Modulation effect in adjacent dual metal single atom catalysts for electrochemical nitrogen reduction reaction
Xiaonan Zheng,Yang Liu,Yu Yan,Xiaoxiao Li,Yuan Yao
MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage &State Key Laboratory of Advanced Welding and Joining,School of Chemistry and Chemical Engineering,Harbin Institute of Technology,Harbin 150080,China
Keywords:Nitrogen reduction reaction Electrocatalysts Density functional theory Dual metal single atom catalysts Modulation effect
ABSTRACT Nitrogen reduction reaction (NRR) is a clean mode of energy conversion and the development of highly efficient NRR electrocatalysts under ambient conditions for industrial application is still a big challenge.Metal-nitrogen-carbon (M-N-C) has emerged as a class of single atom catalyst due to the unique geometric structure,high catalytic activity,and clear selectivity.Herein,we designed a series of dual metal single atom catalysts containing adjacent M-N-C dual active centers (MN4/M’N4-C) as NRR electrocatalysts to uncover the structure-activity relationship.By evaluating structural stability,catalytic activity,and selectivity using density functional theory (DFT) calculations,5 catalysts,such as CrN4/M’N4-C (M’=Cr,Mn,Fe,Cu and Zn),were determined to exhibit the best NRR catalytic performance with the limiting potential ranging from −0.64 V to −0.62 V.The CrN4 center acted as the main catalytic site and the adjacent M’N4 center could enhance the NRR catalytic activity by modulation effect based on the analysis of the electronic properties including the charge density difference,partial density of states (PDOS),and Bader charge variation.This study offers useful insights on understanding the structure-activity relationship of dual metal single atom catalysts for electrochemical NRR.
Ammonia (NH3) is of great significance and widely used in agriculture,industry,and sustainable energy conversion [1,2].In industry,Haber–Bosch method is currently used for large-scale ammonia production at high temperature and high pressure over Fe-based or Ru-based catalysts,which requires high energy input and emits a large amount of greenhouse gases [3,4].Therefore,it is urgentlyneeded to develop green and sustainable ways for NH3production under mild conditions [5–8].Among the NH3synthesis methods,electrochemical nitrogen reduction reaction (NRR) using water as the hydrogen source offers a promising way to replace the high-energy-consuming and environment-polluting Haber–Bosch method [9].
However,it is difficult to cleave and dissociate the N≡N bond due to its high total bond energy (941 kJ/mol) [10].For this reason,the performance of the NRR electrocatalyst has very big upgrade space due to a low yield rate of NH3and faradaic efficiency(FE) [11–13].Multiple materials including pure metals [14,15],alloys [16],metal compounds [17,18]and nonmetals [19,20]have been applied for experimental studies of NH3synthesis in both theory and experiment.Single-atom catalysts (SACs),isolated metal atoms anchored to supports,have been applied in many fields such as oxygen reduction reaction (ORR) [21],CO2reduction reaction(CO2RR) [22]and oxidation of formaldehyde [23,24]due to the 100% atomic utilization,high activity and selectivity,and durable stability [25–27].In a series of single metal atom catalysts,many metal-nitrogen-carbon (M-N-C,M=Fe,Co,Ni,Mn,Mo,Y,Sc,etc.)have successfully developed for electrocatalysis [28–31].In particular,inspired by the natural metalloenzyme called cytochrome c oxidase (CcO) with the adjacent Cu and Fe sites [32–34],several dual metal single atom catalysts with adjacent M-N-C dual active centers have been synthesized recently and exhibited excellent stability and catalytic performance [35–40].For example,a highly dispersed Fe–Cu dual-atom nanozyme has been successfully constructed to mimic Cytochrome c oxidase for catalyzing ORR [37].The dual-metal catalysts with neighboring Fe-N4-C and Co-N4-C active centers are also reported as efficient ORR catalysts [36,39].In addition,a dual metal single atom catalyst consisted of Cu-N4and Zn-N4on the N-doped carbon support was prepared and showed high ORR activity [35].
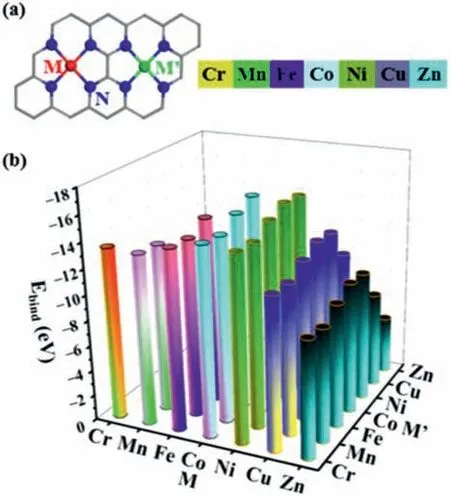
Fig.1.(a) The structures of adjacent dual metal active sites on MN4/M’N4-C and(b) the calculated Ebind values.
