.05 V for G1c suggests that the moderate electron interaction is likely mediated between the ring-closed DTE units across thetrans-Pt(PEt3)2spacer [61].
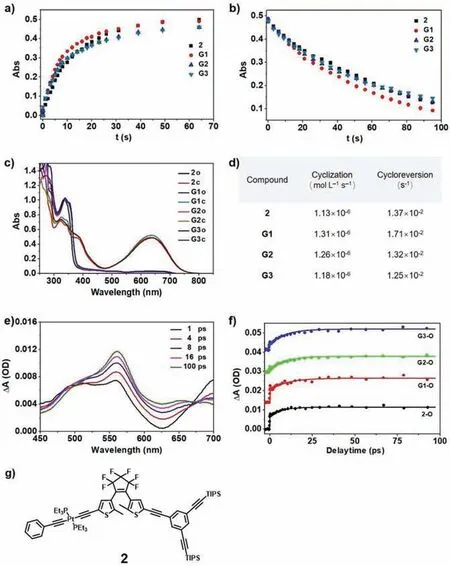
Fig.4.Cyclization-cycloreversion kinetics studies of organometallic complex 2 and the DTE dendrimers Gn.(a and b) Absorption spectral changes at 637 nm of 2 (2.0×10–5 mol/L),G1 (6.7× 10–6 mol/L),G2 (2.2× 10–7 mol/L) and G3 (9.5× 10–8 mol/L)upon UV irradiation at 365 nm (a) and visible-light irradiation (> 530 nm) (b).(c)UV–vis absorption spectra of ring-open and ring-closed form of 2 and Gn.(d) The rate constants of cyclization and cycloreversion processes of 2 and Gn.(e) Femtosecond transient absorption spectra of sample 2 under 365 nm excitation.(f) Kinetics of sample 2,G1,G2 and G3 monitored at 560 nm.(g) Structure of model complex 2.
To better understand the photochromic process of the DTE dendrimers,a model complex 2 was prepared from the precursor 1 (Scheme S2 in Supporting information).The cyclization and cycloreversion kinetics of DTE dendrimers Gn and the model 2 were investigated by irradiation by UV (365 nm) or visible light (>530 nm) (Fig.4).As shown in Fig.4a,the cyclization kinetics of Gn and 2 show that the photochromic cyclization reaction followed the zeroth-order reaction kinetics;the relationships between absorbance and exposure time (Avs.t) display a good linearity upon irradiation with UV light (365 nm).Due to the darker color of the solution that affected the absorption of UV light upon irradiation with UV light,the first nine points are plotted to on the graph.The slopes of the Avs.t lines give the zeroth order rate constant,ko-c.According to this method,all thekvalues for the cyclization process (ko-c) of the dendrimers Gn and the complex 2 are readily determined to be 1.13× 10–6,1.31× 10–6,1.26× 10–6,and 1.18×10–6mol L–1s–1,respectively.Similarly,the cycloreversion kinetic curves shown in Fig.4b indicate that the photochromic cyclization reaction followed first-order reaction kinetics.The rate constantkof the cycloreversion process of DTE dendrimers Gn and the complex 2 are calculated to be 1.71× 10–2,1.32× 10–2,1.25× 10–2,and 1.37× 10–2s−1,respectively.Importantly,the rates of photochromic process for the DTE dendrimers and the complex 2 are essentially identical,indicating that each switching units behaved independently (Fig.4d).Upon irradiation at 365 nm,both the DTE dendrimers Gn and the model organometallic complex 2 undergo photocyclization with the similar maximum at 637 nm.The molar absorptivity of G1–G3 is 3,9 or 21 times that of 2,confirming that the PSS (in terms of the total number of photochromic units) for Gn is essentially identical to 2.
The dynamics of cyclization of diarylethene derivatives were previous studied by time-resolved spectroscopy [62–65]and the ring-close reaction was found to take place within several ps.Therefore,we further investigated mechanism of the cyclization reaction for model complex 2 and DTE dendrimers Gn by using femtosecond transient absorption (TA) spectroscopy.As shown in Fig.4e the upper panel,complex 2 shows one broad excited state absorption (ESA) band (450–600 nm) with two peaks at 500 and 560 nm as well as a second ESA band (620–750 nm) with its maximum at 700 nm immediately after photo excitation.In the next 100 ps,the intensity of the 560 nm ESA signal gradually increases while the intensity of the red side ESA band decreases.Finally,a new ESA peak centered at 650 nm shows up and this peak matches well with the steady-state absorption of complex 2 (Fig.4c) in the ring-closed form,indicating the finish of ring-close reaction in this compound.After 100 ps (Fig.4e the lower panel),there is bare any spectra evolution and the whole ESA signal decay together in our 7.0 ns detection time window.The initial build-up of the ESA signal were used to analyze the time profiles of the ring-close reaction in previous time-resolved studies [63–65]and we chose the kinetic trace at 560 nm to reveal the time scale of ring closure for complex 2 in this study.The best-fit (τ2,Table S1 in Supporting information) shows that the build-up lifetime for complex 2 is 7.4± 0.8 ps and this value is in line with those determined for diarylethene derivatives in literatures [62–64].Thus,we conclude that the ring closure reaction of our model complex 2 should finish in ∼7.0 ps after photo excitation.
