Water-soluble pillar[4]arene[1]quinone: Synthesis,host-guest property and application in the fluorescence turn-on sensing of ethylenediamine in aqueous solution,organic solvent and air
Jin Wang,Moupan Cen,Jian Wang,Di Wang,Yue Ding,Guohua Zhu,Bing Lu,Xiaolei Yuan,Yang Wang,Yong Yao
Sc hool of Chemistry and Chemical Engineering,Nantong University,Nantong 226019,China
Keywords:Pillar[5]arene Host-guest chemistry Fluorescence sensing Pillar[4]arene[1]quinone Ethylenediamine
ABSTRACT Water-soluble pillar[5]arenes are a class of typical macrocycles and have aroused tremendous attention for its easy to modify,abundant host-guest properties and extensive applications.However,up to now,all the reported water-soluble pillar[5]arenes acted as the host molecules,whereas they failed to be postsynthetically modified,which seriously impeded the development of the pillar[5]arene-based supramolecular chemistry.In this work,a new water-soluble pillar[5]arene,pillar[4]arene[1]quinone,was designed and synthsized with eight quaternary ammonium groups as well as a quinone units.Such a new water-soluble pillar[4]arene[1]quinone was capable of forming 1:1 stable complex with sodium 1-octanesulfonate in aqueous solution.Since the 1,4-quinone unit of WP[4]Q[1]could react with ethylenediamine (EDA) to form a conjugated quinoxaline structure,so pillar[4]arene[1]quinone could apply to the facile fluorescence turn-on sensing of EDA in aqueous solution,organic solvent and air.
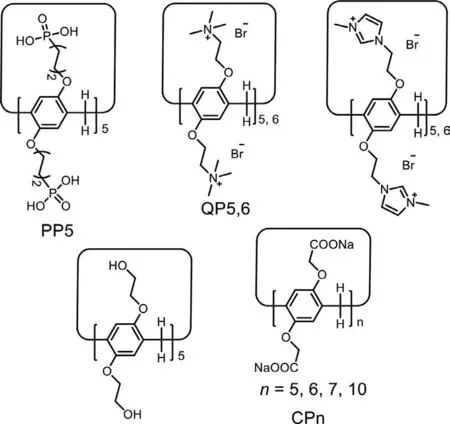
The design,synthesis and host-guest investigation of macrocyclic compounds laid the foundation for the supramolecular chemistry [1-4].In particular,water-soluble macrocycles have been a hotspot insupramolecular studies since most biological processes are performed in aqueous media [5,6].Pillar[n]arenes refer to the newest classical macrocycles that are prepared from the hydroquinone units connectedvia–CH2–bridges [7-10].They exhibit a rigid pillar-like architecture and2nfunctionalizable sites,which makes modification easily [11-16].Over the past 12 years,pillar[n]arenes have been greatly developed in various aspects (e.g.,syntheses,host-guest interactions,self-assembly properties and application in controlled drug release,cancer therapy,TNT adsorption and cell aggregation) [17-23].Among various pillar[n]arenes,water-soluble pillar[n]arenes arouse the particular attention from scientists for their nontoxic and excellent host-guest properties in aqueous solution [24].Thus far,scientists have developed several main water-soluble pillar[n]arenes by introducing negatively,neutrally or positively charged groups into the cavity portals of pillar[n]arenes (Scheme 1) [25-34].
For instance,Ogoshi and co-workers prepared the notable negatively charged carboxylatopillar[5]arene (CP5,Scheme 1).These researchers demonstrated its complexation behavior to cationic viologen salt [24].Subsequently,Prof.Li investigated highly selective binding of CP5 towards other cationic guests,basic amino acids (e.g.,lysine,arginine and histidine),peptides and lysine metabolites [25].Recently,Prof.Yang reported a phosphoryl pillar[5]arene (PP5,Scheme 1) stabilizedβ-NaYF4:Yb/Er upconversion nanoparticle with a high water dispersibility especially in a physiological environment and a superior capability of controlled cargo release and cell imaging [33].Moreover,the authors reported the quaternary ammonium functionalized pillar[5]arene (QP5,Scheme 1) modified Cu2-xSe nanoparticles for the target photo-thermal cancer therapy [34].
