[4+1]Cyclization of benzohydrazide and ClCF2COONa towards 1,3,4-oxadiazoles and 1,3,4-oxadiazoles-d5
Y Wng,Shiqing Mu,Xin Li,Qiuling Song,b,c,∗
a Institute of Next Generation Matter Transformation,College of Materials Science &Engineering and College of Chemical Engineering at Huaqiao University,Xiamen 361021,China
b Guangdong Provincial Key Laboratory of Catalysis,Southern University of Science and Technology,Shenzhen 518055,China.
c State Key Laboratory of Organometallic Chemistry and Key Laboratory of Organofluorine Chemistry,Shanghai Institute of Organic Chemistry,Chinese Academy of Sciences,Shanghai 200032,China
Keywords:Transition-metal-free catalyzed[4+1]Cyclization Halofluorinated compounds 1,3,4-Oxadiazoles
ABSTRACT A facile synthesis of 1,3,4-oxadiazoles and 1,3,4-oxadiazoles-d5 via [4+1]cyclization of ClCF2COONa with non-amine compounds containing amino groups is developed.Of note,this is the first time that halofluorinated compounds are used as C1 synthon to construct deuterated nitrogen-heterocyclic compounds.The current protocol features simple operation,readily accessible raw materials,wide substrate scope and valuable products
Owing to the diverse beneficial effects brought by fluorine atoms,fluorine chemistry has drawn much attention from chemists over the past years [1–10].Especially,halofluorinated compounds,which are recognized as versatile building blocks,have been emerging as one of the hottest research topic in fluorine chemistry[10–30].Various reaction modes of XCF2R have been established by our group [10–24]and others [25–30].For example,besides the double cleavage of XCF2R,several intriguing utilities of XCF2R such as playing the role of difluorocarbene precursors [25–27],serving as C1 synthonviaquadruple cleavage,and reacting with amines to construct nitrogen-containing compounds have been demonstrated [12–18].Despite those achievements,the reaction between the amino groups in hydrazine compounds and XCF2R has not yet been explored.Previously,our group reported the first example for synthesis of deuterated formamide from halofluorinated compounds and amines (Scheme 1A) [13].However,the utilization of ClCF2COONa as C1 synthon and D2O as deuterium sources to construct deuterated nitrogen-heterocyclic compounds has not been documented so far.
1,3,4-Oxadiazoles are a very important class ofN-heterocyclic compounds,which are extensively found in pharmaceuticals and biologically active molecules [31–36].In view of the importance of 1,3,4-oxadiazoles and deuterated compounds in pharmaceuticals,we reported an effective [4+1]cyclization of ClCF2COONa to synthesize 1,3,4-oxadiazoles and more importantly,1,3,4-oxadiazolesd5in decent yields and with excellent deuteration ratios under transition-metal-free conditions.To the best of our knowledge,the direct construction of 1,3,4-oxadiazole-d5has never been documented up to date.Thus our protocol provide a facile and straightforward route for divergent construction of 1,3,4-oxadiazoles and 1,3,4-oxadiazoles-d5viaa transition-metal-free [4+1]cyclization,which represents the first example of efficient one-pot construction of 1,3,4-oxadiazoles-d5(Scheme 1B).

Scheme 1.Transition-metal-free catalyzed synthesis of 1,3,4-oxadiazoles.
To explore the feasibility of our hypothesis,we selected benzohydrazide (1a) and ClCF2COONa (2a) as the model substrates for our proof-of-concept study (Table 1).To our delight,when the reaction was carried out in the presence of base Cs2CO3,the expected 2-phenyl-1,3,4-oxadiazole (3a) was obtained in 73% yield(Table 1,entry 1).Inspired by this result and in light of our previous researches,a range of bases were screened,which turned out that K3PO4was the best choice to deliver the desired product 3a (90% yield) (Table 1,entries 2–4).Next,we examined the reaction temperature.Nevertheless,elevating or lowering the temperature are both unfavorable for the reaction (Table 1,entries 5 and 6).In order to enhance the yield of 3a,we also inspected a number of solvents (Table 1,entries 7–10).However,no superior results gained and it was found that CH3CN was still the optimized solvent.Gratifyingly,when we reduced the amount of both ClCF2COONa and the base to 1.5 equiv.,the targeted 2-phenyl-1,3,4-oxadiazole (3a) could be isolated in 93% yield (Table 1,entry 12).
