Electrochemical utilization of methanol and methanol-d4 as a C1 source to access (deuterated) 2,3-dihydroquinazolin-4(1H)-one
Mingzhu Liu,Liang Xu,Yu Wei
School of Chemistry and Chemical Engineering,Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan,Shihezi University,Shihezi 832003,China
Keywords:Electrochemistry Heterocycles Dihydroquinazolinone Aminobenzamide C1 source
ABSTRACT Herein,an electrocatalytic protocol for the synthesis of 2,3-dihydroquinazolin-4(1H)-one has been disclosed.Methanol is activated and utilized as the C1 source to cyclize with 2-aminobenzamides.This cyclization reaction proceeds conveniently (room temperature and air atmosphere) without any homogeneous metal catalysts,external oxidants,or bases.A wide variety of N,N-disubstituted 2,3-dihydroquinazolin-4(1H)-ones are obtained via this approach.Moreover,when methanol-d4 is used,a deuterated methylene motif is incorporated into the N-heterocycles,providing an efficient approach to the deuterated N-heterocycles.
Incorporation of C1-motif to the organic skeleton,such as methyl,methylene and methenyl,has always been an important research topic in organic synthesis.Such transformations have been demonstrated to be an effective way to modify the chemical and biochemical properties of organic molecules [1,2].A variety of reagents are utilized as the C1-precursors and applied in these transformations,such as carbon monoxide,formic acid,HCHO [3].With an increasing demand for high environmental friendliness and atom economy [4],methanol (MeOH),one abundant,biodegradable,and renewable feedstock,which can be effi-ciently obtained from fossil fuels and agricultural products [5,6],has gradually gained accelerating application as the C1 source in the last decades [7,8].An array of transition-metal-catalyzed MeOH utilization reactions have emerged,forging a variety of C-C and Cheteroatom bonds [9].Among these,Ir- and Ru-based catalysts are most frequently used [10-16].In this regard,a metal-free protocol with MeOH as C1 source should be beneficial to address the cost and metal residue issues [17,18].
On the other hand,electrochemical synthesis,which directly employs electrons as the redox agent,has recently gained renaissance as an environmentally benign protocol and enabled many previous challenging transformations [19-27].Electrochemical utilization of MeOH as the C1 source instead of methoxy source remains rather unexplored.The only examples were disclosed in 2020.Pan,Tang,Wang and co-workers achieved the electrochemical activation of MeOH,realizingα-methoxymethylation and aminomethylation of propiophenones under metal- and oxidantfree conditions [28].Zhao group disclosed a pathway for direct C–H methylthiolation of electron-rich aromatics,using KSCN as sulfur source and methanol as the methylation reagent [29].This leaves ample space to investigate the electrochemical toolkit of MeOH as the C1 source.
As our continued efforts to discover new methods to constructN-heterocycles [30-33],we envisioned that electrochemical activation of MeOH with 2-aminobenzamides would provide an alternative paradigm to access 2,3-dihydroquinazolin-4(1H)-one skeleton,which exist in many naturally occurring alkaloids and drug molecules (Scheme 1a) [34-38].To the best of our knowledge,there are no precedents achieving this transformation,no matter under thermochemical,photochemical,or electrochemical conditions [39,40],although one protocol for the construction of quinazolinones has been disclosedviaIr-catalyzed activation of MeOH as the C1 source (Scheme 1b) [41].
Herein,the success of this hypothesis was reported(Scheme 1c).A range ofN,N’-disubstituted dihydroquinazolinones,which could only be obtained from aldehyde/ketone previously[42],are accessedviathis approach under metal-,oxidant,and additive-free conditions.
