Ingeniously designed Ni-Mo-S/ZnIn2S4 composite for multi-photocatalytic reaction systems
Jing Chen,Yumei Tng,Shiho Wng,Lingin Xie,Cheng Chng,Xiolei Cheng,Mingming Liu,Longlu Wng,∗,Linhui Wng
College of Electronic and Optical Engineering &College of Microelectronics,Jiangsu Province Engineering Research Center for Fabrication and Application of Special Optical Fiber Materials and Devices,Nanjing University of Posts and Telecommunications,Nanjing 210023,China
b State Key Laboratory of Organic Electronics and Information Displays &Jiangsu Key Laboratory for Biosensors,Institute of Advanced Materials (IAM) &Institute of Flexible Electronics (Future Technology),Nanjing University of Posts and Telecommunications,Nanjing 210023,China
Keywords:Molybdenum disulfide (MoS2)Ni-Mo-S nanosheets Multi-reaction systems Hydrogen evolution reaction (HER)Atomic doping strategy
ABSTRACT Molybdenum disulfide (MoS2) with low cost,high activity and high earth abundance has been found to be a promising catalyst for the hydrogen evolution reaction (HER),but its catalytic activity is considerably limited due to its inert basal planes.Here,through the combination of theory and experiment,we propose that doping Ni in MoS2 as catalyst can make it have excellent catalytic activity in different reaction systems.In the EY/TEOA system,the maximum hydrogen production rate of EY/Ni-Mo-S is 2.72 times higher than that of pure EY,which confirms the strong hydrogen evolution activity of Ni-Mo-S nanosheets as catalysts.In the lactic acid and Na2S/Na2SO3 systems,when Ni-Mo-S is used as co-catalyst to compound with ZnIn2S4 (termed as Ni-Mo-S/ZnIn2S4),the maximum hydrogen evolution rates in the two systems are 5.28 and 2.33 times higher than those of pure ZnIn2S4,respectively.The difference in HER enhancement is because different systems lead to different sources of protons,thus affecting hydrogen evolution activity.Theoretically,we further demonstrate that the Ni-Mo-S nanosheets have a narrower band gap than MoS2,which is conducive to the rapid transfer of charge carriers and thus result in multi-photocatalytic reaction systems with excellent activity.The proposed atomic doping strategy provides a simple and promising approach for the design of photocatalysts with high activity and stability in multi-reaction systems.
Nowadays,with the rapid development of industrialization and the rapid growth of people’s demand,traditional fossil fuels are increasingly exhausted [1–4].Society is facing the dual pressure of environmental pollution and energy shortage.Therefore,there is an urgent need for mankind to actively explore and develop new energy sources while making effective use of fossil fuels in order to gradually replace the existing fossil energy sources [5–7].As an important energy carrier,molecular hydrogen as an ideal green energy is characterized by high energy density and high safety.Photocatalysis technology provides a feasible tactic to produce hydrogen energy since Fujishima and Honda demonstrated photoelectrochemical reaction to prepare hydrogen from water splitting by using TiO2photoelectrode in 1972.The key of catalytic hydrogen production lies in the development and utilization of catalytic materials [8–11].
For photocatalytic catalysts,electrons and protons produced in sunlight are mainly used to reduce hydrogen ions to produce hydrogen [12–14].For a catalyst with excellent performance,it is indispensable for hydrogen production to have a catalyst that can absorb visible light and generate a large number of charge carriers and a co-catalyst with abundant active centers [15].Among many photocatalysts,ZnIn2S4has become one of the most promising materials for visible light hydrogen evolution due to its narrow band gap and good chemical stability,which has attracted extensive attention of researchers [16–19].However,the high rate of photoexcited charge recombination and narrow light absorption range make ZnIn2S4unable to utilize solar energy effectively,which limits its photocatalytic efficiency [20].As a co-catalyst,MoS2has attracted much attention due to its low cost,high activity and high abundance,which can be comparable to Pt in improving the effi-ciency of photocatalytic hydrogen evolution to the greatest extent[21–26].Now a large number of studies have proved that MoS2,as an excellent photocatalytic material,is mainly suitable for hydrogen production under acidic conditions,but has poor performance under alkaline conditions [27–32].More recently,Jian Zhanget al.have reported that Ni-MoS2nanosheets with chemical composition of Ni0.13Mo0.87S2were prepared by hydrothermal reaction,which proved that nickel doping can enhance the electrocatalytic hydrogen evolution of MoS2under alkaline conditions [33].Although much efforts have been made to improve the hydrogen evolution efficiency of photocatalysts in acidic or alkaline conditions,there is a lack of studies on the development of photocatalytic material suitable for multi-reaction systems,which have good performance in both acidic and alkaline conditions or even neutral conditions[34–42].Therefore,it is of great significance to develop a photocatalyst with multi-reaction systems for practical application.
In this paper,we report a simple hydrothermal strategy for the synthesis of Ni-Mo-S nanosheets.The HER activity of Ni doped with MoS2substrate in multi-reaction systems was systematically studied.It is found that the introduction of nickel ions (Ni2+) in the precursor solution and the medium environment play an important role in regulating the intrinsic properties of MoS2.Our theoretical calculations show that the monatomic Ni dopant occupies the Mo site in MoS2to expose the high density unsaturated sulfur site,leading to significant electronic structure changes on the MoS2catalytic inert base plane to promote water dissociation and subsequent catalytic processes.This is the reason for the enhancement of HER catalytic activity of MoS2in acidic,neutral and alkaline solutions.The results show that the combination of Ni-Mo-S nanosheets and appropriate semiconductor ZnIn2S4is a reasonable strategy for the construction of an efficient photocatalytic system.