Though the syntheses,host-guest properties,and applications of water-soluble pillar[n]arenes have been extensively investigated,to the best of our knowledge,all the reported watersoluble pillar[n]arenes were perfunctionalized pillar[n]arene with2nidentical substituents at their reactive positions,whereas watersoluble pillar[n]arenes with postsynthetic reactive positions have been rarely developed thus far.In this study,a novel watersoluble pillar[5]arene (WP[4]Q[1],Scheme 2) was designed and prepared,which comprised eight quaternary ammonium groups and a quinone unit.The as-prepared WP[4]Q[1]could form a 1:1 stable complex with sodium 1-octanesulfonate (G1) in aqueous solution.It is noteworthy that,WP[4]Q[1]could further apply to facile fluorescence turn-on sensing of ethylenediamine (EDA) in aqueous solution,organic solvent and air (Scheme 2).
Scheme 1.Structures of previously reported water-soluble pillar[n]arenes.
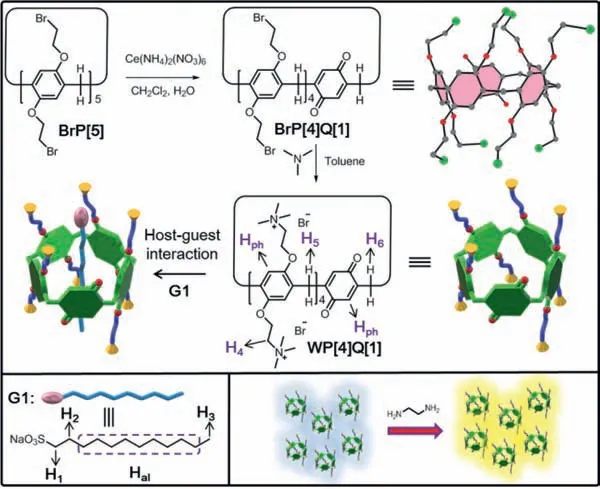
Scheme 2.Synthesis of water-soluble pillar[4]arene[1]quinone (WP[4]Q[1]),the illustration of WP[4]Q[1]form a 1:1 stable complex with sodium 1-octanesulfonate(G1) and the application in fluorescence turn-on sensing of ethylenediamine (EDA).
As outlined in Scheme 2,to synthesize WP[4]Q[1],the key intermediate BrP[4]Q[1]should be prepared first.A mixture of 1 equiv.of BrP[5]and 1 equiv.of Ce(NH4)2(NO3)6was stirred in CH2Cl2/H2O mixture at ambient temperature for 1 h.Though many species were detected in the product mixture by conducting the TLC analysis,pure BrP[4]Q[1]was successfully obtained as red solids by using the column chromatography with a yield of 78%.The structure of BrP[4]Q[1]was fully characterized by conducting1H NMR (Fig.S1 in Supporting information),13C NMR (Fig.S2 in Supporting information),MS (Fig.S3 in Supporting information)and single crystal X-ray analyses (Fig.S4 in Supporting information).The crystal of BrP[4]Q[1]was grown through a slow evaporation of the solution of BrP[4]Q[1]in a dichloromethane/methanol mixture (1:1,v/v).The crystal structure directly demonstrated that BrP[4]Q[1]contained four 1,4-bis(2-bromoethoxy)benzene units and a quinone unit (Fig.1a).Furthermore,BrP[4]Q[1]exhibited a pentagon-like cyclic structure,complying the classical pillar[5]arene (Fig.1c).
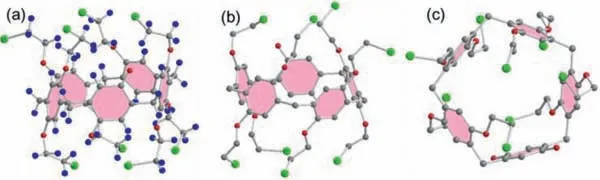
Fig.1.Crystal structures of BrP[4]Q[1]: (a) side view with all atoms,(b) side view with hydrogen omitted for clarity,(c) top view.Carbon atoms are represented in gray,oxygen atoms in red,hydrogen atoms in blue,and bromine atoms in purple.