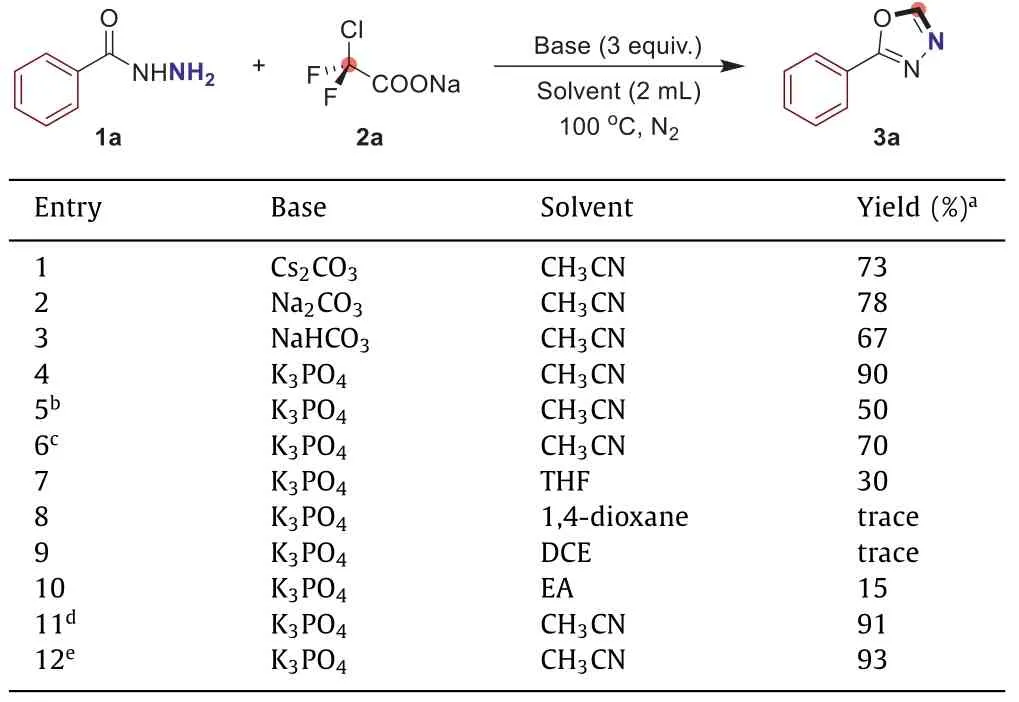
Table 1 Screening of optimized reaction conditions.
With the optimized conditions in hand,we next investigated the substrate generality of this [4+1]cyclization (Scheme 2).To our delight,a wide range of easily accessible benzohydrazides(1a–1u) could react smoothly with ClCF2COONa (2) to provide the desired products 2-substituted-1,3,4-oxadiazoles (3a–3u) in good yields by using CH3CN as solvent and K3PO4as base.The structure of 3a was unambiguously confirmed by X-ray single crystal analysis.As showcased in Scheme 2,a variety of benzohydrazides bearing both electron-donating and electron-withdrawing groups were good candidates in this transformation,affording the desired 2-substituted-1,3,4-oxadiazole products (3a–3m) in 53% to 98% yields.Moreover,halo-substituted benzohydrazides (1n–1r)were also proven to be good candidates in this cyclization reaction,enabling to formation of the desired 2-substituted-1,3,4-oxadiazole products 3n–3r in 78% to 88% yields.In addition topara-substituted benzohydrazides,ortho- andmeta-substituted benzohydrazides could also work well in this reaction,providing the corresponding 1,3,4-oxadiazoles 3q and 3r in good yields.To our delight,heterocyclic benzohydrazides and alkyl hydrazide demonstrated decent reactivity in this cyclization reaction as well,finishing the targeted products 3s–3u in moderate to good yields.Of note,the indole was well-tolerated under the standard conditions without the difluoromethylation of the free N-H in indole.