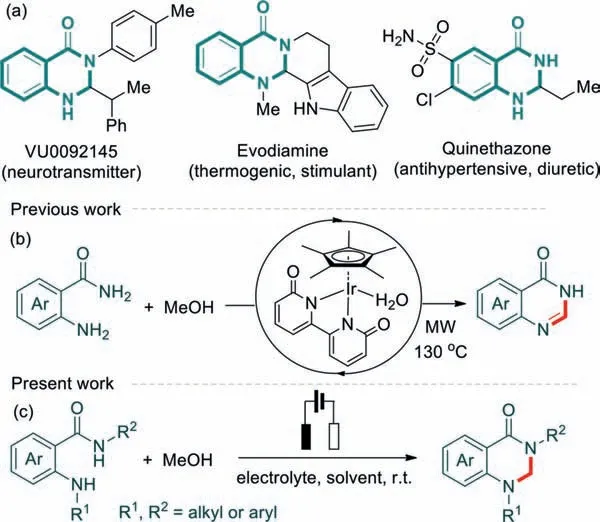
Scheme 1.(a) Biologically active molecules containing the 2,3-dihydroquinazolin-4(1H)-one skeleton;(b) Ir-catalyzed activation of methanol as a C1 source for the construction of quinazolin-4(3H)-ones;(c) Electrochemical synthesis of 2,3-dihydroquinazolin-4(1H)-ones.
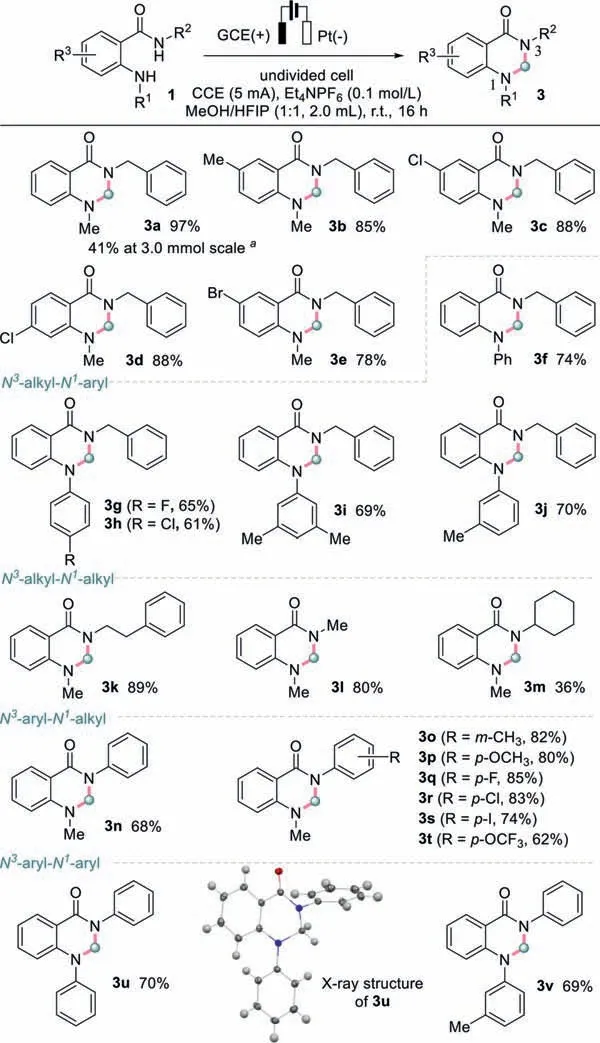
Scheme 2.Substrate scope of 2,3-dihydroquinazolin-4(1H)-one from MeOH.Reaction conditions: undivided cell,1 (0.3 mmol),solvent (2.0 mL),Et4NPF6 (0.1 mol/L),5 mA,r.t.,16 h.a1a (3.0 mmol),solvent (10.0 mL),Et4NPF6 (0.1 mol/L),5 mA,r.t.,36 h.
