Electrochemical determination of paraquat using a glassy carbon electrode decorated with pillararene-coated nitrogen-doped carbon dots
Hao Zhang,Kun-Tao Huang,Ling Ding,Jie Yang,Ying-Wei Yanga,,∗,Feng Liang
a The State Key Laboratory of Refractories and Metallurgy,School of Chemistry and Chemical Engineering,Wuhan University of Science and Technology,Wuhan 430081,China
b International Joint Research Laboratory of Nano-Micro Architecture Chemistry,College of Chemistry,Jilin University,Changchun 130012,China
Keywords:Electrochemical detection Carbon dots Macrocyclic chemistry Supramolecular chemistry Pesticide detection
ABSTRACT An electrochemical sensor (carboxylatopillar[5]arene-coated nitrogen-doped carbon dots,namely CCDs)based on carboxylatopillar[5]arene (CP[5]) functionalized nitrogen-doped carbon dots (N-CDs) has been developed in a facile and economic manner.To improve the performance of this electrochemical sensor in pesticide detection,the optimal solution pH (pH 7) and loading amount of CCDs on the electrode(0.50 mg/mL) have been determined.By virtue of the good conductivity of N-CDs and the molecular recognition property of CP[5],CCDs modified glassy carbon electrode,namely CCDs/GCE,shows excellent anti-interference capability,selectivity,stability,and reproducibility in the sensitive detection of paraquat.The peak currents are proportional to the paraquat concentration (from 0.1 μmol/L to 10 μmol/L) with a detection limit of 6.4 nmol/L (S/N=3),indicating a great potential in pesticide detection.In comparison with the electrochemical sensors that require expensive metal nanoparticles and complex preparation processes,CCDs/GCE exhibits excellent detection capability of paraquat with lower cost and simpler preparation processes.
The appearance of pesticides prompts the rapid development of modern agriculture,but also brings potential risks to the surrounding environment and human beings [1,2].Particularly,the abuse of highly toxic pesticides,such as paraquat (PQ,N,N′-dimethyl-4,4′-bipyridinium dichloride,also known as methyl viologen) and diquat,generates toxic residues in agricultural products,which may cause irreversible damage to human liver,lung,and kidney,rendering the establishment of a simple and effective approach for pesticide detection extremely necessary [3,4].Certain methods for pesticide detection,including high-performance liquid chromatography,Raman spectroscopy,gas chromatography-mass spectrometry,fluorescence,capillary electrophoresis,and electrochemical method,are in continuous development and undoubtedly fruitful [5-15].However,most of these methods require expensive equipment and complicated operational processes.Considering the potential demand of miniaturized devices and rapid detection,electrochemical method has received extensive attention due to the simple operation,rapid analysis,simple equipment requirements,and relatively low cost [16,17].Moreover,electrochemical sensors rely on the electrocatalytic reaction between electrodes and analytes,and produce an electrical signal for the analyte,which have a significant impact on the test performance of electrochemical detection [18].Therefore,the development of a rapid,reliable,and sensitive electrochemical sensor will further promote the application of electrochemical approaches in pesticide detection.
Recently,carbon dots (CDs),as a class of zero-dimensional carbon-based nanomaterial,have made outstanding achievements in electrochemical sensing due to the excellent solubility,low toxicity,small size effect,easy modification,and high electrochemical activity [19-21].Furthermore,heteroatom doping,including nitrogen,phosphorus,and boron atoms,can effectively improve the electrochemical performance of CDs [22].Nitrogen-doped carbon dots (N-CDs) have been widely used for electrochemical detection in virtue of the excellent electrochemical activity and easy availability [23].In addition,abundant amino and carboxyl groups on the surface of N-CDs are beneficial for surface modification,which will further improve the sensitivity,selectivity,reproducibility,and stability of electrochemical sensors.