TA experiments were also carried out on DTE dendrimers Gn under the same condition and the spectra are shown in Fig.S15(Supporting information).Very similar time-resolved spectra behavior was observed for sample G1–G3,suggesting these samples could have the same cyclization reaction mechanism.Kinetic traces at 560 nm (Fig.4f) clearly demonstrate that sample G1–G3 should have almost the identical signal build-up process.The best fit lifetimes (Table S1) are determined to be 9.9± 1.5 ps,10.8± 0.8 ps and 11.4± 1.1 ps for G1–G3,respectively,and these values agree with each other within experimental uncertainty.Since the total number of photochromic units in sample G1–G3 is also the same in our TA experiments,the almost identical ESA build-up lifetimes and TA signal intensities are the direct evidence to show that the ring closure time is ∼10 ps no matter there are 3,9 or 21 DTE units in our DTE dendrimers.
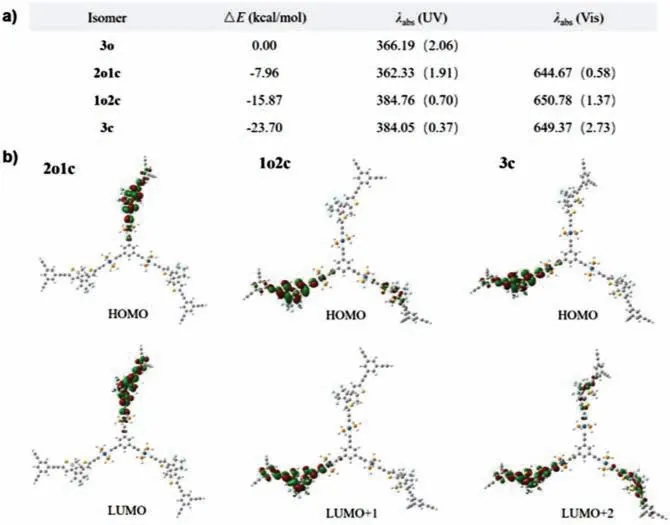
Fig.5.Computational studies of G1.(a) Relative energies (ΔE (kcal/mol)) and main visible absorption bands (obtained after convolution) for the different isomers of G1.(b) Molecular orbitals of three isomers of G1.The suffix “o” indicates the ringopen form and “c” indicates the ring-closed form.3o,2o1c,1o2c and 3c are G1 isomers with three ring-open form,two ring-open form,one ring-open form and three ring-closed form DTE units.
TD-DFT calculations were performed on G1 to learn more about the independent switching units.The simulated absorption spectra of the four structures obtained upon successive DTE cyclisations are given in Fig.5,whereas Fig.5a lists the corresponding and the relative total energies.The relative energies regularly increase by 7.96 kcal/mol for each electrocyclization,a typical cost of unconstrained DTE unit.Indeed,a strong UV band corresponding to the lowest energetic transition of each isolated ring-closed DTE unit is found in G1 (3o),whereas in G1 (1c2o),the intensity of this band decreases while a new band corresponding to the second energetic transition of ring-closed form DTE at the longer wavelengths appears.The nature of the molecular orbital transitions assigned to the main visible spectra in the different isomers was investigated.The energy gap of the molecular orbital transitions between HOMO and LUMO of G1 (2c1o) corresponding to the absorption band atca.645 nm is 2.4 eV,which is same as the energy gap of G1 (1c2o)and G1 (3o).Besides,as shown in the simulated molecular model of G1,the benzene core could prevent the effective energy transfer between the photochromes due to the twists between the phenyl ring and platinum-acetylide units (Fig.5b).All these observations are consistent with the experimental trends and hint that the three DTE could be viewed as independent photochromic entities rather than a conjugated ensemble.
In summary,a new family of miltiple DTE metallodendrimers was prepared by using platinum-acetylide moieties as bridges.UV–vis absorption spectra and multinuclear NMR spectroscopy suggested the high transformation rate,high conversion yield and excellent fatigue resistance of the resultant DTE dendrimers.Cyclization-cycloreversion kinetics studies demonstrate that twenty-one DTE units are rather independent,i.e.,the electronic feature of a DTE being is unaffected by the nature of its neighbors.This approach to arranging chromophores is expected to be general and allow for straightforward synthetic access to systems,in which multiple photochemical and redox-active units are arranged with a high degree of order and retention of molecular properties,thus allowing for their promising applications in various photonic devices such as optical memory and organic lightemitting diodes in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.11674101,21873030,91850202,and 21871092),and the Fundamental Research Funds for the Central Universities.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.048.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
