Cleavage/cross-coupling strategy for converting β-O-4 linkage lignin model compounds into high valued benzyl amines via dual C–O bond cleavage
Le Ji,Cho-Jun Li,Huiying Zeng,∗
a The State Key Laboratory of Applied Organic Chemistry,Lanzhou University,Lanzhou 730000,China
b Department of Chemistry,FQRNT Centre for Green Chemistry and Catalysis,McGill University,Montreal,Quebec H3A 0B8,Canada
Keywords:β-O-4 Linkage Lignin C-O bond cleavage Cross-coupling reaction Palladium-catalyzed
ABSTRACT Lignin is the most recalcitrant of the three components of lignocellulosic biomass.The strength and stability of the linkages have long been a great challenge for the degradation and valorization of lignin biomass to obtain bio-fuels and commercial chemicals.Up to now,the selective cleavage of C–O linkages of lignin to afford chemicals contains only C,H and O atoms.Our group has developed a cleavage/crosscoupling strategy for converting 4-O-5 linkage lignin model compounds into high value-added compounds.Herein,we present a palladium-catalyzed cleavage/cross-coupling of the β-O-4 lignin model compounds with amines via dual C–O bond cleavage for the preparation of benzyl amine compounds and phenols.
Conversion of renewable biomass to chemicals and fuels is becoming an ideal way to build a sustainable development due to the gradual depletion of fossil resources and environmental pollution concerns [1–5].Lignocelluloses (cellulose,hemicellulose and lignin)are the most representative biomass resources [6].Among them,due to the complexity of lignin structure,the utilization of lignin is the most challenge [7,8].Until now,lignin has been discarded as a waste product of the pulp and paper industry,and is generally burned to supply thermal energy.From the perspective of sustainable development,lignin is a unique candidate for the sustainable production of organic aromatic molecules [9–11].The development of effective methods to access valuable chemicals from lignin is not only highly desirable but also challenging.More recently,some impressive achievements have been made.The selective cleavages of C–O or C–C linkages to obtain oxygen-containing compounds(phenols [12–14],ketone [15,16],acids [17,18]and aldehydes [19–21]) and alkane (cycloalkanes [22,23],alkene [24]and other small molecules compounds [25]) have been reported (Scheme 1a).However,these transformations typically afford chemicals containing only C,H,and O atoms.For converting lignin to high value-added compounds,cross-coupling reaction of lignin and its model compounds is highly desirable.
Nitrogen-containing compounds are important organic building block for synthetic dyes,pharmaceuticals and polyurethane precursors,which are usually generated from non-renewable fossil resource [26–29].For sustainable development of resource and society,synthesis of nitrogen-containing compounds from renewable biomass is highly desirable.Toward to this aim,our group has developed a cleavage/cross-coupling strategy for converting lignin 4-O-5 linkage model compounds to high value-added nitrogencontaining compoundsviadual C–O bond cleavage (Scheme 1b)[30,31].Due to the continuing interest of our research group on the degradation and valorization of renewable lignin and its model compounds [32–42],it is also advantageous to use the cleavage/cross-coupling strategy to convert other lignin linkage to high value-added compounds.Meanwhile,considering that theβ-O-4 structural motifs are the most abundant in all the linkages of lignin [43–47],its efficient cleavage is of great significance to the degradation of lignins.Herein,we present a palladium-catalyzed direct cross-coupling of theβ-O-4 lignin model compounds with aminesviadual C–O bond cleavages for the preparation of benzyl amine compounds and phenols (Scheme 1c).Very recently,Li and co-workers reported similar method during the preparation of the current report [48].
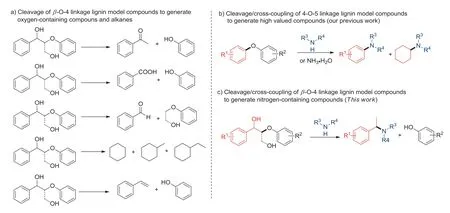
Scheme 1.Cleavage strategy vs. cleavage/cross-coupling strategy for valorization of lignin model compounds.
