An acid-base responsive linear-cyclic polymer rotaxane molecular shuttle with fluorescence signal output
Zhnqi Co,Dongpu Wu,Mengzhen Li,Fn Yng,Zhiki Li,Wnki An,Song Jing,Xin Zheng,Coyun Niu,Dhui Qu
a College of Science,Henan Agricultural University,Zhengzhou 450002,China
b Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering,Feringa Nobel Prize Scientist Joint Research Center,Frontiers Science Center for Materiobiology and Dynamic Chemistry,Institute of Fine Chemicals,School of Chemistry and Molecular Engineering,East China University of Science and Technology,Shanghai 200237,China
c College of Chemistry and Environmental Engineering,Shenzhen University,Shenzhen 518060,China
Keywords:Supramolecular polymer Rotaxane molecular shuttle Stimuli responsive Fluorescence signal Topological structure
ABSTRACT The preparation of intelligent-responsive materials with controllable topology structure has long been a significant objective for chemists in the field of materials science.In this paper,we designed and prepared a linear-cyclic reversible topological structure polymer based on the bistable [1]rotaxane molecular shuttle.A ferrocene-functionalized [1]rotaxane and naphthalimide fluorophore group are introduced into the both ends of the polymer,which exhibit distance-induced photo-electron transfer effect.The structural transformation between linear and cyclic state of polymer is demonstrated by simple acidbase stimuli,accompanying visual fluorescence changes.The transformation process was characterized by 1H NMR spectra and fluorescence spectra.This work provides a novel strategy to construct functionalized polymers with topological structure.
During the past decades,polymers with cyclic structure have attracted considerable attention from chemists and biochemists because of its topological dissimilarities and distinctive properties that are different from the linear or branched counterparts [1,2].The research suggests that cyclic polymers have unique applications in mickle fields including microelectronics [3],biomedicine[4],wastewater treatment [5]and especially in novel polymer materials [6].The major difficulty in this field is the synthesis and purification processes [7],which limits the widespread application of cyclic polymers.The two commonly used synthetic methods for cyclic polymers are ring-closure [8]and ring-expansion strategies[9].The former can construct cyclic polymersviacoupling strategy to connect the two end groups of a linear polymer [10,11],while the latter involves the insertion of cyclic monomer units into an activated cyclic chain [12,13].In addition,there is a novel strategy could be utilized to prepare cyclic polymers,i.e.,taking advantage of reversible structure transformation behavior between linear and cyclic polymer of novel stimuli-responsive materials [14,15].
Mechanically interlocked molecules (MIMs) [16-21],especially bistable rotaxanes [22-25],are usually served as the basic backbone for the construction of stimuli-responsive materials due to their unique bistable structure [26-32].Bistable rotaxanes with distinguishable recognition sites,usually be utilized to prepare various functional molecular machines or devices [33-39].As a significant member of bistable rotaxanes family,[1]rotaxane is composed of cyclic and rod-like component which is tightly linked together by covalent bonds,has been applied to prepare various molecular machines [40-42].Takata and co-workers [43,44]reported series of researches on topology transformation between cyclic polymers and linear polymers based on [1]rotaxane protocol,and provide foundation for large-scale preparation cyclic polymers.These pioneering works demonstrate that [1]rotaxane is of great significance to design and construct linear-cyclic topology polymers.However,the characterization methods in those studies were complicated,and the topology transformation was controlled by sophisticated chemical reaction [45].Thus,it is necessary to develop more simple means with obvious phenomenon to drive topological structural transformation.
