Visible-light-initiated 4CzIPN catalyzed multi-component tandem reactions to assemble sulfonated quinoxalin-2(1H)-ones
Zhiwei Wng,Qishun Liu,Ruisheng Liu,Zhongyin Ji,Yn Li,Xiohui Zho,Wei Wei,∗
a School of Chemistry and Chemical Engineering,Qufu Normal University,Qufu 273165,China
b Qinghai Provincial Key Laboratory of Tibetan Medicine Research and Key Laboratory of Tibetan Medicine Research,Northwest Institute of Plateau Biology,Chinese Academy of Sciences,Qinghai 810008,China
Keywords:Visible-light Multi-component reaction Radical process Sulfonated quinoxalin-2(1H)-ones
ABSTRACT A mild and efficient photochemical multi-component tandem reaction of quinoxalin-2(1H)-ones,alkenes and sulfinic acids is reported.This tandem reaction could be conveniently carried out at room temperature by employing 4CzIPN as the metal-free photocatalyst and dioxygen (air) as the environmentally benign oxidant.A number of sulfonated quinoxalin-2(1H)-ones were obtained in satisfactory yields with favorable functional group tolerance.Radical trapping experiment and fluorescence quenching experiments were performed to elucidate this visible-light mediated radical reaction process.
3-Functionlized quinoxalin-2(1H)-ones have received considerable synthetic pursuit of chemists owing to their important biological activities and pharmacological effects [1,2].In this regard,the C3−H functionalization of quinoxalin-2(1H)-ones represent a direct and powerful tool for modification at the C3 position of quinoxalin-2-ones due to their high reaction efficiency[3,4].Up to date,some functional groups such as amino [5,6],acyl [7–10],oxyalkyl [11–13],aryl [14–16],phosphoryl [17,18],cyano [19,20],sulfenyl [21,22]and alkoxyl [23,24]substitutes were efficiently incorporated into framework of quinoxalin-2(1H)-ones by two-component functionalization reactions.Despite these advances,there is still a great demand to develop the feasible and efficient strategies for the construction of more complex and diverse 3-substituted quinoxalin-2-ones.Multi-component tandem reaction has emerged as an important protocol in synthetic chemistry due to its vast applications for building complex and diverse organic molecules in an atom- and step-economic manner [25–32].In this context,some multi-component reactions have been developed for the synthesis of 3-functionlized quinoxalin-2-ones [33–37].Nevertheless,most of these reactions involve the utilization of metal catalyst,high temperature or stoichiometric amounts of peroxides/organic oxidants,which limited their wide applications in synthetic chemistry.
Visible-light photoredox-catalysis has attracted continuous interest in synthetic chemistry in term of its mild,clean and sustainable features [38–51].In particular,visible-light-mediated multicomponent reaction has become a powerful tool to synthesize various valuable organic molecules [52–57].Recently,two photocatalytic three-component tandem reaction strategies were applied to construct C3 trifluoroalkyl or per-fluoroalkyl quinoxalin-2(1H)-ones from quinoxalin-2(1H)-ones,alkenes and fluoroalkyl reagents through the cascade formation of two C-C bonds [58,59].Sulfone group plays a prevalent role in vast biologically active compounds,artificial drugs and functional materials [60–62].Thus,there is an increasing demand to develop mild,efficient and environmentallybenign strategies to introduce sulfone group into organic framework.With our continued interests in the preparation of organic sulfones [63–66],herein,we wish to describe a simple and convenient visible-light-initiated 4CzIPN (2 mol%) catalyzed threecomponent reaction of quinoxalin-2(1H)-ones,alkenes and sulfinic acids to access various sulfonated quinoxalin-2(1H)-ones at room temperature (Scheme 1),in which the C-S and C-C bonds were efficiently formed in a single operation.