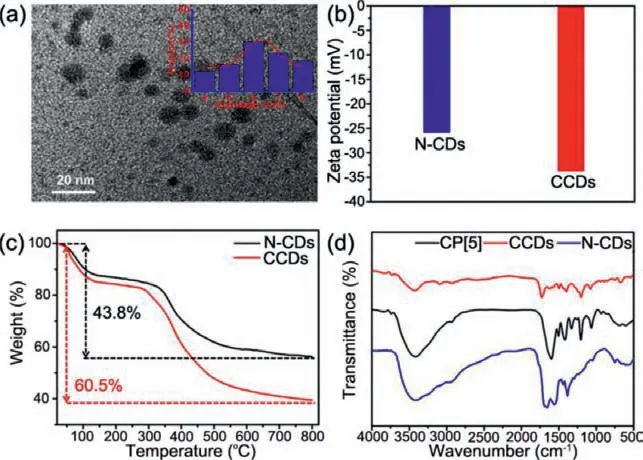
Fig.1.(a) TEM image and the particle size distribution (inset image) of CCDs.(b)Zeta potentials of N-CDs and CCDs.(c) TGA curves of N-CDs and CCDs.(d) FT-IR spectra of CP[5],N-CDs and CCDs.
To accurately identify and capture pesticide molecules,modification of supramolecular macrocycles on sensors has been considered as an effective method [24-28].Unique cavity and stable structure ensure that certain supramolecular macrocycles can recognize and capture pesticide moleculesviahost-guest interactions,effectively enhancing the detection ability of electrochemical sensors [27-32].For example,in 2019,Diao and co-workers reported on the preparation of silver nanoparticles/water-soluble pillar[5]arene functionalized graphene oxide modified glassy carbon electrode (GCE) and realized excellent performance of PQ detection,due to the host-guest recognition between the water-soluble pillar[5]arene and paraquat [13].Similarly,Zhao and co-workers reported the control assembly of pillar[6]arene modified Ag nanoparticles on the surface of covalent organic framework in 2019,fabricating a rapid,ultrasensitive,and highly selective electrochemical sensing platform for the determination of PQ [33].Although these electrochemical sensors have shown excellent detection capability of PQ,complex preparation processes and the use of expensive metal nanoparticles limit their further applications [34].
Herein,we facilely synthesized N-CDs in one step by a hydrothermal process,and carboxylatopillar[5]arene (CP[5])was covalently attached to the surface of N-CDs by the EDC-NHS coupling reaction,forming an electrochemical sensor(carboxylatopillar[5]arene-coated nitrogen-doped carbon dots,denoted as CCDs) with a simple and low-cost manner (Scheme 1).Meanwhile,the electrochemical characteristics of CCDs modified electrode (CCDs/GCE) were studied.In virtue of the strong supramolecular interactions between CP[5]and PQ [35,36],CCDs/GCE showed good current response toward PQ.In addition,we explored the effect of the solution pH and the loading amount of CCDs on the detection of PQ.CCDs/GCE performed excellent anti-interference capability,selectivity,stability,and reproducibility in the detection of PQ.
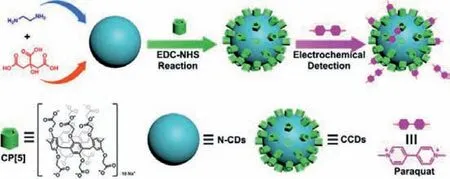
Scheme 1.Schematic representation of the synthesis of CCDs via attaching CP[5]on the surface of N-CDs by EDC-NHS coupling reaction,and excellent electrochemical performance in PQ detection exhibited by CCDs/GCE.
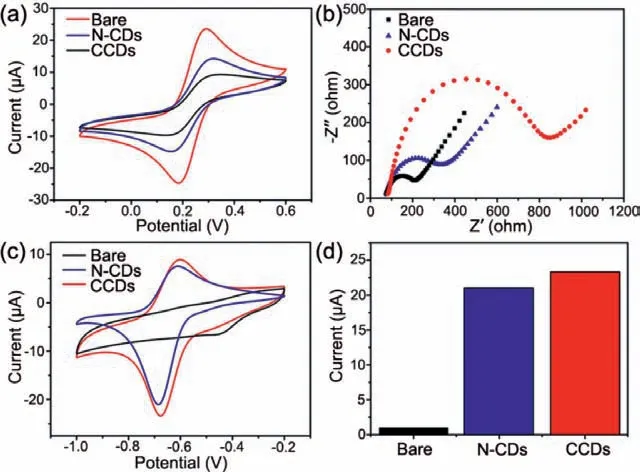
Fig.2.(a) CV responses and (b) EIS plots of bare GCE,N-CDs or CCDs modified electrodes in 0.1 mol/L KCl solution containing 2 mmol/L [Fe(CN)6]3-/4−at a scanning rate of 50 mV/s.(c) CV responses and (d) relative peak current histogram of bare GCE,N-CDs or CCDs modified electrodes in 0.1 mol/L PBS solution (pH 7,used as electrolyte solution) containing 10 μmol/L PQ at a scanning rate of 50 mV/s.