Here,we report a more convenient tactics for construction topology structure polymers between linear and cyclic transformation depending on simple acid-base stimuli,and this transformation process could be demonstrated by obvious visible fluorescence changes.As shown in Scheme 1,the target polyrotaxane Poly-1-H contains a ferrocene-functional [1]rotaxane at one end,and an electron-deficient 4-morpholinnaphthalimide (MA) at the other end.The electron-rich ferrocene unit and MA group are bridged by a chain polymer structure,and the two exist distance-induced photoelectron transfer (PET) process.There are two different recognition stations for the dibenzo-24-crown-8 (DB24C8) macrocycle at two ends of the polymer structure,that’s dibenzylammonium(DBA) andN-methyltriazolium (MTA) site.The polyrotaxane Poly-1-H shows linear structure at the initial state and present relatively strong fluorescence,then the linear polymer transfer to cyclic structure Poly-1 under base environment,leading to the fluorescence intensity decrease due to the PET process between ferrocene unit and MA group.And then the cyclic structure returns to its linear state under acid stimuli,accompanying the fluorescence intensity recovering to its initial state.What is more,the structure transformation process is accompanied by visual fluorescence changes.We expected this work will put forward a small step towards to preparation topological structure polymers.
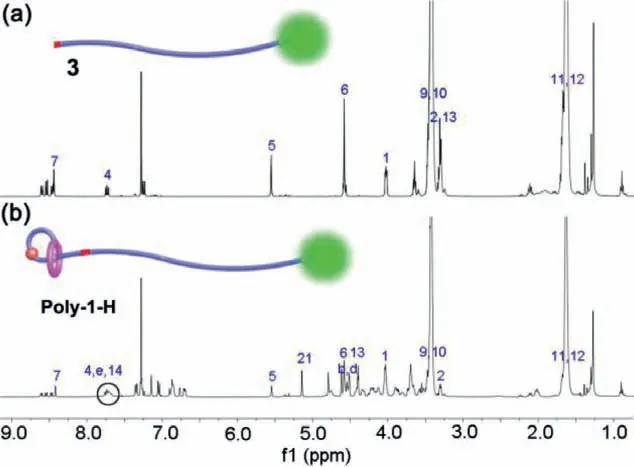
Fig.1.1H NMR spectra (400 MHz,CDCl3,298 K) of intermediate 3 (a) and polyrotaxane Poly-1-H (b).The black circle in the bottom indicates the presence of the triazole proton 14.

Scheme 2.The synthetic routes of the target polyrotaxane Poly-1-H.
The synthetic routes of the target polyrotaxane Poly-1-H are shown in Scheme 2.The key alkyl intermediate 2 [46]has an electron-rich ferrocene unit and a DBA site for DB24C8 macrocycle.The commercially available polytetrahydrofuran,as the basic backbone of the chain polymer,is introduced into the azide-terminally modified intermediate 3.The Poly-1-H was obtained with a yield about 37% with aMnof 3.2 kDa and a PDI of 1.03 through the classical “CuAAC” click reaction between compound 2 and intermediate 3 in the presence of [Cu(CH3CN)4]PF6.The compounds not reported previously are characterized by1H NMR,MALDI-TOF-MS,FT-IR spectrum and size exclusion chromatography (Figs.S2-S21 in Supporting information).
The intermediate 3 and polyrotaxane Poly-1-H were characterized by1H NMR,FT-IR spectra and MALDI-TOF-MS as discussed below.In the1H NMR spectra of compound 3 (Fig.1a),the single peak signal of the methyl proton H6and the proton H7on MTA station were located at 4.56 ppm and 8.42 ppm,respectively.And the signal peaks of representative proton on MA group were obvious at 4.00 ppm (H1),7.72 ppm (H4),5.53 ppm (H5).What is more,the methylene group of polymer structure (H9,H10) from 3.38 ppm to 3.42 ppm and the methylene group (H11,H12) from 1.57 ppm to 1.63 ppm could also be observed.In addition,the FT-IR spectra (Fig.2a) signal peak at 2096 cm−1of intermediate 3 indicates the presence of azide functional group.After the click reaction between compound 2 and intermediate 3,there were several obvious signal changes of the1H NMR and FT-IR spectra.Firstly,in the1H NMR spectra of polyrotaxane Poly-1-H (Fig.1b),the emergence of the peak at 7.68 ppm indicates the generation of the new triazole proton H14.Secondly,the signal peak of the methylene (H13) next to the azide group shifted from 3.28 ppm (Fig.1a) to 4.38 ppm(Fig.1b),and other special proton signal peaks of compound 2 and intermediate 3 could also be observed in the1H NMR spectrum of the Poly-1-H (Fig.1b).Thirdly,the FT-IR spectra (Fig.2b) of Poly-1-H at 2096 cm−1disappears completely compared with that of intermediate 3,indicating a highly efficient click reaction.What is more,the MALDI-TOF-MS (Fig.S19 in Supporting information) of Poly-1-H were in accordance with the calculated value of Poly-1-H lost one PF6−.All above evidence proved that target polyrotaxane Poly-1-H had been synthesized successfully.