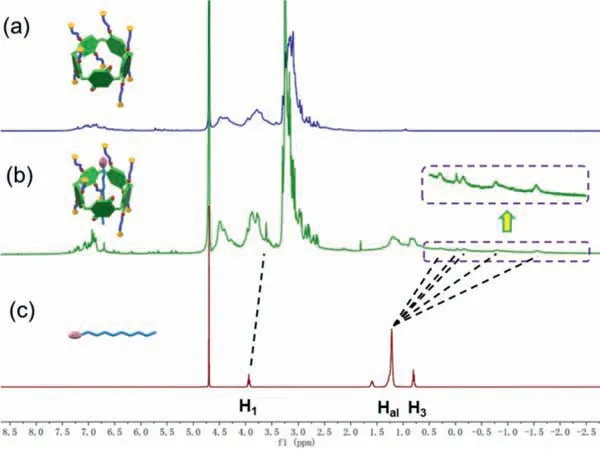
Fig.2.1H NMR spectra (D2O,293 K,400 MHz) of (a) 5.00 mmol/L WP[4]Q[1];(b)5.00 mmol/L WP[4]Q[1]+5.00 mmol/L G1;(c) 16.00 mmol/L G1.
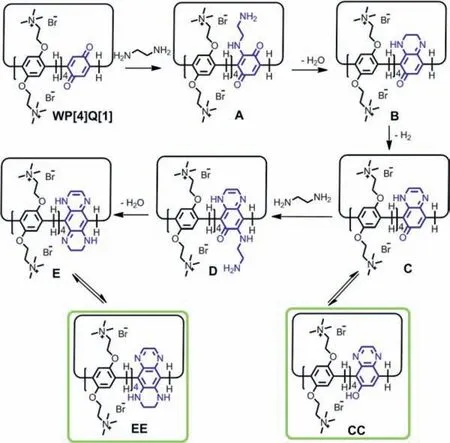
Scheme 3.Proposed aqueous solution reaction mechanism for WP[4]Q[1]with EDA.
Subsequently,WP[4]Q[1]was synthesized by the treatment of BrP[4]Q[1]with excess trimethylamine in reflux toluene/CH3CH2OH mixture,the as-prepared WP[4]Q[1]comprising eight cationic trimethylammonium groups and a quinone unit were synthesized as the dark red solid and characterized by using1H NMR (Fig.S5 in Supporting information),13C NMR (Fig.S6 in Supporting information) technologies.
Since WP[4]Q[1]comprised eight cationic groups,its binding mode with sodium 1-octanesulfonate (G1) in aqueous solution was investigated with1H NMR,2D NOESY NMR,and ESI-MS technologies.The1H NMR spectrum (Fig.2) of an equimolar solution of WP[4]Q[1]and G1 exhibited the clear complexation induced upfield shifts of the signals corresponding to H1and Halon G1,thereby demonstrating that the formation of a threaded structure.The assignment and correlations of the protons of WP[4]Q[1]and G1 were validated from the NOESY NMR spectrum (Fig.S7 in Supporting information) [35,36].The NOESY NMR examination displayed the unequivocal correlation peaks between the signals of alkyl chain protons (H1,Haland H3) of G1 and those of the aromatic protons Hph,H4,and the bridge protons H5,6of WP[4]Q[1].The desired complexes were further demonstrated by using LRESIMS (Fig.S8 in Supporting information).The equimolar mixture of WP[4]Q[1]and G1 had the mass fragment of [WP[4]Q[1]+G1–8Br––Na++H2O]7+/7:m/z223.4 (100%) and [WP[4]Q[1]+G1–3Br––Na++2H++H2O]4+/4:m/z495.6 (35%),indicating the formation of 1:1 complexes between WP[4]Q[1]and G1.As revealed from a mole ratio plot for the complexation between WP[4]Q[1]and G1,the stoichiometry of the complex between WP[4]Q[1]and G1 was 1:1 (Fig.S9 in Supporting information),complying with the mentioned result of LRESIMS.The association constant (Ka) of WP[4]Q[1]⊃G1 was calculated as (2.35 ± 0.06) × 105L/mol in aqueous solution by conducting a nonlinear curve-fitting analysis (Fig.S10 in Supporting information).ThisKa value exceeded the correspondingKa value of the previously reported complex between an anionic water-soluble pillar[5]arene and cationic viologen.This result was explained that compared with anionic water-soluble pillar[5]arene,one unit of WP[4]Q[1]was replaced by 1,4-quinone,thereby making G1 threaded into the cavity of WP[4]Q[1]more easily.