In this work,zinc acetate dihydrate (Zn(CH3COO)2·2H2O),indium chloride tetrahydrate (InCl3·4H2O),thiourea (CH4N2S),ammonium molybdatetetrahydrate ((NH4)6Mo7O24·4H2O),nickel sulfate hexahydrate (NiSO4·6H2O),triethanolamine (TEOA),lactic acid,sodium sulfide nonahydrate(Na2S·9H2O),sodium sulfite(Na2SO3)were purchased from Sinopherm Chemical Reagents Co.,Ltd.(Shanghai,China).All reagents and materials can be used without additional purification.The deionized water used in the experiment was from local sources.
ZnIn2S4was synthesized by solvothermal method.First,0.08 g of Zn(CH3COO)2·2H2O,0.15 g of CH4N2S and 0.10 g of InCl3·4H2O are weighed in a 100 mL beaker,and then 16 mL of ethylenediamine and water (the concentration is 1:1) mixed solution is added to the beaker and stirred for 30 min.After the solid is completely dissolved,the reaction mixture is transferred into a 25 mL Teflon-lined stainless steel autoclaveand sealed.The stainless steel autoclave with Teflon liner was heated at 160 °C for 12 h and then cooled to room temperature.The black turbidity liquid was centrifuged,washed with ethanol and centrifuged several times,and then placed in a vacuum drying oven to dry at 60 °C for 8 h.
Ni-Mo-S was synthesized by hydrothermal method.(NH4)6Mo7O24·4H2O,NiSO4·6H2O and CH4N2S were used as molybdenum,nickel and sulfur sources,respectively.In order to study the influence of different proportions of Ni and Mo precursors on hydrogen evolution performance,the ratio of Ni and Mo was changed by changing the amount of added nickel source.0.71 g (NH4)6Mo7O24·4H2O,0.53 g NiSO4·6H2O and 3.21 g CH4N2S(the Ni and Mo precursor ratio is 1:2) were dispersed in 80 mL deionized water by rapid stirring to prepare mixed aqueous solution.The solution was then transferred to a 100 mL stainless steel autoclave lined with Teflon.After 24 h of heating at 200 °C,it was naturally cooled to room temperature and repeatedly washed with deionized water to remove impurities and unreacted precursors.Finally,the obtained material was dried in a vacuum oven at 50 °C for 48 h.
The 20 mg ZnIn2S4and 2 mg Ni-Mo-S powders synthesized by the above method were uniformly mixed by mortar grinding to prepare ZnIn2S4/Ni-Mo-S composite materials.
The morphology of the catalyst was observed by Hitachi S-4800 scanning electron microscope (SEM),and the microstructure of the catalyst was studied by TECNAI G2 F30 S-TWIN transmission electron microscope (TEM).High-resolution TEM (HRTEM) photomicrographs were obtained with an energy dispersive X-ray spectrometer (EDX) at 200 kV.X-ray photoelectron spectroscopy (XPS)analysis was performed on a Thermo Escalab 250XI spectrometer using a monochromatic Al Kσsource (1486.6 eV).The spectral binding energy was C 1s binding energy at 284.6 eV for reference.The XPS data was analyzed using Casa XPS software.Ultravioletvisible diffuse reflection (UV-vis DRS) absorption spectra were obtained on a Hitachi UV-4100 spectrometer,using BaSO4as the reference standard.Photoluminescence (PL) spectra were measured using an F-4700 FL type fluorescence spectrophotometer with an excitation wavelength of 400 nm.
In this study,the band structure and the density of states of Ni-Mo-S are realized by the ultrasoft pseudopotential method of plane waves based on density functional theory (DFT).The Cambridge Sequence Total Energy Package (CASTEP) code was used for geometric optimization,and the exchange correlation interaction was processed by the Perdew-Burke-Ernzerhof of the generalized gradient approximation (GGA-PBE).In the process of geometric optimization,the total energy was 2.0 × 10−5eV/atom,the maximum force was 0.05 eV/˚A,the maximum displacement was 0.02 ˚A,and the maximum stress was 0.1 GPa.Convergence tests are required before geometric optimization.According to the convergence test,the tolerance of self-consistent field (SCF) is 5.0 × 10−6eV/atom,and the plane wave energy cutoff (Ecut) is determined to be 350 eV.The Brillouin region with 3 × 3 × 2 K-grid was sampled by Monkhorst−Pack for electron convergence test.