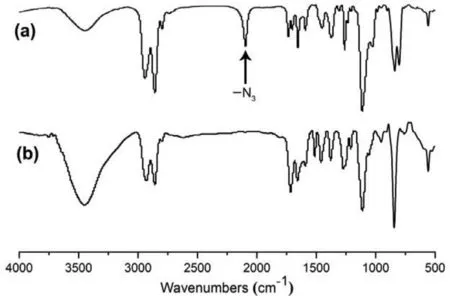
Fig.2.FT-IR spectra of intermediate 3 (a) and polyrotaxane Poly-1-H (b).
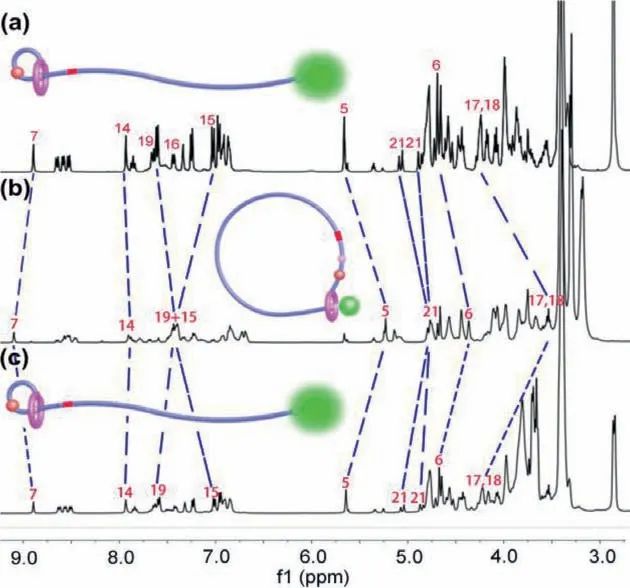
Fig.3.Partial 1H NMR spectra (400 MHz,CD3COCD3,298 K) of (a) Poly-1-H,(b)deprotonation with addition of 5.0 equiv.of DBU to sample a,(c) reprotonation with addition of 10.0 equiv.of TFA to sample b.The proton assignment corresponds to the structure as shown in Scheme 2.
The reversible topology transformation of polyrotaxane between linear structure Poly-1-H and cyclic state Poly-1 was particularly characterized by1H NMR spectra and fluorescence spectra.In the initial state,the polymer rotaxane molecules Poly-1-H present linear structure and the DB24C8 macrocyclic components were located at the DBA recognition site (Fig.3a).After addition of 5.0 equiv.of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) to the solution of Poly-1-H,the DBA site was deprotonation and the DB24C8 macrocycle shifted from DBA site to MTA site.There are several obvious characteristic signal peak changes (Fig.3b).The signal peaks of proton of H5,H6and H7on or neighboring the MTA site were shifted with aΔδof −0.42,−0.31 and 0.22 ppm,respectively,due to its interaction with the DB24C8 macrocycle.The signal peaks of methylene proton H17and H18on the DBA station were shifted with aΔδof −0.69 ppm.The1H NMR signal changes showed that the macrocycle moved to the MTA recognition site under the action of DBU and the polymer rotaxane molecular demonstrated cyclic structure.After the reprotonation of the -NH- group of the DBA site with 10.0 equiv.of CF3COOH (TFA),the1H NMR signal peaks of Poly-1-H recovered to its original state (Fig.3c).