To verify the successful modification of CP[5]onto CCDs,various characterization methods were used.From the transmission electron microscopy (TEM) image,the average particle size of CCDs was 6.3 ± 2.4 nm and showed no observable size change compared with N-CDs (Fig.1a and Fig.S1 in Supporting information).Due to the abundant amino and carboxyl functional groups introduced by ethylenediamine and citric acid [37],the obtained samples had good water solubility (Fig.S2 in Supporting information).For the same reason,the characteristic bands of -COO−(1654 and 1407 cm−1) and N-H (1575 cm−1) were evidently observed in CCDs and N-CDs from the Fourier transform infrared (FT-IR) spectra (Fig.1d)[38-40].Moreover,the characteristic peaks at 785 and 937 cm−1correspond to the C-H out-of-plane deformation and ring skeletal vibrations of the benzene rings in CP[5],respectively,indicating the existence of CP[5]modified on CCDs [38].To further illustrate this point,the zeta potentials of N-CDs and CCDs were measured.Owing to the electronegativity of carboxyl functional groups on CP[5],the zeta potential of CCDs was −33.8 mV,more negative than NCDs (−25.9 mV,Fig.1b).These results revealed that CP[5]was successfully modified on the surface of CCDs.
Further,the amount of CP[5]on CCDs was evaluated by thermogravimetric analysis (TGA).As depicted in Fig.1c,the pyrolysis of the amino and oxygen groups on the surface of N-CDs resulted in an abrupt mass loss at roughly 200 °C.With the continued increasing temperature,N-CDs began to decompose and lost 43.8 wt% until 800 °C [13,41].Similarly,60.5 wt% weight loss of CCDs was observed at 800 °C,illustrating that approximately 16.7 wt% mass loss was caused by the pyrolysis of CP[5]modified on CCDs.
As the electrochemical sensors,electrochemical properties of these materials are of paramount importance.In view of the SEM images (Fig.S3 in Supporting information),both N-CDs and CCDs were homogeneously dispersed on the electrodes,which was beneficial for the signal transduction to the surface electrode and the next electrochemical testing.From the cyclic voltammograms(CV) curves (Fig.2a),bare GCE showed a standard redox peaks of [Fe(CN)6]3-/4−at a scanning rate of 50 mV/s.Relatively,the redox current on N-CDs/GCE became smaller due to the electrostatic repulsion between N-CDs and [Fe(CN)6]3-/4−.Meanwhile,CP[5]molecules with ten carboxyl groups further increased the electronegativity of CCDs,and the hydrophobic cavity of CP[5]cannot recognize [Fe(CN)6]3-/4−,so CCDs/GCE exhibited the least redox current among them [13].The CV curves exhibited that the modification of CP[5]prevented the electron transfer between CCDs/GCE and [Fe(CN)6]3-/4−.
On the other hand,the charge transfer resistances (Rct) of GCE,N-CDs/GCE,and CCDs/GCE were measured in the presence of equivalent 2.0 mmol/L [Fe(CN)6]3-/4−by the electrochemical impedance spectroscopy (EIS).As expected,N-CDs/GCE performed much largerRct(350Ω) than that of bare GCE (210Ω),attesting that N-CDs film on GCE inhibited interfacial charge transfer(Fig.2b).In addition,due to the poor electrical conductivity of CP[5],CCDs/GCE had the largestRctvalue (837Ω).Thus,the interface properties of these modified electrodes have been verified by CV and EIS.
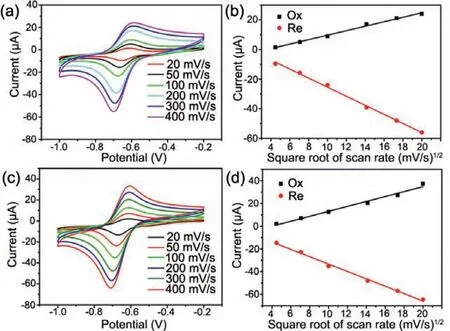
Fig.3.CV responses of (a) N-CDs/GCE and (c) CCDs/GCE with 10 μmol/L PQ in pH 7.0 PBS at a scanning rate ranging from 20 mV/s to 400 mV/s.Linear relationships between peak currents and square root of scanning rate of (b) N-CDs/GCE and (d)CCDs/GCE.