Photocatalytic hydrogen evolution experiments were carried out in a sealed quartz flask of 100 mL at room temperature and atmospheric pressure.A 300 W xenon lamp (PLS-SXE300,Beijing Xendetong Technology Co.,Ltd.,China) was used as the light source,and equipped with a cut-off filter (Kenko L-42) to remove the ultraviolet light with a wavelength less than 420 nm,and the photocatalytic reaction was carried out at 10 cm away from the light source.In an acidic or alkaline reaction system,the prepared 20 mg ZnIn2S4photocatalyst powder and 2.35 mg Ni-Mo-S co-catalyst powder were weighed.The prepared photocatalyst was dispersed in 100 mL mixed aqueous solution containing 15 mL lactic acid (pH 2.5) or 0.35 mol/L Na2S·9H2O and 0.25 mol/L Na2SO3(pH 13.2) as sacrifice reagents.In the neutral reaction system,20 mg of Eosin Y (EY) dye sensitizer and 20 mg of Ni-Mo-S photocatalyst powder were dispersed in 100.0 mL of mixed aqueous solution containing 15 mL of triethanolamine (pH 7) as sacrifice reagent.The mixed aqueous solution was ultrasonic treated for 10 min and transferred to 100 mL sealed quartz bottle reactor for hydrogen evolution.Before the photocatalytic reaction,the system was degassed by bubbling argon for 20 min to ensure that the reaction took place in an inert environment.During the photocatalytic reaction,the temperature of the reaction system was kept at 25 °C by circulating water pump and irradiation source.In order to eliminate the precipitation of the mixed solution and ensure the uniformity,the magnetic stirring (400 r/min) was maintained throughout the photocatalytic hydrogen production test.Gas chromatography with A built-in thermal conductivity detector (TCD) and a 5 ˚A molecular sieve column was used for online analysis of hydrogen production,with nitrogen as the carrier gas.
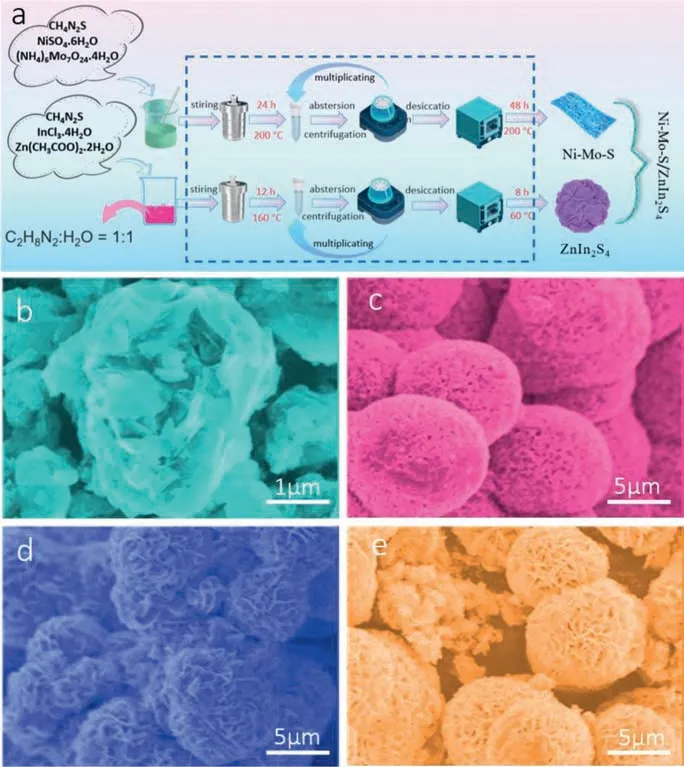
Fig.1.(a) Schematic illustration of the synthesis of Ni doped MoS2/ZnIn2S4 composite.SEM images of (b) ZnIn2S4, (c) MoS2,(d) Ni-Mo-S (Ni:Mo=1:2),(e) Ni-Mo-S(Ni:Mo=1:1).
The schematic diagram of the preparation process of ZnIn2S4and Ni-Mo-S is shown in Fig.1a.The micromorphology of ZnIn2S4and Ni-Mo-S samples with different Ni loads were observed by scanning electron microscopy (SEM).Fig.1b shows the bract structure of pure ZnIn2S4composed of many lamellar petals.Figs.1c-e show SEM images of MoS2,Ni-Mo-S (Ni:Mo=1:2) and Ni-Mo-S(Ni:Mo=1:1) respectively.It can be seen that their aggregates are similar in morphology.The diameter of microspheres is about 1.0 μm to 4.0 μm,and the edges are serrated,indicating that the microspheres are made up of a large number of interlaced flakes.This also shows that the doping of Ni2+with different mole ratio hardly changes the morphology of MoS2.Due to the similar layered structure of ZnIn2S4and Ni-Mo-S,the Ni-Mo-S nanosheets can be closely loaded on the ZnIn2S4nanoflowers for self-assembly to form heterojunctions.
The detailed microstructure of ZnIn2S4,Ni-Mo-S and Ni-Mo-S/ZnIn2S4samples were studied by TEM and HRTEM.The TEM in Fig.2a shows that ZnIn2S4has a lamellar structure with relatively transparent characteristics,indicating that ZnIn2S4nanoscale flowers have ultrathin properties.In Fig.2b,the gauze mesh edges of the nanoflower can be clearly observed to further verify that the synthesized Ni-Mo-S was formed by stacking and selfassembling thin nanosheets.This is consistent with the SEM image in Fig.1d.It is also found that the edge of Ni-Mo-S nanosheets is curly.This phenomenon may be attributed to the instability of the ultra-thin nanosheet,which forms a closed structure by rolling up to eliminate dangling bonds at the edges and minimizing its surface energy.As can be seen from the TEM pattern of Ni-Mo-S/ZnIn2S4composite in Fig.2c,the accumulation of Ni-Mo-S/ZnIn2S4nanosheets is relatively loose,which may be due to the mutual blocking of the formation and stacking of Ni-Mo-S and ZnIn2S4nanosheets during the synthesis process.The intersecting lattice fringes in the HRTEM image (Fig.2d) confirm the close contact between the Ni-Mo-S nanosheets and the ZnIn2S4nanoflowers.The lattice spacing of 0.35 nm corresponds to plane (102) of ZnIn2S4.The lattice spacing of 0.27 nm and 0.2 nm matched well with the (100) plane of MoS2and (111) plane of Ni,respectively,indicating that Ni is successfully doped into MoS2to form Ni-Mo-S.The map of EDX elements in Fig.2e shows that Mo,Ni,S,In and Zn are uniformly distributed in the whole Ni-Mo-S/ZnIn2S4composite.Therefore,it is reasonable to believe that two-dimensional Ni-Mo-S/ZnIn2S4heterostructures with close interfacial contact have been constructed.