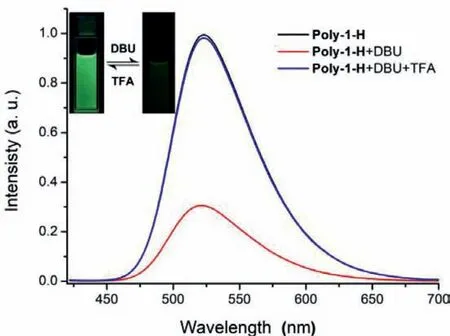
Fig.4.Normalized fluorescence spectral changes in the CH2Cl2 solution of Poly-1-H (1.0 × 10−5 mol/L).The mixture obtained after adding excess DBU to the solution of Poly-1-H and the mixture obtained after adding excess TFA to the DBU-added solution of Poly-1-H.The excitation wavelength was 410 nm.
In order to further prove the topological structure transformation between linear and cyclic polymer,the photophysical properties of polyrotaxane Poly-1-H was special discussed.The UV-vis absorption spectra (Fig.S1 in Supporting information) of Poly-1-H showed a strong absorption peak atλmax=410 nm and its fluorescence spectroscopy exhibited a very remarkable emission peak atλmax=521 nm in CH2Cl2solution (Fig.4),which was the characteristic emission peak of MA fluorophore and further proved that the MA fluorophore was attached to the polymer structure successfully.In this initial state,the Poly-1-H exhibited a linear structure and the DB24C8 macrocycle was relatively far from the MA fluorophore.After addition excess DBU to the CH2Cl2solution of Poly-1-H,the UV-vis absorption spectra had no change (Fig.S1 in Supporting information),while the fluorescence spectroscopy intensity atλmax=521 nm reduced 70% compared to its initial state(Fig.4).And this phenomenon could be explained by the strong PET process between electron-rich ferrocene group and electronpoor MA fluorophore,which resulted from the short distance between ferrocene functionalized DB24C8 macrocycle and MA fluorophore.The fluorescence intensity decrease clearly shows that the polymer structure changes from linear to cyclic state.The fluorescence intensity recovered to its initial state after excess TFA addition to the DBU-added CH2Cl2solution of Poly-1-H.This could be explained by the reprotonation of the DBA recognition site under excessive TFA,and the macrocyclic component moved to its original position along the rod-like component.And the polymer structure transformed from cyclic to linear state and the transformation process was accompanied by visual fluorescence changes (Fig.4).All the above evidence proved that the polymer rotaxane molecular Poly-1-H could realize the reversible topology transformation responding to external acid-base stimuli.
Although a low molecular weight polytetrahydrofuran was applied as the backbone of the polymer rotaxane,this still was a good program to construct cyclic polymer from linear polymer based on stimuli-responsive of bistable [1]rotaxane molecular shuttle.Simultaneously,the PET process between the electron-rich ferrocene group and the electron-deficient MA group exhibited obvious advantages,such as operation simple,phenomena obvious.This scheme provides a possible solution for the subsequent convenient construction of the topological structure of ring polymers.
In conclusion,we have successfully constructed a reversible linear-cyclic topological structure polymer based on bistable [1]rotaxane molecular shuttle with controllable fluorescence signal output.The reversible topology transformation presents visible fluorescence changes responding to external acid-base stimuli.It’s a good idea to utilize the fluorescence signal changes and simple acid-base stimulation for constructing linear-circular topological structure polymer,which provides the foundation for preparing more sophisticated topological polymers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21901063,U20041101),Young Talents Personnel Fund of Henan Agricultural University (No.30500604).We acknowledge Molecular Scale Lab for mass spectrometry characterization.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.001.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