Owing to the two-step reduction of PQ,two reduction peaks at−0.63 V (PQ1) and −1.07 V (PQ2) were observed in the CV curve(Fig.S4 in Supporting information) [13].And the peak of PQ1 with a higher reduction peak current and a lower negative working potential was selected for further study,improving the effect of electrochemical detection.As in Fig.2c,the reduction peaks of PQ on GCE,N-CDs/GCE,and CCDs/GCE in 0.1 mol/L PBS solutions at a scanning rate of 50 mV/s were totally different.The bare GCE had no obvious reduction peak,indicating the negligible electrochemical activity of unmodified GCE towards the detection of PQ.Correspondingly,the N-CDs/GCE would attract positively charged PQ by electrostatic interaction,leading to an enhancement in response current.Furthermore,due to the electronegative cavity of CP[5],PQ was captured by CP[5]s on the surface of CCDsviahost-guest interactions,and the CCDs/GCE showed the highest response current[41,42].The CP[5]rings on CCDs effectively enhanced the response current by attracting PQ to the CCDs.These results illustrated that the combination of N-CDs and CP[5]effectively increased the electrochemical activity of this electrochemical sensor.
To further confirm the influence of scanning speed on the electrochemical sensors,CV curves of N-CDs/GCE and CCDs/GCE in PBS solutions (pH 7) were recorded at a scanning rate ranging from 20 mV/s to 400 mV/s (Figs.3a and c).With an increased scanning rate,both reduction and oxidation currents of N-CDs/GCE and CCDs/GCE became larger.Moreover,the peak currents were linearly related to the square root of scanning rate,suggesting a diffusioncontrolled kinetic process of the redox reaction on N-CDs/GCE and CCDs/GCE (Figs.3b and d).
Similarly,the solution pH and the loading amount of CCDs on the electrode were also considered.In Fig.4a and Fig.S5 (Supporting information),the peak currents of CCDs/GCE increased with the pH shifted from 5 to 7,illustrating that the acidic environment inhibited the electrochemical activity of CCDs/GCE.When the pH increased from 7 to 10,a large amount of hydroxide hindered the adsorption of PQ on CCDs/GCE,resulting in an obvious decrease of the peak currents.Therefore,CCDs/GCE showed the best electrochemical performance at pH 7.In addition,the effect of loading amount of CCDs was inspected at pH 7.Notably,dropping the same volume of suspension with different concentrations onto the GCE was an effective means to adjust the loading amount of CCDs.As showed in Fig.4b,the peak currents of CCDs/GCE increased first and then decreased as the concentration of CCDs suspension raised from 0.25 mg/mL to 2.00 mg/mL,and a maximum peak current appeared at 0.50 mg/mL.Although the increase of loading amount could improve the adsorption to PQ,the thicker film on GCE possessed a larger charge transfer resistance.Thus,0.5 mg/mL CCDs suspension was selected for PQ detection.
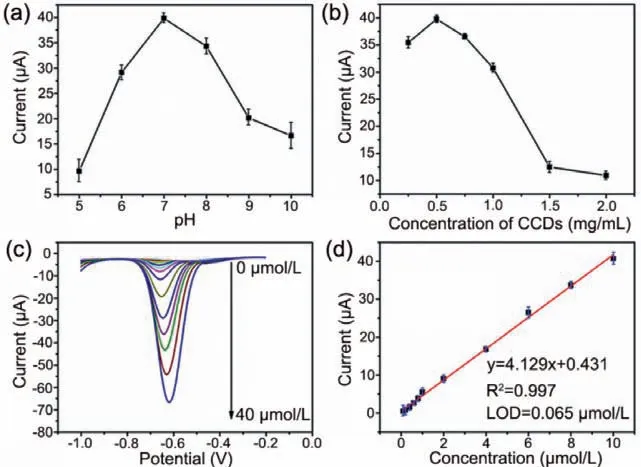
Fig.4.(a) Peak currents of 10 μmol/L PQ on CCDs/GCE in different pH solutions.(b) Peak currents of 10 μmol/L PQ on CCDs/GCE in a pH 7 solution with the different concentrations of CCDs.(c) DPV curves of CCDs/GCE at 0.1 mol/L PBS (pH 7)with the concentration of PQ ranging from 0 to 40 μmol/L.(d) Linear relationship between peak currents and PQ concentrations (0.1 to 10 μmol/L).