Initially,N,N’-disubstituted 2-aminobenzamide 1a and MeOH were chosen as the model substrate to explore the reaction conditions in an undivided cell.The desired aminal-type product 3a could be obtained fortunately.After an extensive screening of an current was cut off,indicating its essential role in this cyclization(Table 1,entry 13).array of variables,the electrolysis could result in a 97% isolated yield of the target product 3a in a mixed solvent of MeOH/HFIP(1:1) at room temperature (Table 1,entry 1),when the reaction was conducted under a constant current of 5 mA in an undivided cell equipped with a glassy carbon electrode (GCE) anode and a platinum cathode.Et4NPF6was explored as the supporting electrolyte.Replacing Et4NPF6with other electrolytes,such as Me4NPF6,nBu4NBF4and Et4NBF4,was found to be detrimental to this cyclization process (entries 2-4).Thereafter,other solvents/C1 sources were investigated.DMF and MeCN were found ineffective for the formation of 3a (entries 5 and 6).The Pt(+)/Pt(−) and GCE(+)/GCE(−) electrode combinations led to slightly decreased yields (entries 7 and 8).Reducing the current slightly affect the result (entry 9),while increasing the current to 8 mA resulted in a 22% decrease in isolated yield (entry 10).When the reaction time was shortened or prolonged,the isolated yield both lowered.Finally,the transformation was totally prohibited when the electric
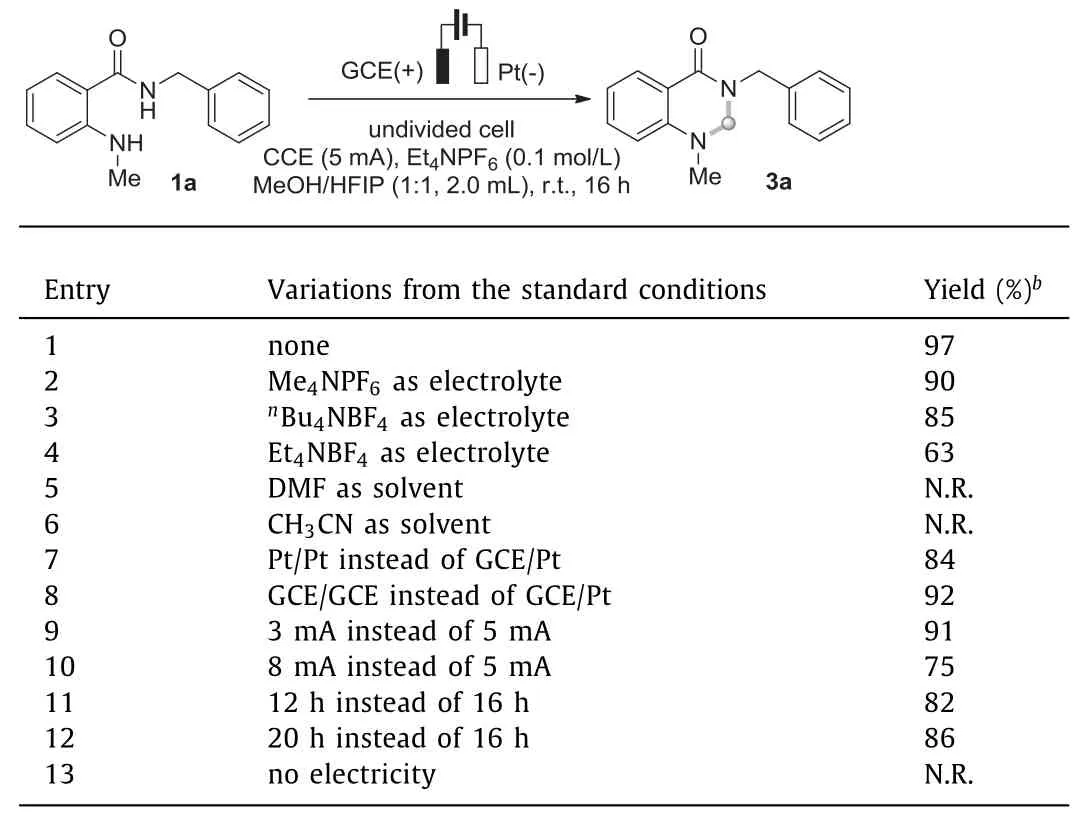
Table 1 Optimization of the reaction conditions.a
With the optimal conditions in hand,the substrate scope for the synthesis of 2,3-dihydroquinazolin-4(1H)-one was then investigatedviathis electrochemical cyclization approach (Scheme 2).

Scheme 3.Substrate scope of 2,3-dihydroquinazolin-4(1H)-one from other alcohols.Reaction conditions: undivided cell,anthranilamide (0.3 mmol),alcohol (1.0 mL),HFIP (1.0 mL),Et4NPF6 (0.1 mol/L),5 mA,r.t.,16 h.a Under 7 mA current.