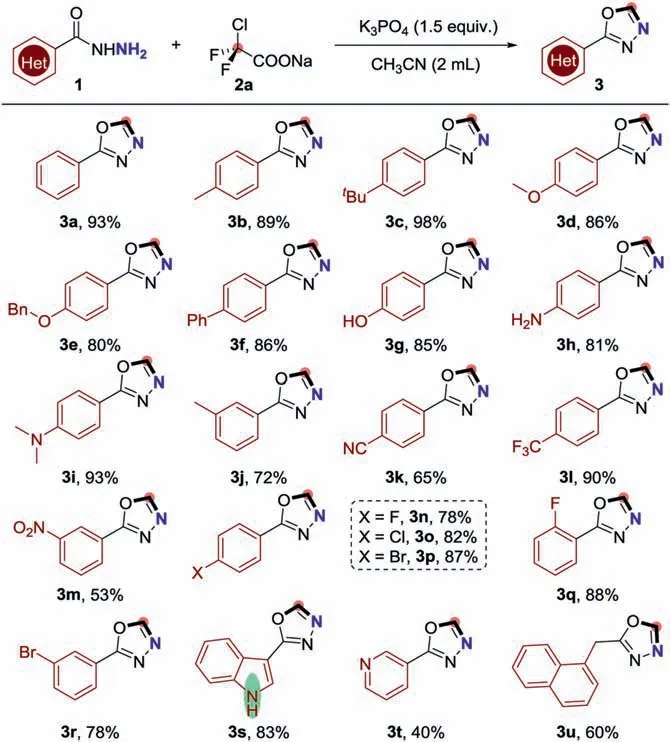
Scheme 2.Substrate scope for the synthesis of 1,3,4-oxadiazoles.Reaction condition: 1 (0.2 mmol),2a (0.3 mmol),K3PO4 (0.3 mmol),CH3CN (2 mL),N2,100 °C,1 h.

Scheme 3.Substrate scope of halodifluoromethyl compounds.Reaction condition:1a (0.2 mmol),2 (0.3 mmol),K3PO4 (0.3 mmol),CH3CN (2 mL),N2,100 °C,1 h.
As summarized in Scheme 3,we also investigated other halodifluoromethyl compounds and it was found that all of them could serve as carbon sources to forge 2-phenyl-1,3,4-oxadiazole under the same conditions by using benzohydrazides 1a as the model substrates.In addition to sodium chlorodifluoroacetate 2a,ethyl chlorodifluoroacetate 2b,ethyl bromodifluoroacetate 2e and ethyl iodifluoroacetate 2g were proved to be good C1 source in this transformation,enabling to deliver 3a in 48% to 70%yields,respectively.When difluorobromoacetic acid 2d was used as C1 source,the yield of 3a could be isolated in 45% yield.Diethyl(bromodifluoromethyl)phosphonate 2h provided the corresponding 2-phenyl-1,3,4-oxadiazole 3a in a comparable 50% yields.Compared to other halodifluoromethyl compounds,chlorodifluoromethane 2c and 2-bromo-2,2-difluoro-N-hexylacetamide 2f displayed lower reactivity,resulting in the target product 3a with 22%and 10% yields,respectively.