Fig.2.TEM images of (a) ZnIn2S4 and (b) Ni-Mo-S (Ni:Mo=1:2).(c) TEM and(d) high resolution TEM images of Ni-Mo-S/ZnIn2S4 (Ni:Mo=1:2) composites.(e)The corresponding EDX mapping images of Mo,Ni,S,In and Zn of Ni-Mo-S/ZnIn2S4(Ni:Mo=1:2) composites.
The composition and chemical states of the prepared Ni-Mo-S/ZnIn2S4composites were measured and analyzed by X-ray photoelectron spectroscopy (XPS).According to the full spectrum shown in Fig.3a,the prepared catalyst is composed of S,In,Zn,Mo and Ni elements.Figs.3b-f show the high-resolution XPS spectra of different elements in the Ni-Mo-S/ZnIn2S4composite.In the XPS signal of composite In 3d (Fig.3b),the peaks of 444.8 eV and 452.4 eV are attributed to In 3d5/2and In 3d3/2,respectively,indicating the presence of In3+[43].The peaks at 1021.5 eV and 1044.6 eV observed in Fig.3c correspond to Zn 2p3/2and Zn 2p1/2respectively,indicating that Zn is in+2 oxidation state [44].In the spectrum of Ni 2p (Fig.3d),the binding energy is located in two strong peaks of 857.1 eV and 870 eV,which are derived from the Ni 2p3/2and Ni 2p1/2corresponding to the Ni2+and Ni-S bonds in Ni-Mo-S,respectively [45].The XPS spectrum of S 2p can be convolved into two peaks (Fig.3e) with the binding energy located at 161.6 eV and 163.3 eV,which are respectively assigned to S 2p1/2and S 2p3/2,corresponding to the −2 reduction state of S [46].In Fig.3f,the peaks with binding energies of 228.2 eV and 232.3 eV are allocated to Mo 3d5/2and Mo 3d3/2,respectively,confirming+4 oxidation state of Mo [47].In addition,the peak value at 225.6 eV corresponds to the transition of S 2s [18].The above XPS results confirm the presence of S,In,Zn,Mo and Ni elements,which is consistent with the chemical composition of Ni-Mo-S/ZnIn2S4.
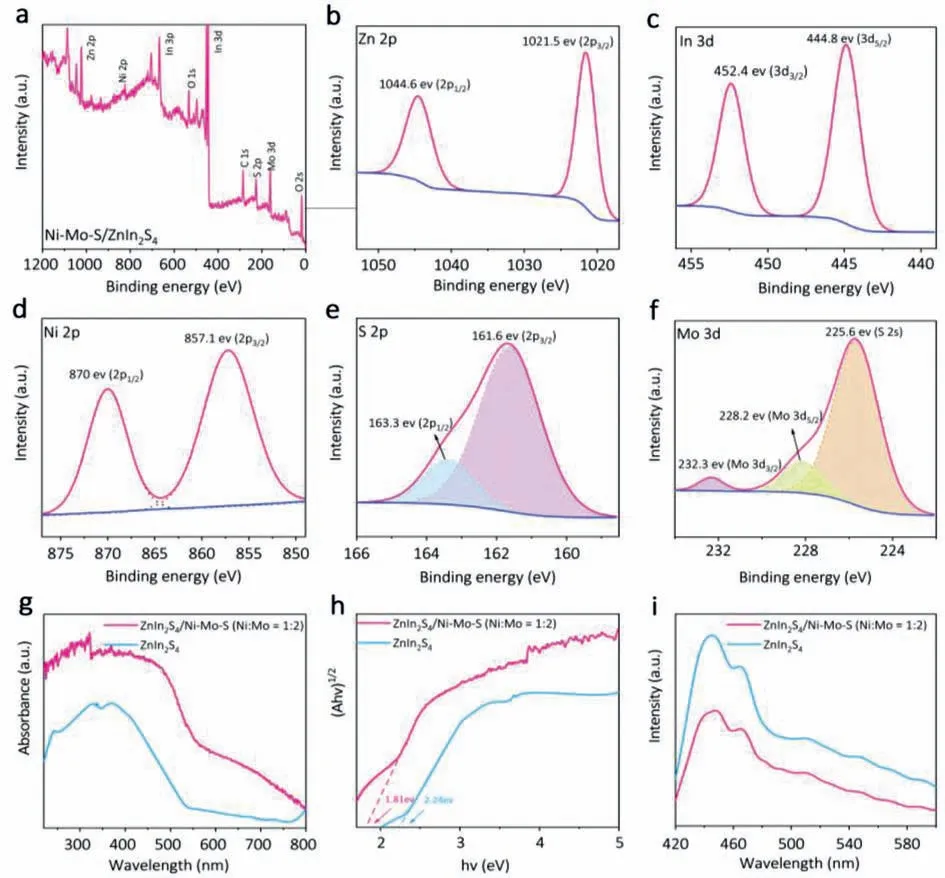
Fig.3.(a) Survey spectra of ZnIn2S4/Ni-Mo-S (Ni:Mo=1:2) composites photocatalyst.High resolution XPS imagesof (b) Zn 2p,(c) In 3d,(d) Ni 2p,(e) S 2p,(f) Mo 3d.(g)UV–vis DRS spectra,(h) Tauc plots,(i) photoluminescence (PL) spectra of ZnIn2S4 and ZnIn2S4/Ni-Mo-S (Ni:Mo=1:2).