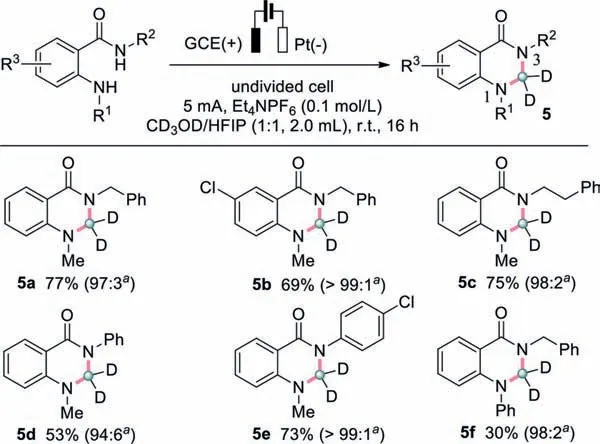
Scheme 4.Substrate scope of 2,3-dihydroquinazolin-4(1H)-one-2,2-d2 from methanol-d4.Reaction conditions: undivided cell,anthranilamide (0.3 mmol),methanol-d4 (1.0 mL),HFIP (1.0 mL),Et4NPF6 (0.1 mol/L),5 mA,r.t.,16 h.a The ratio of deuterium-incorporation.
At first,variants on the phenyl of anthranilamide were explored.5-Methyl (1b),5-Cl (1c) and 5-Br (1e) substituted anthranilamides afforded the corresponding dihydroquinazolinone compounds in good yields,indicating the compatibility of the conditions with electron-withdrawing and donating groups.Halogen (Cl and Br)substituted anthranilamide reacted smoothly with methanol,affording 3c-3e in isolated yields of 78%-88%.When the methyl on the aniline motif of 1a was replaced by a phenyl,N3-benzyl-N1-phenyl-substituted 3f was obtained in 74% yield.Also,the electrondonating and electron-withdrawing groups on theN1-aryl group were well tolerated to access the corresponding products 3g-3j in 61%-70% yields.Similarly,3n-3t could be obtained in moderate to good yields,affording diverseN3-aryl-N1-methyl substituted substrates.Methoxy,trifluoromethoxy,methyl,and halogens were all compatible herein.When two methyls were present on the aniline and benzamide motifs,3l was obtained in 80% yields.In the same while,probably due to the steric effect,a bulky cyclohexyl resulted in a dramatically decreased yield to 36% (3m).N1,N3-Diphenyl substrate 3u could be accessed in 70% yield.The presence of the aminal motif,with methylene between two nitrogen atoms,was confirmed unambiguously by X-ray single-crystal diffraction.
Then,different alcohols were treated under such electrochemical cyclization conditions (Scheme 3).Generally,lower isolated yields were obtained probably due to steric hindrance,in comparison with the MeOH case.Without modifying the conditions in Scheme 2,ethanol reacted with anthranilamide derivatives to access corresponding products 4a-4c with methyl on the aminal carbon in 45%-60% yields.When secondary alcohols were used,increasing the current to 7 mA resulted in the products in moderate yields (4d,48%).
Deuterium is a stable isotope of hydrogen.Deuterated ingredients can be utilized in mechanistic,spectroscopic,and tracer studies during the discovery and development of pharmaceuticals [43-45].Therefore,the incorporation of deuterium into potential drug substances has seen a great progress in recent years.It was envisioned that methanol-d4,a readily available and relatively cheap deuterated solvent,might introduce a deuterated methylene motif in this electrosynthesis process [46].As illustrated in Scheme 4,methanol-d4was found to engage in the cyclization process smoothly,leading to 2,2-deuterated dihydroquinazolin-4(1H)-ones.Generally,compared with its methanol counterpart,deuterated methanol resulted in the products in decreased yields.For example,5a could be isolated in 77% yield (Scheme 4),which was 20% lower than the value of undeuterated 3a (97%,Scheme 2).Also,an array of variants from the starting anthranilamides were explored,demonstrating this protocol to be compatible with differentN,N’-disubstituted substrates.