Initially,we started our investigation usingβ-O-4 model compound 1a and 1-heptanamine 2a as model substrates to optimize the reaction conditions.Several palladium catalysts were examined using toluene as the solvent (Table 1,entries 1-3).When the reaction was performed in the presence of Pd/C (10 mol%),HCO2Na (3 equiv.),cross-coupling product 3a and phenol 4a were obtained in 67% and 93% yield,respectively (entry 1).Other palladium catalysts,such as Pd(OH)2/C and Pd/Al2O3showed lower catalytic activity than Pd/C (entries 2 and 3).When the reaction was explored under air atmosphere,the yield was slightly decreased (entry 4).As expected,the reaction did not occur without the catalyst (entry 5).When the reaction was performed in the absence of hydrogen donor,the yield of 4a was reduced to 42%,with crosscoupling product 3a being only a trace amount together with a large amount of unreduced imine product (entry 6).A variety of hydrogen sources (HCO2H,HCO2NH4,NaBH4,isopropanol and H2)were investigated,giving lower yield than HCO2Na (entries 7–11).Adjusting the amount of HCO2Na,we found that a lower yield was obtained when it was reduced to 2 equiv.(entry 12).On the contrary,the best yield (87% and 95%) was obtained when the amount of HCO2Na was increased to 5 equiv.(entry14).Increasing the amount of 2a to 2 equiv.gave the highest yields (entry 16).Further increasing the amount of 2a led to a lower yield (entry 17).The yield of 3a and 4a did not increase when the temperature was raised or lowered (entries 18–20).Adjusting the loading of the catalyst led to lower yields (entry 21) (see Supporting information for details).
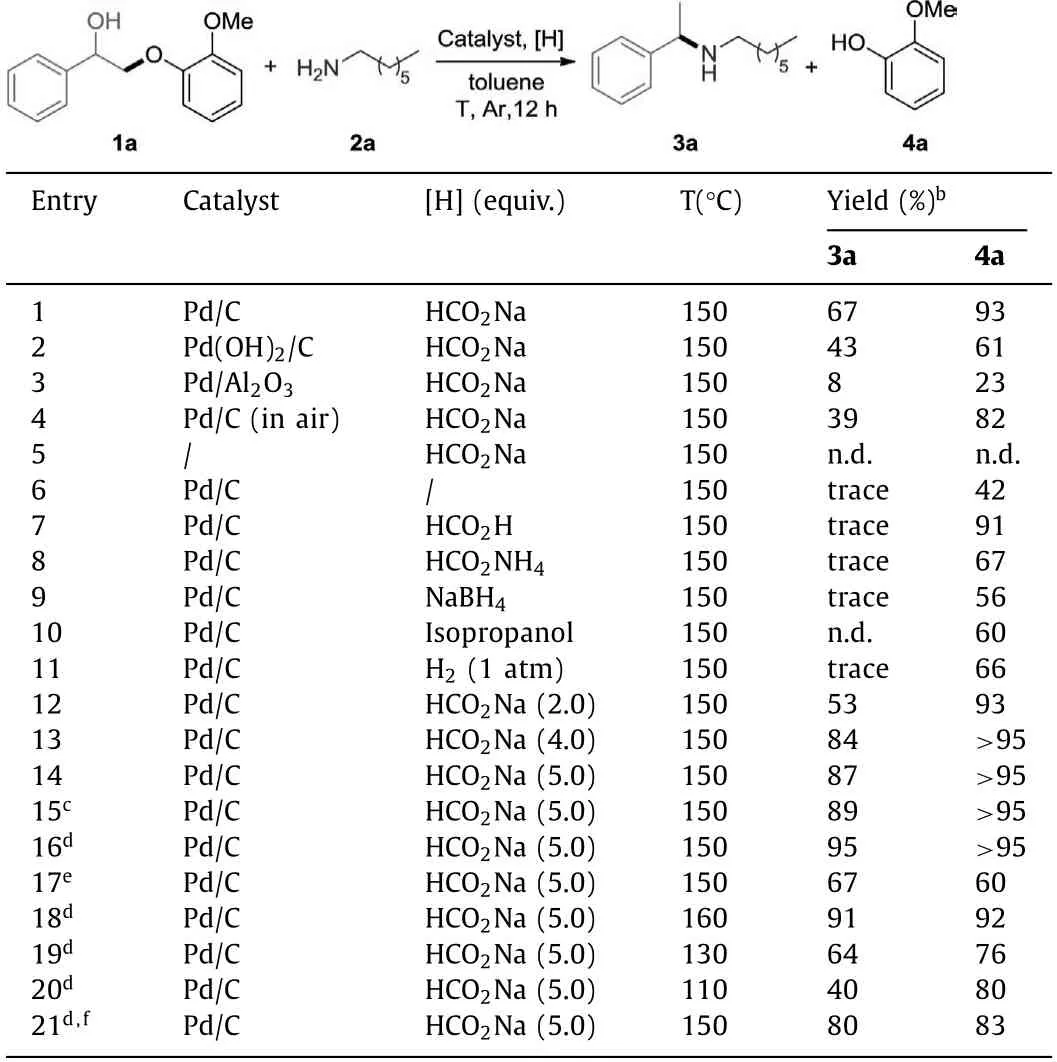
Table 1 Optimization of the reaction conditions.a