The optical absorption characteristics of ZnIn2S4/Ni-Mo-S(Ni:Mo=1:2) composites were measured by UV-visible diffuse reflectance spectroscopy (DRS) (Fig.3g).The absorption band of pure ZnIn2S4ends in the visible light region around 540 nm,showing a weak light absorption characteristic.ZnIn2S4/Ni-Mo-S nanocomposites show a wider range of light absorption and stronger absorption intensity.ZnIn2S4/Ni-Mo-S has a characteristic absorption corresponding to ZnIn2S4in the region of 500–700 nm,and the enhanced absorption is related to the absorption of Ni-Mo-S.Tauc plots of ZnIn2S4and ZnIn2S4/Ni-Mo-S were plotted based on the results of UV-vis DRS,as shown in Fig.3h.In general,the semiconductor band gap energy (Eg) is estimated by the following equation: (αhν)1/n=A(hv–Eg).The band gap energies of ZnIn2S4,ZnIn2S4/Ni-Mo-S are calculated to be 2.24 eV and 1.81 eV,respectively.The narrower band gap of ZnIn2S4/Ni-Mo-S composites is conducive to charge separation.This shows the advantages of ZnIn2S4/Ni-Mo-S composite materials,which can make ZnIn2S4more effective use of visible light and enhance the photocatalytic performance.In order to study charge migration in the composite photocatalyst,we measured the photoluminescence (PL) spectra of pure ZnIn2S4and ZnIn2S4/Ni-Mo-S composites,as shown in Fig.3i.It is well known that the stronger the intensity of PL peak is,the greater the probability of recombination of electron holes in the corresponding photocatalyst [48].The PL peak of ZnIn2S4/Ni-Mo-S composite is significantly lower than that of pure ZnIn2S4,which proves that ZnIn2S4/Ni-Mo-S can effectively inhibit the recombination of electron hole pairs,which is helpful to the separation of electron holes and improve photocatalytic performance.
Emerging experimental results and theoretical predictions suggest that MoS2is a promising alternative to inexpensive,earthabundant and visible responsive catalysts [49,50].However,catalytic activity is limited by the low active site exposure and the high kinetic energy barrier of water decomposition in EY/TEOA and Na2S/Na2SO3system.Accordingly,the photocatalytic hydrogen evolution performance of Ni-doped MoS2as cocatalyst in lactic acid,EY/TEOA and Na2S/Na2SO3systems were evaluated by photocatalytic hydrogen generation.
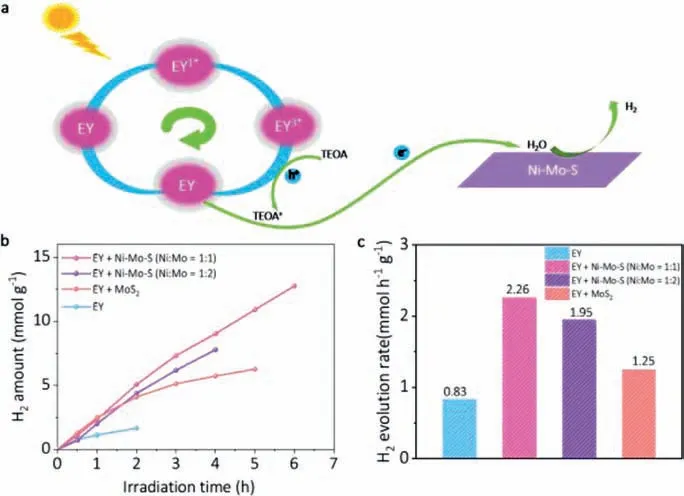
Fig.4.(a) Diagram of carrier migration process of Ni-Mo-S in EY/TEOA aqueous solution (pH 7) under visible light irradiation.(b,c) Comparison of photocatalytic H2 evolution activities over EY,MoS2/EY,Ni-Mo-S/EY (Ni:Mo=1:2) and Ni-Mo-S/EY(Ni:Mo=1:1)
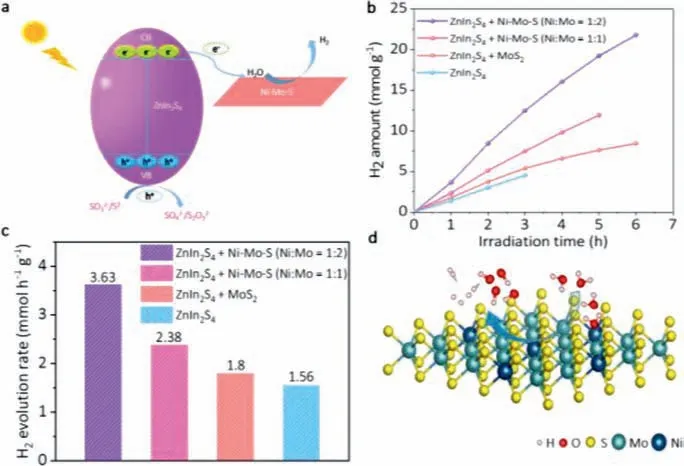
Fig.5.(a) Schematic of the charge carrier migration process of Ni-Mo-S/ZnIn2S4 composites in Na2S/Na2SO3aqueous solution (pH 13.2) under visible light irradiation.(b,c) Comparison of photocatalytic H2 evolution activities over ZnIn2S4,MoS2/ZnIn2S4,Ni-Mo-S/ZnIn2S4 (Ni:Mo=1:2) and Ni-Mo-S/ZnIn2S4 (Ni:Mo=1:1).(d) Atomic structure diagram of Ni-Mo-S nanosheets for H2O dissociation.