Deuterium incorporation plays an important role in isotope labelling,which can be used in the investigation of reaction mechanism,as well as in nuclear magnetic resonance spectroscopy(NMR) and mass spectrometry (MS) [37].In addition,more and more deuterated drugs are reported [38],which has created an urgent need for synthetic methods to effectively generate deuterated building blocks.Grounded in our current successful 1,3,4-oxadiazoles formationviathe base promoted [4+1]cyclization reaction,we also invented a mild and generic method for the deuteration of 1,3,4-oxadiazoles using D2O as an inexpensive deuterium source (Scheme 4).Delightfully,when we replaced CH3CN with anhydrous CH3CN and add 20 equiv.of D2O for this cyclization reaction,and the yields were barely affected along with excellent deuteration ratios (Scheme 5).Both aryl and alkyl benzohydrazides were inspected under the corresponding reaction conditions,the corresponding target products (4a-4p) were procured in up to 88%yields accompanied with excellent deuteration ratios.
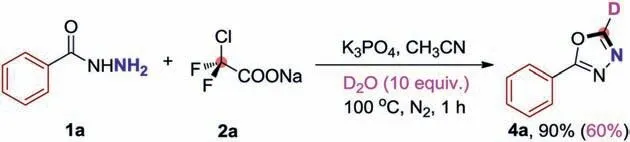
Scheme 4.Isotope-labelling experiments.
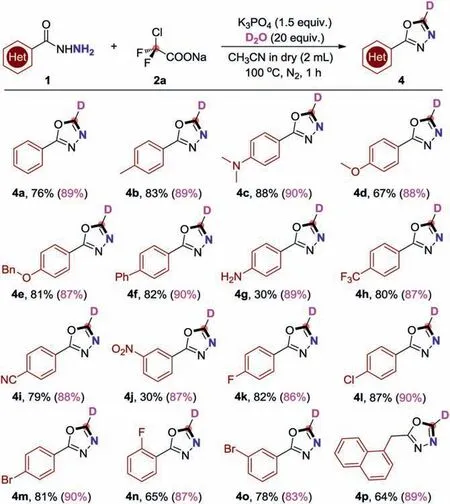
Scheme 5.Deuteration with D2O as the deuterium source.Reaction condition: 1(0.2 mmol),2a (0.3 mmol),K3PO4 (0.3 mmol),D2O (20 equiv.),anhydrous CH3CN(2 mL),N2,100 °C,1 h.All yields are the isolated yields,and the numbers in parentheses are the deuteration ratios of the corresponding deuterated products.
We also studied the scalability of this [4+1]cyclization reaction.When current protocol was scaled up ten times,the desired 1,3,4-oxadiazoles 3h and 3p could be readily obtained in 75% and 73% yields without the loss of efficiency,respectively (Schemes 6a and b).Further transformations of the 1,3,4-oxadiazoles were executed.The ethyl 1-(4-(1,3,4-oxadiazol-2-yl)phenyl)-1H-imidazole-4-carboxylate 5 could be obtained in moderate yieldviaa Cucatalyzed [3+1+1]type cyclization of ClCF2COONa for the assembly of imidazolesviathein-situgenerated isocyanides(Scheme 6c) [12].The synthetic utility of this approach was also showcased by the assembly of 2-(4′-methoxy-[1,1′-biphenyl]-4-yl)-1,3,4-oxadiazole 7viaa Pd-catalyzed Suzuki-coupling (Scheme 6d)[39].

Scheme 6.Scale-ups and synthetic transformations.
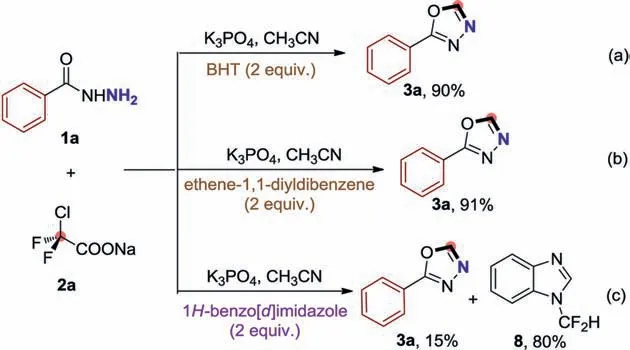
Scheme 7.Control experiments.