With the optimized reaction conditions in hand,we explored the substrate scope of various ligninβ-O-4 model compounds (Table 2).The simplest model compounds 2-phenoxy-1-phenylethan-1-ol (1b) was successfully cleaved and cross-coupled with 1-heptanamine to generate desired products 3a and 4b in high yields (entry 1).A methoxy group substituted on different positions (ortho-,para-andmeta-position) of the phenyl ring (phenolic part) has no effect,obtaining the corresponding products with good to high yields (entries 2–4).Methoxyl group substituted on both aromatic rings or another aromatic ring (left part)also successfully reacted to form the desired products (entries 5-8).Aromatics (1i and 1j) with a weak electron-donating methyl substituent were used in the reaction,generating targeted products in moderate to high yields (entries 9 and 10).Model compounds with hydroxymethyl substituent onβ-position was the most similar to lignin,which was also successfully cleaved by the ether bond to form C-C bond cleavage products (entries 11–14,3a and 3c) and phenols (4a,4b and 4d).The C-C bond cleavage may be the result of a retro-aldol process when the benzyl alcohol part was oxidized to the corresponding ketone by palladium catalyst.
We also investigated the scope of various amines for this cleavage/cross-coupling reaction (Table 3).First,the transformation of ligninβ-O-4 model compounds 1a with different primary amines was tested,delightfully,obtaining good to excellent yields for all these primary amines (Table 3,entries 1–10).It is interesting to note that the use of cyclopropylmethylamine as substrate did not generate any three-membered rings opening product under the palladium-catalyzed reductive condition (entry 3).Trifluoromethyl group also remained intact in the product without further reduction (entry 7).In addition,secondary amines such as pyrrolidine,piperidine and morpholine also successfully coupled to afford the corresponding product (3n,3o and 3p) with good to high yields (entries 11–13).When benzylamine,acyclic secondary amines (such as dipentylamine and dipropylamine) and anilines(such as aniline andN-methylaniline) subjected to the C-O ether bond cleavage,phenols 4a was obtained with high yields (83%-93%) (entries 5 and 14–17).However,the