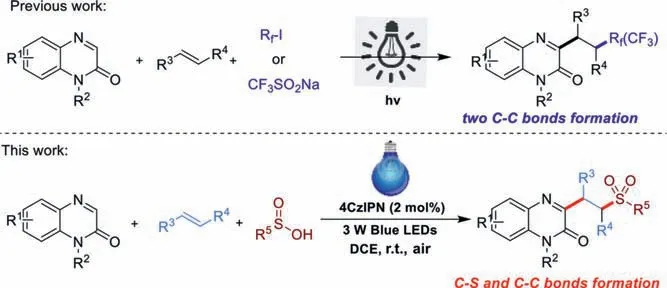
Scheme 1.Visible-light-mediated multi-component reaction for the synthesis of 3-functionlized quinoxalin-2-ones.
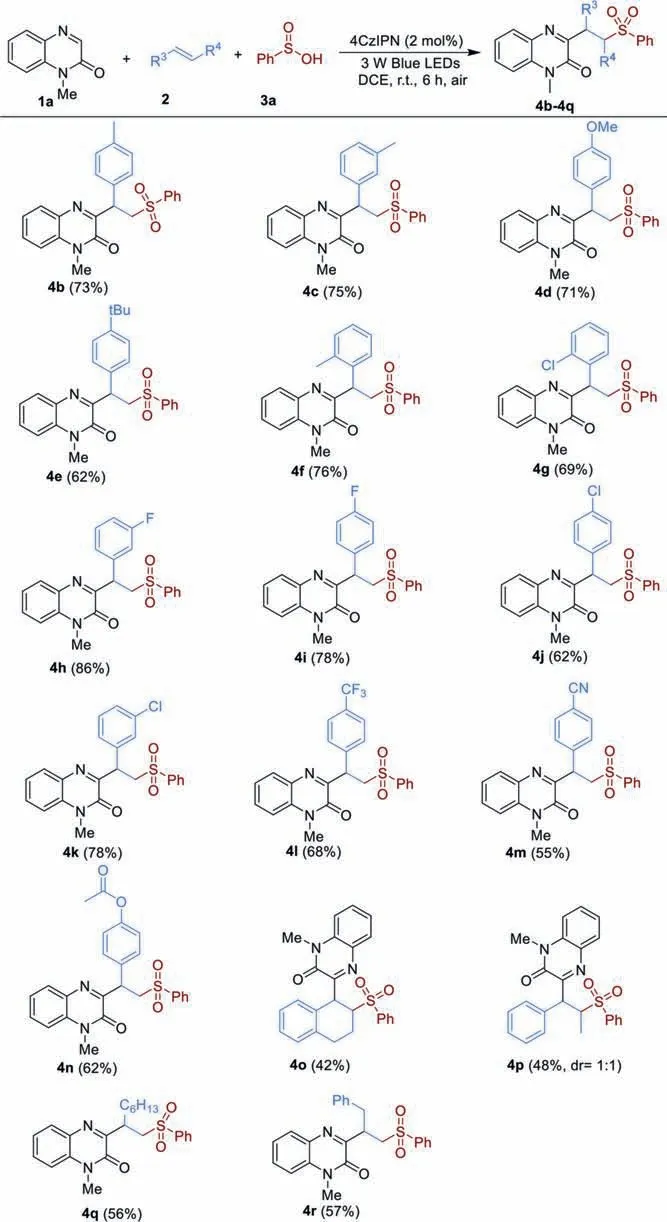
Scheme 2.Substrate scope of various alkenes.Reaction condition: 1a (0.1 mmol),2(0.2 mmol),3a (0.2 mmol),4CzIPN (2 mol%),DCE (2 mL),3 W blue LED lamps,r.t.,air,6 h.Isolated yields based on 1a.

Scheme 3.Substrate scope of various quinoxalinones and sulfinic acids.Reaction condition: 1 (0.1 mmol),2a (0.2 mmol),3 (0.2 mmol),4CzIPN (2 mol%),DCE (2 mL),3 W blue LED lamps,r.t.,air,6 h.Isolated yields based on 1.