In the EY/TEOA system,we discussed the formation mechanism of hydrogen generation using EY as sensitizer,TEOA hole scavenger and Ni-Mo-S nanosheet as catalyst.Fig.4a shows the schematic diagram of the electron transfer process of EY/Ni-Mo-S under visible light irradiation.The reaction is triggered by the light excitation of EY to produce the triple excited state (EY3∗).Then,through reduction quenching,electrons are extracted from TEOA to produce free radical EY−.Electrons are easily transferred from highly reductive EY−to Ni-Mo-S nanosheets to reduce H+generated by H2O decomposition to form an excited state H∗.Two excited states H∗react with each other to produce H2.In Fig.4b,the hourly H2dynamic productivity of different photocatalytic materials in the process of HER was tracked by a constant online detection system.It can be seen that the hydrogen evolution performance was significantly improved after the addition of cocatalyst in EY.With the increase of the ratio of Ni:Mo in Ni-Mo-S,the amount of hydrogen evolution increased.When the value of Ni:Mo is equal to 1:1,the catalytic performance is the best,and the maximum H2production rate reaches 2.26 mmol h−1g−1(Fig.4c).Therefore,Ni doped MoS2nanosheets (Ni-Mo-S) can promote photocatalytic hydrogen evolution in EY/TEOA aqueous solution.
In the Na2S/Na2SO3system,the slow HER kinetics of MoS2catalyst is improved by the design of hydrolysis dislocation by doping Ni atoms into MoS2nanosheets.The introduced Ni site can not only effectively reduce the energy barrier of initial water dissociation,but also promote the desorption of OH−[33,51].Fig.5a shows the Ni-Mo-S/ZnIn2S4composite under visible light irradiation,with Na2S/Na2SO3as the hole sacrifice agent,and the photogenerated electrons are excited from valence band (VB)of ZnIn2S4to conduction band (CB).Since the CB of ZnIn2S4is more negative than that of Ni-Mo-S,the photogenerated electrons on ZnIn2S4CB can be quickly transferred to Ni-Mo-S,which promotes the separation of photogenerated electron-hole pairs and inhibits the recombination.Under the action of electrons,the H+of H2O decomposition is reduced to the excited state H∗,and the two excited states H∗combine to produce H2.Fig.5b shows the H2dynamic productivity per hour of different photocatalytic materials in the HER process.It can be found that when the Ni:Mo value of co-catalyst Ni-Mo-S nanosheets is 1:2,the hydrogen evolution performance is the best,and the maximum H2production rate can reach 3.63 mmol h−1g−1(Fig.5c).In Fig.5d,the doping of Ni in MoS2not only activates the S atoms around it,but also accelerates the dissociation of water and promotes the desorption of the formed OH−,which greatly improves the activity of hydrogen production.Therefore,Ni-Mo-S as co-catalyst in Na2S/Na2SO3aqueous solution can also improve the efficiency of hydrogen production.
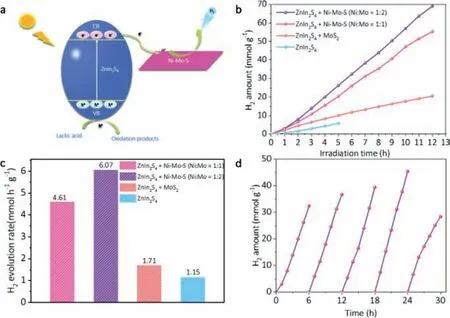
Fig.6.(a) Schematic of the charge carrier migration process of Ni-Mo-S/ZnIn2S4 composites in lactic acid aqueous solution (pH 2.5) under visible light irradiation.(b,c) Comparison of photocatalytic H2 evolution activities over ZnIn2S4,MoS2/ZnIn2S4,Ni-Mo-S/ZnIn2S4 (Ni:Mo=1:2) and Ni-Mo-S/ZnIn2S4 (Ni:Mo=1:1).(d) Cycling test of catalytic H2 generation from Lactic acid solution over the Ni-Mo-S/ZnIn2S4 (Ni:Mo=1:2) nanocomposite.