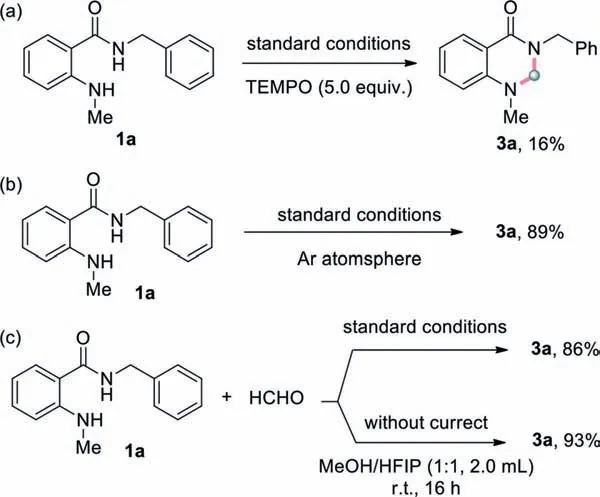
Scheme 5.Control experiments.
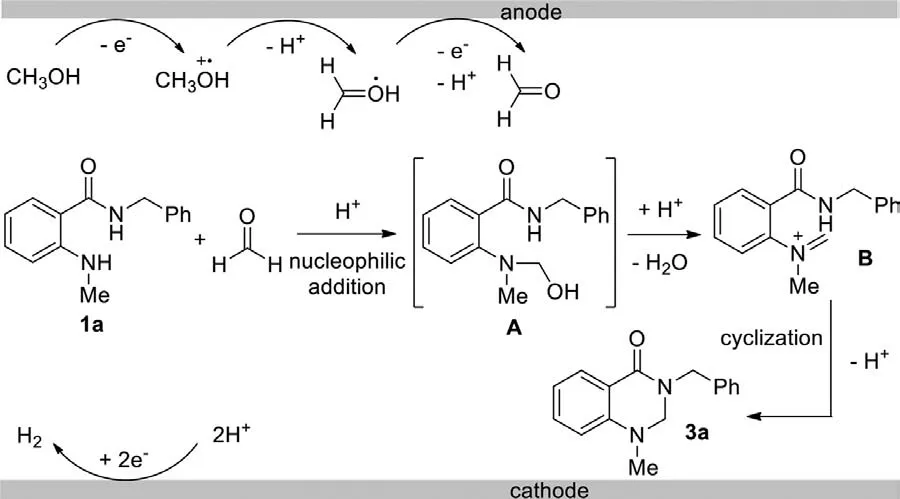
Scheme 6.Plausible reaction mechanism.
The mechanism of the reaction was explored by several control reactions.When 5.0 equiv.of 2,2,6,6-tetramethylpiperidineNoxyl (TEMPO),a radical scavenger,was added into the standard reaction conditions,the yield of the desired product is only 16%(Scheme 5a),which implied the involvement of radical species during the reaction.Oxygen has also been determined to be not critical to this transformation (Scheme 5b).The reaction between 1a and formaldehyde under standard conditions led to the formation of 3a in 86% isolated yield (Scheme 5c),indicating aldehyde might be a possible intermediate during this cyclization reaction.This envision was further reiforced since the cyclization process between 1a and formaldehyde could proceed efficiently in the absence of current (Scheme 5c).
Based on the above observations and previous reports,a plausible mechanism of this electrochemical cyclization was proposed,which was shown in Scheme 6.The proposed mechanism was initiated by sequential oxidation of methanol to produce formaldehyde at the anode [47].During the oxidation process,proton was released into the mixture,which could catalyze the nucleophilic addition of the amine motif onto formaldehyde and lead to the intermediate A.Then,enamine cation B was generated via the dehydration process.The final cyclization would afford the target product.During this process,H2was released on the cathode via the consumption of the proton.
In summary,an efficient electrochemical protocol for the construction of 2,3-dihydroquinazolin-4(1H)-one skeleton has been developed herein.Using methanol or methanol-d4as the C1 source,anthranilamide derivatives could be cyclized at room temperature to afford the desired (deuterated)N-heterocycles conveniently,under homogeneous metal-based catalyst,external oxidant and base-free conditions.This electrochemical methodology is expected to not only complement the existing methods towards the privileged dihydroquinazolinone skeleton,but also provide a chance to access deuteratedN-heterocycles efficiently and economically.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful for the financial support from the National Natural Science Foundation of China (No.22061036),the program for youth science and technology innovation leader of Xinjiang Bingtuan (Nos.2019CB026,CXRC201601).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.019.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