Initially,the three-component reaction of 1-methylquinoxalin-2(1H)-one (1a),styrene (2a) and benzenesulfinic acid (3) was chosen as model reaction to optimize the reaction conditions.The initial reaction was performed in CH3CN by the irradiation of 3 W blue LED lamps at room temperature using 4CzIPN (1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene) as photocatalyst (2 mol%).To our delight,the corresponding product 4a would be obtained in 54% yield (Table 1,entry 1).Subsequently,a number of solvents were examined,and replacement of CH3CN with ether such as DME,THF or 1,4-dioxane gave a significantly decreased yield(Table 1,entries 2–4).Halogenated solvents such as CHCl3and 1,2-dichloroethane (DCE) afforded the good reaction efficiency,and DCE was elicited as the best mediator for the reaction (Table 1,entry 5).By contrast,the relatively lower yields were observed when dimethylformamide (DMF) and dimethyl sulfoxide (DMSO)were used as the reaction solvents (Table 1,entries 7 and 8).Next,the catalytic activities of various photocatalysts were investigated(Table 1,entries 9–14).In addition to 4CzIPN,other photocatalysts such as Eosin Y,Na2-Eosin Y,rhodamine B,Ru(bpy)3Cl2·3H2O,or Mes-Acr+ClO4−can also promote the reaction,but each of them was less effective than 4CzIPN.A screen of the amount of photocatalyst revealed that 2 mol% of 4CzIPN was the best choice,the decrease or increase of 4CzIPN loading would lead to the lower yields (Table 1,entries 15 and 16).In addition,a critical survey of light source showed that blue LED was the best-supporting light source,other light sources such as green or white LED only gave the desired product 4a in moderate yields (Table 1,entries 17 and 18).When the reaction was carried out under N2or O2,and the product 4a was obtained in 73% and 46%,respectively (Table 1,entries 19 and 20).Control experiments without either photocatalyst or light were performed to show that this transformation did not occur at all (Table 1,entries 21 and 22).
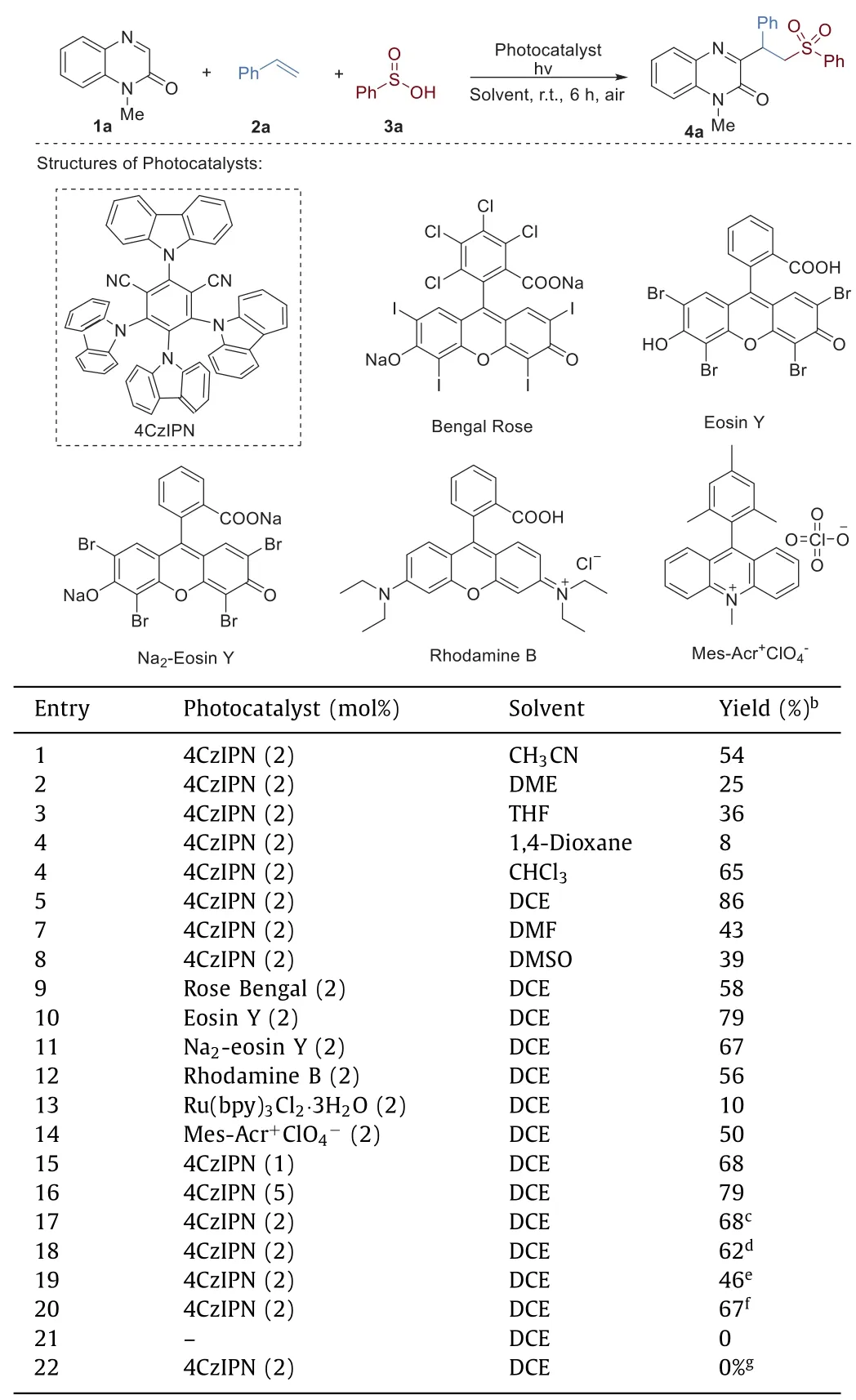
Table 1 Screening of the reaction conditions.a