Then we studied the photocatalytic hydrogen evolution performance of Ni-Mo-S nanosheets as co-catalyst in lactic acid system.Fig.6a shows Ni-Mo-S/ZnIn2S4composite material under visible light irradiation with lactic acid as sacrificial agent.Photogenerated electrons excited by ZnIn2S4are transferred to Ni-Mo-S nanosheets.Under the action of electrons,H+in solution is reduced to the excited state H∗,which then combines with H+in solution to produce H2.Fig.6b shows the kinetic process of H2production by different samples at HER per hour.Pure ZnIn2S4photocatalyst shows poor photocatalytic H2production activity with a hydrogen production rate of 1.15 mmol h−1g−1(Fig.6c).Compared with Fig.5,the hydrogen production rate of pure ZnIn2S4in the Na2S/Na2SO3system is higher (1.56 mmol h−1g−1),indicating that Na2S/Na2SO3is more suitable for the electron donor in the ZnIn2S4system.When ZnIn2S4is combined with MoS2nanosheet,it can be observed that the H2yield is increased compared with that of pure ZnIn2S4,because MoS2acts as a cocatalyst to accelerate the separation of electron-hole pairs.However,due to the existence of MoS2inert substrate surface,the hydrogen evolution performance is still limited.The ability of H2generation is significantly improved when the Ni doped MoS2nanosheets (Ni-Mo-S) were coupled with ZnIn2S4to form heterogeneous composite photocatalyst.When the Ni:Mo value of co-catalyst Ni-Mo-S nanosheets is 1:2,the hydrogen evolution performance is the best.The maximum hydrogen production rate of H2can reach 6.07 mmol h−1g−1,which is 5.28 times that of pure ZnIn2S4.
Therefore,the Ni-Mo-S co-catalyst in lactic acid system can greatly enhance the efficiency of hydrogen production.Table S1(Supporting information) provides the photocatalytic hydrogen evolution activity of some recently studied ZnIn2S4-based photocatalysts.
It is well known that the stability of catalysts plays an important role in practical applications.The hydrogen evolution reaction of the optimized Ni-Mo-S/ZnIn2S4(Ni:Mo=1:2) photocatalyst is carried out to evaluate its photocatalytic stability.As shown in Fig.6d,5 cycles were tested,each cycle being 6 h.In the first 4 cycles,the hydrogen production rate of each cycle is gradually improved,and the hydrogen production performance is getting better and better.The reduced activity of the photocatalyst in the fifth cycle may be due to the consumption of sacrificial agents.However,after the fifth cycle,the hydrogen production efficiency is still about 88% of the initial efficiency,and the surface Ni-Mo-S/ZnIn2S4photocatalyst still retains its original activity to a large extent after 30 h reaction.Therefore,the prepared Ni-Mo-S/ZnIn2S4(Ni:Mo=1:2) as a photocatalyst in acidic medium has high stability and good recovery,which is conducive to large-scale production and application.
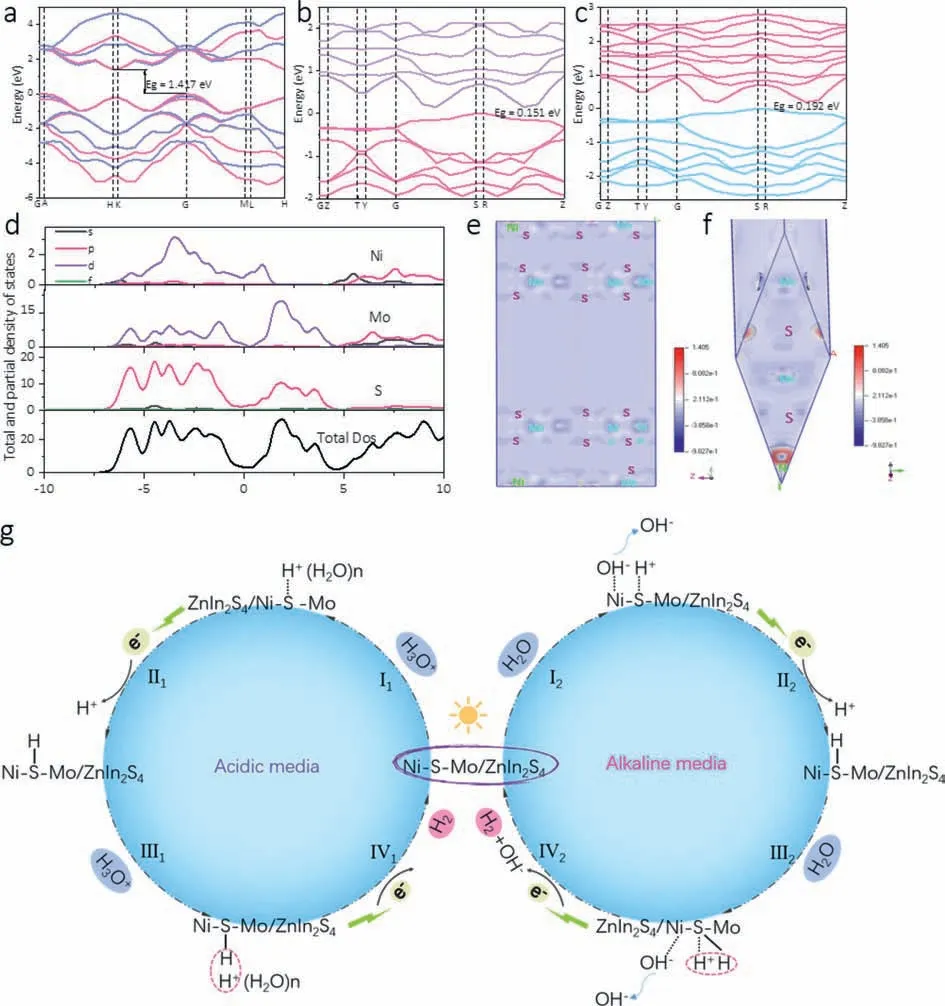
Fig.7.The band structure of (a) pure MoS2,(b) Ni replaces the edge Mo position of MoS2.(c) Ni replaces the middle Mo position of MoS2.(d) The partial and total density of states.(e,f) The charge density difference of MoS2 where the edge Mo is replaced by Ni.(g) Correlation of HER mechanism in lactic acid and Na2S/Na2SO3 system.Left:HER mechanism of Ni-Mo-S/ZnIn2S4 in lactic acid aqueous solution;Right: HER mechanism of Ni-Mo-S/ZnIn2S4 in Na2S/Na2SO3 aqueous solution.