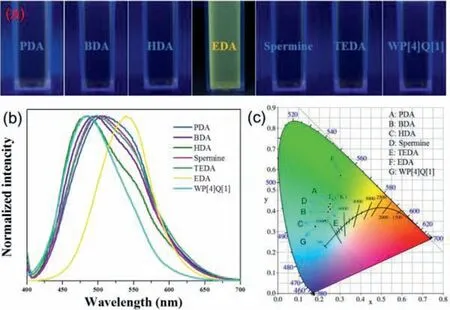
Fig.3.(a) Photographs of aqueous solution of WP[4]Q[1]under 365 nm UV irradiation through the addition of different diamines.(b) Normalized fluorescence spectra and (c) fluorescence CIE chromaticity diagram of aqueous solution of WP[4]Q[1]through the addition of different diamines.λex=365 nm.
Ethylenediamine (EDA) refers to a vital chemical raw material,which can strongly irritate to mucous membrane and skin[37].Exposure to the EDA vapor may cause conjunctivitis,bronchitis,pneumonia or pulmonary edema.The exposure to the aqueous solution of EDA can also cause liver and kidney damage.The WP[4]Q[1]here contained a 1,4-benzoquinone unit,which could react with EDA to generate fluorescent compounds undergoing a ring-formation reaction [38,39].Given this,the authors raised a question that whether WP[4]Q[1]can be applied in detecting EDA both in solution and gas.
First,the aqueous solution of WP[4]Q[1]toward diamines with different lengths of alkyl chains (EDA,1,3-propanediamine (PDA),1,4-butylenediamine (BDA),1,6-hexylenediamime (HDA),spermine and triethylenediamine (TEDA)) were investigated by using the fluorescence spectroscopies.According to Figs.3a,after adding of various diamines into the aqueous of WP[4]Q[1],only the addition of EDA made the fluorescence color changed from blue to yellowgreen under the 365 nm UV light,while others were not significantly different from the original color.Besides,the corresponding fluorescence spectrum confirmed that the addition of EDA induced the distinct blue shifts of the fluorescence,whereas others did not cause any obvious fluorescence change (Fig.3b).The CIE chromaticity diagram (Fig.3c) corresponding to fluorescence spectra demonstrated the different fluorescence colors before and after the addition of diamines.Different color changes from dark red to brown and orange were observed when WP[4]Q[1]solids were exposed to different aliphatic amine vapors after several days,as shown in Fig.S15 (Supporting information).Furthermore,the detection limit was calculated to be 9.60 × 10−7mol/L when the concentration of WP[4]Q[1]was 2 × 10−5mol/L (Fig.S14 in Supporting information).These results indicated that WP[4]Q[1]has highly sensitivity response for EDA.
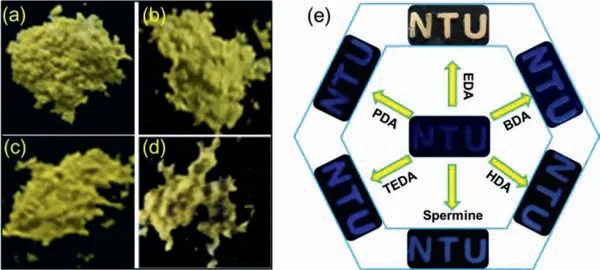
Fig.4.Fluorescent photographs of powder WP[4]Q[1]under the 365 nm irradiation after immersing into organic solvents which contain 10−5 mol/L of EDA for 5 min:(a) Acetone,(b) Hexane,(c) Acetonitrile and (d) CHCl3.(e) Fluorescent photographs of WP[4]Q[1]based thin and solid “NTU” shape film before and after being exposed to different diamine vapor.