We then turned our attention to shed light on the mechanism of this [4+1]cyclization reaction.Radical trapping experiments were investigated firstly (Scheme 7).When 2,6-di-tert-butyl-4-methylphenol (BHT) and ethene-1,1-diyldibenzene were served as radical scavengers under the standard conditions,this cyclization reaction has no significant influence (Schemes 7a and b,and Scheme S7b in Supporting information),indicating that a radicaltype process might not be involved in this [4+1]cyclization.Based on our previous quadruple cleavage of halogenated difluoro compounds,difluorocarbenes (:CF2) was generated in-situ.To verify the production of difluorocarbene in this reaction,the difluorocarbene trapping experiment was conducted.When benzimidazole was added as difluorocarbene trapper,the 1-(difluoromethyl)-1Hbenzo[d]imidazole (8) was obtained in 80% yield (Scheme 7c).
Based on the above experimental results and previous literatures [40,41],a possible reaction mechanism for this cyclization reaction of benzohydrazides and ClCF2COONa is depicted in Scheme 8.Firstly,difluorocarbene is formedviathe dechlorination and decarboxylation of ClCF2COONa.Thein-situgenerated difluorocarbene is immediately captured by the benzohydrazides 1 with the assistance of a base to afford the Intermediate A,which rapidly undergoes the tautomerism to obtain Intermediate B.Simultaneously,the Intermediate B to give the intermediate C in the presence of base.The intermediate C further goes through the intramolecular cyclization and protonation or deuterate to affords the target product 3.
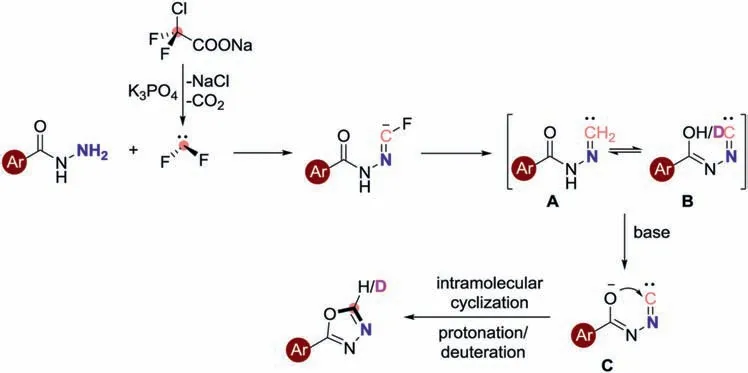
Scheme 8.Proposed reaction mechanism for the synthesis of 1,3,4-oxadiazole derivatives.
In conclusion,we have disclosed a novel transition-metalfree [4+1]cyclization of ClCF2COONa for the assembly of 1,3,4-oxadiazoles and 1,3,4-oxadiazoles-d5under mild conditions.The desired products were obtained in decent yields and excellent deuteration ratios with a wide substrate scope by using ClCF2COONa as C1 synthon.When D2O was employed as deuterium sources,a sequence of 1,3,4-oxadiazoles-5d were obtained in good yields and excellent deuteration ratios.The current protocol also represents the first example of construction of deuteratedN-heterocyclic compounds using ClCF2COONa and hydrazine compounds as starting materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support from the National Natural Science Foundation of China (Nos.21772046,2193103),and the Guangdong Provincial Key Laboratory of Catalysis (No.2020B121201002) is gratefully acknowledged.The authors also thank the Instrumental Analysis Center of Huaqiao University for analysis support.Y.Wang thank the Subsidized Project for Cultivating Postgraduates’Innovative Ability in Scientific Research of Huaqiao University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.089.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