corresponding crosscoupling products amines were generated in lower yields (0–32%)instead of acetophenone (5) with moderate to good yields.Inorganic nitrogen resource,ammonia,was used instead of organic amines,generating C-O ether bond cleavage product acetophenone(5) and guaiacol (4a) in lower yields (entry 18).
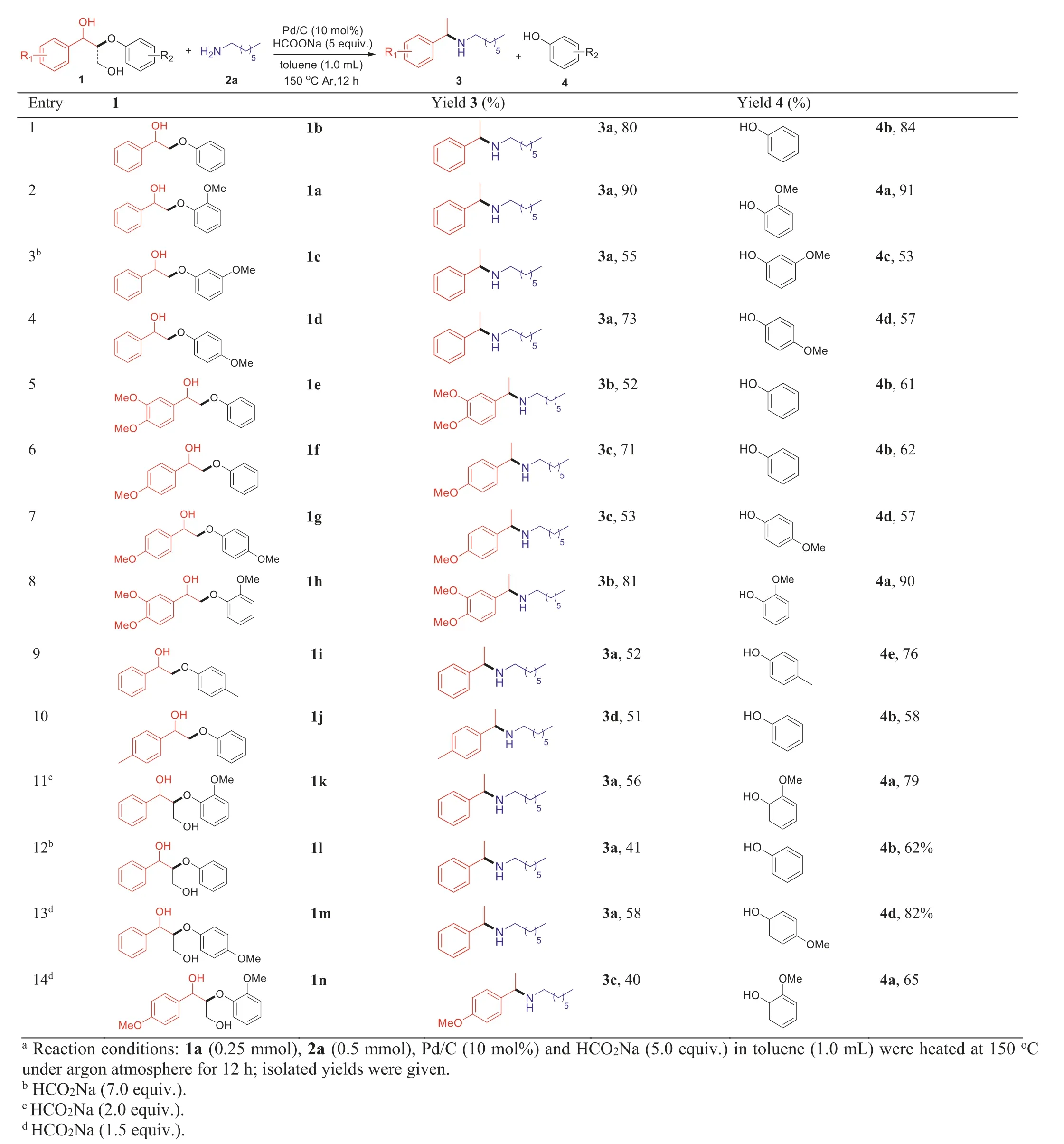
Table 2 Cleavage/cross-coupling of lignin β-O-4 model compounds with 1-heptanamine.a

Table 3 Cleavage/cross-coupling of lignin β-O-4 model compound 1a with amines.a
To investigate the reaction mechanism,a series of control experiments were carried out.Methyl ether compounds 6 and tertiary alcohol 7 did not generate the desired product,indicating that the hydrogen of hydroxyl and benzyl position was very important,which benefited the reactionviadehydrogenation to give a ketone intermediate (Schemes 2a and b).When we used the ketone 8,mimicking the possible intermediate under standard conditions,the cross-coupling product and phenol were obtained with excellent yields (Scheme 2c).This result demonstrated that the ketone was the intermediate for this transformation.To further investigate the cleavage of the ether bondviaenol or enamine,betadimethyl substituted compound 9 was used for this transformation under standard conditions,cross-coupling product 10 (31% yield),C–O bond cleavage product 11 (15% yield) and phenol 4a (41%yield) were obtained (Scheme 2d).This information excluded C–O bond cleavageviaenol or enamine process.alphaD-isotope labelling compound 12 was then chosen as substrate for investigating the whereabouts of D atom (Scheme 2e).The result showed that no D atom was incorporated into the product.Based on our previous work [42],we propose that D atom might exchange with solvent.Therefore,we used deuterated toluene-d8as a solvent.It was found that there was D atom in the product,indicating that the deuterium atoms in toluene participated in the reaction and was transferred to the amination product and guaiacol.