To better understand the fluorescence transformation mechanism,we performed the solution-phase reaction of WP[4]Q[1]towards excess EDA in aqueous solution.The time-dependent fluorescence spectra revealed that the week-blue fluorescent WP[4]Q[1]was transformed into the strong yellowgreen fluorescent compounds in the solution (Fig.S11 in Supporting information).In this study,when nonfluorescent WP[4]Q[1]are exposed to EDA vapor,EDA located into the cavity of WP[4]Q[1]to,form a [2]pseudorotaxane (Fig.S16 in Supporting information).Subsequently,1,4-benzoquinone could react with EDA to generate fluorescent compounds undergoing a ringformation reaction.Hypothesized a reaction mechanism for WP[4]Q[1]in aqueous solution,as illustrated in Scheme 3.Such a mechanism involves a series of tandem reactions (e.g.,Michael addition,condensation,dehydration,and intermolecular hydrogen migration).Next,the MS study was further conducted to confirm the mentioned molecular structure transformations.According to Fig.S12 (Supporting information),compound B ([B-6Br]6+/6=249.3206,calcd.249.1367),compound CC([CC-3Br]3+/3=576.3603,calcd.576.5203,and[CC-2Br]2+/2=904.2363,calcd.904.2399),compound EE ([EE-2Br]2+/2=924.2626,calcd.924.2612) were identified.Products CC and EE all contained conjugated quinoxaline structure,which might account for the fluorescence of the modified pillar[n]arenes both in solution and in the solid state.
Furthermore,whether WP[4]Q[1]can detect EDA in organic solvents arouses the attention from the authors.According to Figs.4a-d,when the powder WP[4]Q[1]was added into the organic solvents (e.g.,acetone,hexane,acetonitrile and CHCl3) that contained 10−5mol/L of EDA for 5 min,the powder WP[4]Q[1]exhibited bright yellow-green fluorescence under the 365 nm irradiation.As opposed to the mentioned,the powder WP[4]Q[1]showed no fluorescence when dispersed in the pure above organic solvents(Fig.S13 in Supporting information).
In aqueous and organic solutions,the fluorescence variations of WP[4]Q[1]in the presence of EDA could be identified,and the WP[4]Q[1]could also act as a gas sensor to detect EDA vapors.In addition,a thin and solid “NTU” shape film was prepared by dropcasting WP[4]Q[1]solution onto a glass slide.As shown by the corresponding fluorescent photograph in Fig.4e,the “NTU” film had low fluorescence under the 365 nm illuminations.However,when the “NTU” film was exposed to EDA vapor (Fig.4e),the 1,4-quinone unit of WP[4]Q[1]reacted with EDA to form a conjugated quinoxaline structure,which led to an emission of bright fluorescence.In contrast,the fluorescence varied slightly when the “NTU” film was also exposed to other diamine vapor (e.g.,PDA,BDA,HDA,spermine and TEDA) (Fig.4e).
In brief,a new water-soluble pillar[5]arene,pillar[4]arene[1]quinone,comprising eight quaternary ammonium groups and a quinone unit,was developed and synthesized successfully.Different technologies,such as1H NMR,13C NMR,MS and single crystal diffraction were adopted to characterize the resultant compounds.Such a new WP[4]Q[1]could form a 1:1 stable complex with sodium 1-octanesulfonate in aqueous solution with a relative high association constant.More importantly,since the 1,4-quinone unit of WP[4]Q[1]could react with EDA to form a conjugated quinoxaline structure,the WP[4]Q[1]can apply for facile fluorescence turn-on sensing of ethylenediamine in aqueous solution,organic solvent and gas.It is anticipated that this new type of water-soluble pillar[5]arene can apply to the fabrication of new types of functional supramolecular smart materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.21801139),the Natural Science Foundation of Jiangsu Province (Nos.BK20180942,BK20190917),the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (No.19KJB150015),the Six Talent Peak Projects in Jiangsu Province (No.XCL-085).We also thank Nantong University Analysis &Testing Center for characterization.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.044.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