Based on the optimal conditions,we subsequently investigated the substrate scope of this photocatalytic multi-component reaction of quinoxalin-2(1H)-ones,alkenes and sulfinic acids(Scheme 2).The results revealed that a number of aromatic alkenes bearing various substituents on the phenyl ring reacted smoothly to give the corresponding products (4b,5f) in moderate to good yields.The reaction efficiency was not significantly affected by the steric hindrance effect and styrene with methyl and chloro groups atorthoposition afforded the desired products (4f and 4g) in 76% and 69% yields,respectively.A variety of functionalities such as halogen,3-trifluoromethyl,cyano and acyloxy groups were well tolerated in this procedure,which can be used as potential handles for further transformations (4g–4n).It was found that internal alkenes such as 1,2-dihydronaphthalene and (E)-prop-1-enylbenzene could be employed in this reaction to give the products 4o and 4p in relatively low yields.In addition to aromatic alkenes,aliphatic alkenes were also suitable substrates,but leading to the corresponding products 4q and 4r in moderate yields.
Furthermore,the scope of various quinoxalinones and sulfinic acids was studied.As shown in Scheme 3,quinoxalinones with a series ofN-protecting groups includingN-ethyl,N-propargyl,Ncyanomethyl,andN-phenyl groups worked well under the standard conditions,which provided the products 5a–5d in good yields.In addition,quinoxalinones bearing substituents on the aromatic ring were also good candidates for this reaction and led to the corresponding products 5e,5f in good efficiencies.It should be noted thatN-free protected quinoxalinone was also suitable for this transformation,providing the corresponding product 5g in 81% yield.With regards to arylsulfinic acids,a number of substituted arylsulfinic acids containing electron-donating or electronwithdrawing groups were all susceptible during the transformation,and the corresponding products were obtained in moderate to good yields (5h–5m).The reaction efficiency was found to be sensitive to the steric hindrance effect of arylsulfinic acids.In comparison with substituents at themetaandparapositions,arylsulfinic acid with chloro group atorthoposition afforded the relatively lower yield (5n).

Fig.1.Quenching of 4CzIPN fluorescence emission in the presence of 1a.