According to the above experimental results,a possible mechanism for the cleavage/cross-coupling reaction of lignin model compound with amine is proposed in Scheme 3.The alcohol A is dehydrogenatively oxidized by palladium to generate ketone intermediate B and simultaneously form palladium hydrogen species.The ketone B may condense with amine to generate imine C.Next,palladium inserts into the C-O bonds of the ketone B or imine Cvia O-oriented orN-oriented process to form intermediate D or E.Finally,the intermediate D or E is reduced by a hydrogen source to obtain phenol H,and ketone F or imine G,as well as regenerates palladium catalyst.The ketone F is condensed with amine and reduced by palladium hydrogen species to obtain the cross-coupling product J.The imine G is also reduced to generate product J.

Scheme 2.Control experiments.
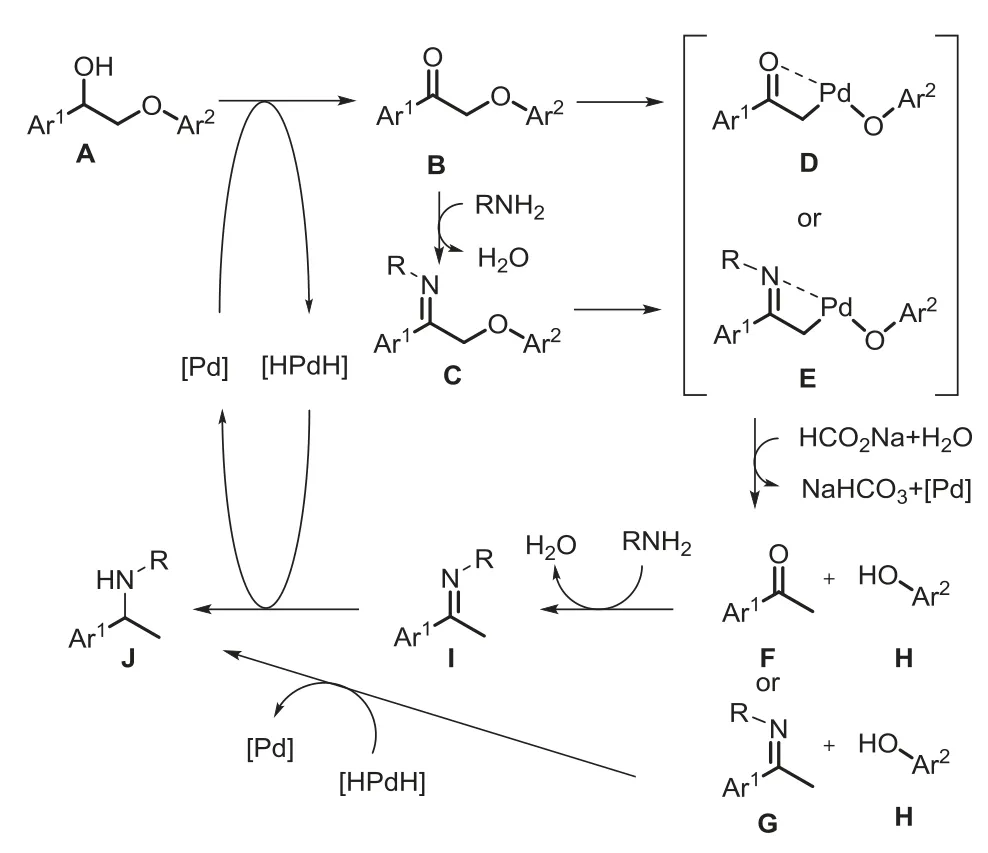
Scheme 3.Proposed reaction pathways for cross-coupling of lignin β-O-4 model compound with amines.
In summary,we have developed cleavage/cross-coupling strategy for convertingβ-O-4 linkage lignin model compounds to high valued benzyl amines via dual C–O bond cleavage and cross coupling reaction.The ligninβ-O-4 model compounds can be directly converted into nitrogen-containing aromatic compounds and phenols.This method provides a novel avenue in the exploitation of novel sustainable feedstocks for chemical production and represents a particularly difficult transformation.Further efforts will be made to apply the present strategy to obtain high-valued chemicals from natural lignins.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the National Natural Science Foundation of China(NSFC,No.21971093),the International Joint Research Centre for Green Catalysis and Synthesis (Nos.2016B01017 and 18JR4RA003)and the 111 project for support of our research.We also thank the Canada Research Chair (Tier I) foundation,the E.B.Eddy Endowment Fund,the Canada Foundation for Innovation,The Natural Sciences and Engineering Research Council (NSERC),and Le Fonds Québécois de la Recherche sur la Nature et les Technologies(FQRNT) to C.-J.Li.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.125.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