Therefore,through the above three diverse systems,we experimentally confirm that the introduction of Ni site in MoS2greatly enhance HER activity.
In order to analyze the electronic structure of Ni-Mo-S,the energy band structure,density of states and charge density difference are calculated.In Figs.7a-c,the band gaps of the pure MoS2and Ni substituting for the edge Mo sites of MoS2and the middle Mo sites of MoS2are calculated respectively.Fig.S1 (Supporting information) further shows its atomic structure.The figure shows that the band gap significantly decreases after the introduction of Ni,from 1.471 eV of pure MoS2to 0.151 eV of minimum band gap.The Significant reduction of the band gap is conducive to rapid charge transfer and improved HER efficiency.
The partial and total density of states of Mo sites at the edge of Ni substituted MoS2are calculated in Fig.7d.It can be clearly seen that the atomic contribution of Ni 3d is the main contribution to the top of the valence band and the bottom of the conduction band of Ni-Mo-S.The charge density difference shows the charge transfer between atoms.In order to understand the electron transfer process in Ni-Mo-S,the charge density difference is calculated,as shown in Figs.7e and f.The results show that more electrons are obtained on the Ni site than on the Mo site.It is theoretically proved that the performance of HER can be greatly improved by introducing Ni into MoS2.
Fig.7g illustrates the optimized HER mechanism of Ni-Mo-S/ZnIn2S4in lactic acid and Na2S/Na2SO3system.Under visible light irradiation,the hydrated ions and water are preferentially adsorbed to the Ni-doped activated S sites (step I1and step I2).In lactic acid aqueous solution,the adsorbed H3O+is reduced on the surface by an electron excited from ZnIn2S4to form the adsorbed H atom (Step II1).Finally,another proton from the adjacent H3O+reacts with the first adsorbed H atom to produce H2(Step III1-IV1).In Na2S/Na2SO3aqueous solution,the mechanism of overcoming the kinetic barrier of hydrolysis dissociation has been found.We believe that water splitting occurs at the Ni site,leading to the fracture of the HO-H bond,forming adsorbed OH−and H+,which are adsorbed at the Ni and S sites,respectively.However,the adsorbed OH−at the Ni site can easily break free and return to the medium,while the adsorbed H+at the S site is reduced by an electron to form the adsorbed H atom (Step II2).The last two adsorbed H atoms react to form H2(Step III2-IV2).Therefore,it is theoretically confirmed that HER dynamics can be greatly improved by introducing Ni sites.
In conclusion,we have shown that the catalytic activity of Ni-Mo-S can be greatly improved in the multi-reaction systems,whether as catalyst or co-catalyst.Firstly,we prepared Ni-Mo-S nanosheets by a simple hydrothermal method.It can be found by TEM that Ni is successfully doped into MoS2.Secondly,in the experiment,the experimental data of the multi-reaction systems proved that Ni-Mo-S as a catalyst has significantly enhanced catalytic hydrogen evolution performance and excellent catalytic stability.Finally,our theoretical calculations show that the doping of Ni plays a crucial role in the Ni-Mo-S nanostructure.Ni-Mo-S has a narrower band gap,and the Ni in Ni-Mo-S has the main atomic contribution at the top of valence band and the bottom of conduction band,and has more electrons at the Ni site.The above experiments and theories have confirmed the key role of Ni doped MoS2in improving its catalytic performance.These findings in the current work provide some new clues for the development of largescale production,multi-reaction systems and inexpensive photocatalyst.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work,there is no professional or other personal interest of any nature or kind in any product,service and/or company that could be construed as influencing the position presented in,or the review of,the manuscript entitled.
Acknowledgments
The authors acknowledge financial support from the National Natural Science Foundation of China (Nos.11974188,11304159),the China Postdoctoral Science Foundation (Nos.2021T140339,2018M632345),the Qing Lan Project of Jiangsu Province,the Natural Science Foundation of Jiangsu Province (Nos.BK20201381,BK20161512),and NUPTSF (No.NY218022).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.103.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