As far as we know,there is no report of dual-metal catalysts for the NRR by now,and the in-depth analysis and understanding of structure-property relationship for the neighboring M-NC catalysts are also insufficient.Inspired by the successful synthesis of adjacent M-N-C catalysts and their potential catalytic activity,we designed a series of non-precious metal-based neighboring M-N-C catalysts (denoted as MN4/M’N4-C),and employed the density functional theory (DFT) method to explore the NRR activity.Firstly,the stability of catalysts is evaluated,and NRR selectivity was investigated by considering∗N2/∗H adsorption.According to the adsorption configuration of N2,we then explore possible reaction pathways in the NRR process and screen promising catalysts for the NRR.Finally,the electronic structure analysis is performed to further understand the interaction on the adjacent dual metal single atom to achieve good NRR catalytic performance.
Seven non-precious metal atoms (Cr,Mn,Fe,Co,Ni,Cu and Zn)were selected to anchor on N-doped graphene in pairs,constructing 28 MN4/M’N4-C catalysts in the present study as shown in Fig.1a,motivated by that the corresponding M-N-C catalysts with N4-coordinated structure have been synthesized [41,42]and this may contribute to the development of adjacent M-N-C dual single atom catalysts.In the optimized geometries,each metal atom bonds with four surrounding N atoms on the graphene substrate with bond length of 1.85∼1.97 ˚A (Table S1 in Supporting information).In particular,the Cu-N and Zn-N bond lengths in CuN4/ZnN4-C were calculated to be 1.91 and 1.94 ˚A (Table S1),which are in agreement with the experimental results from the extended X-ray absorption fine structure (EXAFS) measurement for Cu/Zn-NC with the Cu-N and Zn-N bond lengths of 2.01 ˚A [35].The dual metal single atom centers of M-N-C and M’-N-C were located in an adjacent position with the distance between two metal atoms ranging from 4.96 ˚A to 5.10 ˚A (Table S1),consistent with the corresponding experimental results that the distance between the two metal atoms is around 5.0 ˚A for the synthesized MN4/M’N4-C catalysts such as CuN4/ZnN4-C,FeN4/CuN4-C and FeN4/CoN4-C [35–37].These show that our computational models for the as-designed catalysts are reliable and reasonable.
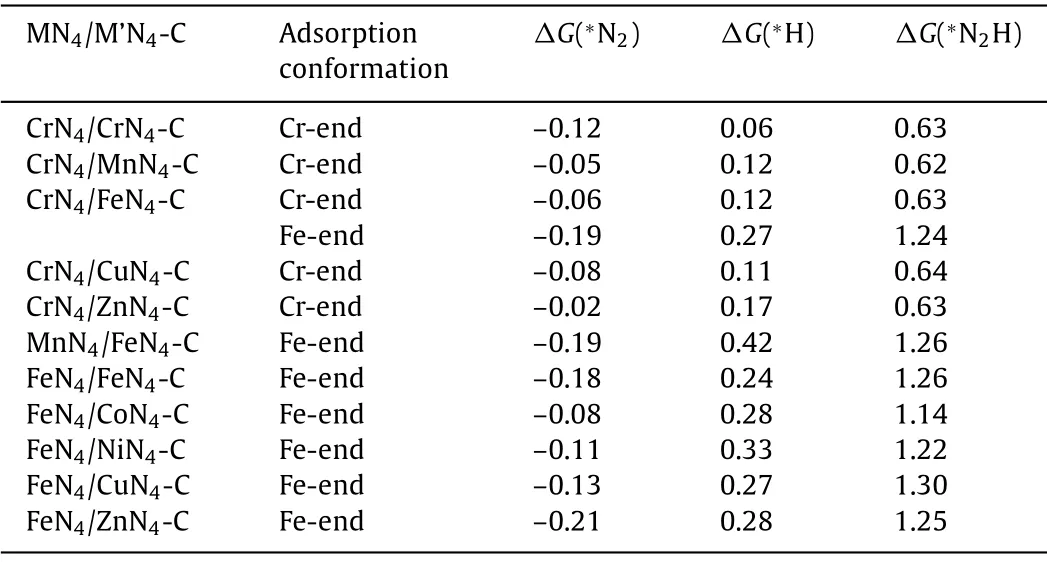
Table 1 The adsorption configurations of N2 molecule and the adsorption energies of N2 molecule,H and N2H on 11 MN4/M’N4-C (The ‘∗’donates the atom adsorbed on the surface) in eV.
Based on the optimized geometries of the as-designed catalysts,we then evaluate their stabilities by calculating the binding energies (Ebind) using equation:Ebind=E(MN4/M’N4-C)–E(NC)–E(M)–E(M’),whereE(MN4/M’N4-C) andE(NC) represent the electronic energies of catalyst andN-doped graphene substrate,respectively;E(M) andE(M’) are the electronic energies of M and M’atoms,respectively.According to this definition,a lowerEbindindicates a higher thermodynamics stability of MN4/M’N4-C.As shown in Fig.1b,one can see that theEbindvalues were all negative ranging from–15.72 eV to–4.48 eV,indicating neighboring dual metal single atom can be stably anchored in the N-doped graphene.In addition,they have the similar stabilities as the experimentally synthesized FeN4/CoN4-C,FeN4/CuN4-C and CuN4/ZnN4-C which have the binding energies calculated in this study with the values of–15.33,–12.54 and–7.45 eV,respectively.It may imply that the asdesigned catalysts could also be synthesized in future.