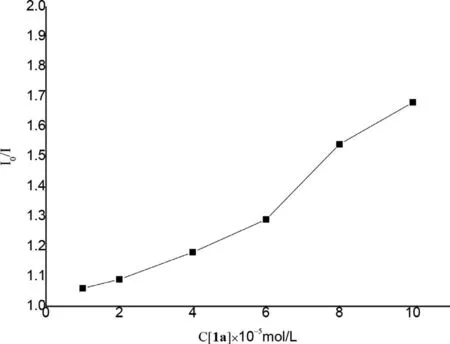
Fig.2.Stern-volmer plots.
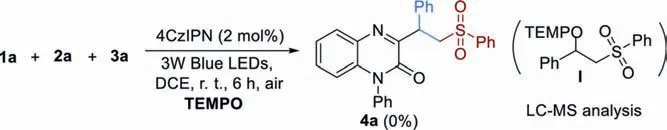
Scheme 4.Radical trapping experiment.
Some control experiments were performed to investigate the possible reaction mechanism.A radical reaction pathway received experimental confirmation from the fact that the model reaction was completely suppressed by the addition of radical scavenger TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) and TEMPO-trapped complex I were detected by LC-MS (Scheme 4).
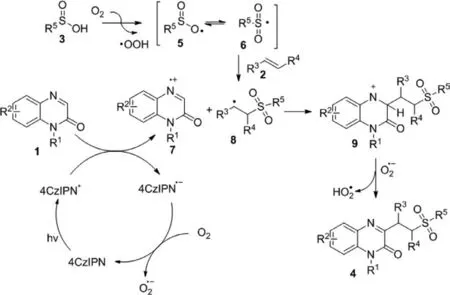
Scheme 5.Possible reaction pathway.
Furthermore,fluorescence quenching experiments were conducted to validate an energy transfer process between substrates and 4CzIPN in the presence of visible-light irradiation.As demonstrated in Figs.1 and 2,the emission intensity of excited 4CzIPN diminished gradually along with the increase of the loading of 1-methylquinoxalin-2(1H)-one (1a).Nevertheless,this fluorescence quenching phenomenon was not obviously observed when styrene(2a) or benzenesulfinic acid (3a) separately interacted with 4CzIPN(Supporting information).The above experimental results indicated that visible-light mediated energy transfer process should occur between excited 4CzIPN and quinoxalin-2(1H)-one.
Based on the above experiments and previous reports[58,59,65–70],we propose a possible mechanism for this visible light-induced multi-component reaction as depicted in Scheme 5.Firstly,photocatalyst 4CzIPN converted into its excited state 4CzIPN∗under visible-light irradiation [59,65].Subsequently,quinoxalin-2(1H)-one 1 and the excited state of 4CzIPN∗underwent single-electron transfer (SET) to provide radical cation 7 and 4CzIPN·−.4CzIPN·−was further oxidized by dioxygen (air) afforded the ground state 4CzIPN and O2·−[59,65,66].At the same time,the oxidation of arylsulfinic acid 3 by O2gaveO-centered radical 5 resonating with sulfonyl radical 6 [67].Then,sulfonyl radical 6 added to the alkene 2 to generate the radical intermediate 8[68–70],which interacted with radical cation 7 affording a nitrogen cation intermediate 9.Finally,the deprotonation of intermediate 9 produced the sulfonated quinoxalin-2(1H)-ones 4.
In summary,a mild and efficient photocatalytic strategy has been proposed for the synthesis of sulfonated quinoxalin-2(1H)-onesviathree-component tandem reaction of quinoxalin-2(1H)-ones,alkenes and sulfinic acids in air.This simple reaction,catalyzed by 4CzIPN in a metal-free fashion,provides satisfactory yields of sulfonated quinoxalin-2(1H)-ones from simple and readily available materials with abroad substrate scope and favorable functional group tolerance.Mechanistic studies showed that radical process might be involved in this transformation.Further investigation of mechanism and synthetic application is currently underway in our laboratory.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by Youth Innovation and Technology Project of high School in Shandong Province (No.2019KJC021),Natural Science Foundation of Qinghai Province of China (No.2020-ZJ-915).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.036.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