After optimization of the test conditions,the detection of PQ on CCDs/GCE was investigated by using differential pulse voltammetry(DPV).As expected,the reduction peak currents on CCDs/GCE gradually increased with the rise of PQ concentration (Fig.4c).Meanwhile,the peak currents were proportional to the PQ concentration from 0.1 μmol/L to 10 μmol/L with a detection limit of 6.4 nmol/L(Fig.4d,S/N=3),which was compared favorably with other modified electrodes reported in the literatures (Table 1) [43-47].However,when the PQ concentration was higher than 10 μmol/L,the peak currents would lose the linear relationship with PQ concentrations.
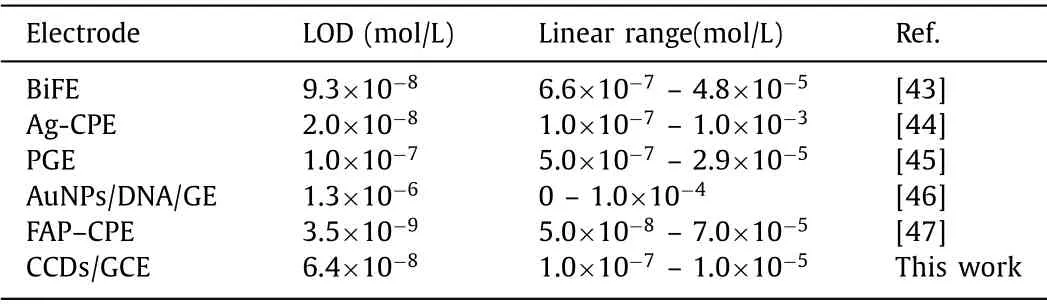
Table 1 Comparison of the modified electrodes.
Naturally,the influence of common inorganic salts in the practical detection had to be taken into account.As shown in Fig.S6(Supporting information),after adding 100-fold concentration of KCl,NaCl,CaCl2,MgCl2,and Na2SO4respectively,the reduction currents of 10 μmol/L PQ on CCDs/GCE showed no significant effect (signal change below 5%),suggesting that CCDs/GCE possessed good anti-interference capability.Moreover,considering the more complex environment in the detection,the interference effects of other organic substances,such as related herbicide (diquat),certain common herbicides (imidacloprid and acetamiprid),phenolics (hydroquinone and catechol),flavonoids (rutinum) and biogenic amine(dopamine) were explored.From Fig.S7 (Supporting information),no significant interference was observed from these substances in the detection of PQ.These results demonstrated a good selectivity of CCDs/GCE.
To estimate the practical applications of CCDs/GCE in real sample analysis,the ordinary tap water was used in the detection and spiking-recovery test was also carried out to ensure the accuracy.As illustrated in Table S1 (Supporting information),the recovery rate was in the range of 97.5% to 104.75% with a relative standard deviation (RSD) of 0.3% to 2.5%,indicating the stability and viability of CCDs/GCE in the practical detection.Furthermore,three equal CCDs/GCE modified by the same way exhibited similar electrochemical signals in three successive DPVs,confirming the excellent reproducibility of CCDs/GCE (Fig.S8 in Supporting information).These experimental results demonstrated CCDs/GCE had a great applied potential in PQ detection.
In conclusion,we prepared an electrochemical sensor based on CP[5]functionalized CCDs in a simple and low-cost manner.Due to the host-guest interaction,CCDs/GCE exhibited excellent electrochemical activity and a good current response to PQ.In the presence of inorganic salts and certain organic substances,CCDs/GCE showed good anti-interference ability and selectivity.Moreover,excellent stability and reproducibility of CCDs/GCE ensured the application potential in tap water samples and cyclic testing.Hopefully,this electrochemical sensor will provide a valuable research direction for pesticide detection.
Declaration of competing interest
The authors declare no declarations of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.21871108),Wuhan University of Science and Technology,and the Jilin Province-University Cooperative Construction Project–Special Funds for New Materials (No.SXGJSF2017-3).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.002.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