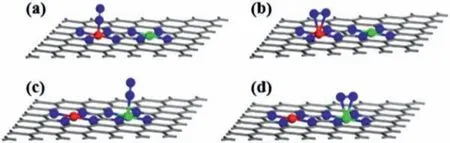
Fig.2.The N2 adsorbed on M atom by (a) end-on and (b) side-on configurations.The N2 adsorbed on M’atom by (c) end-on and (d) side-on configurations.
We next investigated the adsorption of N2molecule on MN4/M’N4-C surfaces.As shown in Fig.2,one can see that N2molecule can adsorb on the surface in perpendicular end-on or parallel side-on configurations.However,two metal atoms locate so far with the distance of around 5 ˚A so that it is not suitable for the formation of the bridge conformation in which two N atoms in N2molecule adsorb on different metal atoms,respectively.Based on the calculatedΔGvalues of N2adsorption listed in Table S2(Supporting information),we found that the end-on configurations are more energetically favorable than the side-on configurations for both M and M’sites.Therefore,the 11 MN4/M’N4-C with negativeΔGvaluesviaN2end-on adsorption configuration are summarized in Table 1.Although the adsorption ability of N2on the 11 MN4/M’N4-C was relatively weak (–0.21 eV to–0.02 eV),it was still an exothermic reaction which could proceed spontaneously.In contrast,the remaining catalysts will be excluded from following study because the adsorption and activation of N2on these catalysts hardly take place at room temperature.
As well known,hydrogen evolution reaction (HER) is the key competition reaction toward NRR,thus,the competitive adsorption between H and N2on the catalysts was studied by comparing the changes of Gibbs free energies (ΔG(∗H) andΔG(∗N2)).The results listed in Table 1 showed that theΔG(∗N2) are all negative,whereas theΔG(∗H) values are all positive.It means that N2is preferentially adsorbed onto the screened 11 MN4/M’N4-C rather than H,preventing the accumulation of H-adatoms and exhibiting good selectivity for electrochemical NRR.
The first hydrogenation step (∗N2+H++e−→∗N2H) is usually considered as the potential determining step (PDS) for electrochemical NRR,and high-activity catalysts should haveΔG(∗N2H)values of less than theΔGmaxof the well-established Ru(0001)stepped surface (0.98 eV) [12],which was set as the criterion for metal-based catalysts due to the highest NRR theoretical activity among bulk metal surfaces [43,44].As shown in Table 1,5 CrN4/M’N4-C (M’=Cr,Mn,Fe,Cu and Zn) presented theΔG(∗N2H)values of about 0.60 eV,which suggests that they meet the requirements above and will be further studied and discussed.
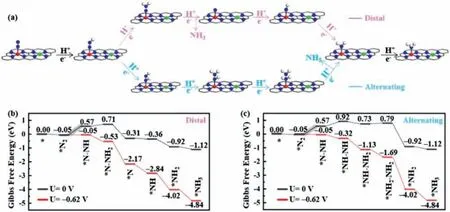
Fig.3.(a) Schematic representation of distal and alternating pathways for NRR in end-on configuration.Gibbs free energy diagrams of the NRR at zero potential (blake lines)and an applied potential (red lines) via the (b) distal and (c) alternating pathway on CrN4/MnN4-C.
Since N2molecule prefers the end-on adsorption configuration on the 5 CrN4/M’N4-C,the typical NRR reaction pathways including distal and alternating pathways were considered to reveal the catalytic mechanism (Fig.3a).In the distal pathway,the first three proton-electron (H++e−) pairs are preferentially added on the distal N atom,leading to the generation of the first NH3molecule,and then another three proton-electron (H++e−) pairs attack the remaining N atom to release the second NH3.In the alternating pathway,six proton-electron (H++e−) pairs alternately hydrogenate two N atoms.The produced NH3can be easily protonated to NH4+and released into solution under the electrochemical conditions [45,46],so the further protonation of∗NH3into NH4+was not considered.
By taking the CrN4/MnN4-C as an example,as shown in Figs.3b and c,it can be seen that the first two steps of the alternating and distal pathways,namely N2adsorption and∗N2H formation,were the same.TheΔG values of the first two steps were–0.05 and 0.62 eV,respectively.After adsorbed N2is hydrogenated,the generated∗N2H intermediate can proceedviadistal or alternating pathways.In the distal pathway (Fig.3b),the next hydrogenation to form∗N-NH2is slightly endothermic with a free energy change of 0.14 eV.One can see that∗N-NH2can be hydrogenated to release the first NH3to form∗N with free energy change of −1.02 eV.The free energy changes for the continuous hydrogenations of∗N to form∗NH,∗NH2and∗NH3were calculated to be −0.05,−0.56 and −0.20 eV,respectively.Regarding the alternating pathways on CrN4/MnN4-C (Fig.3c),the second hydrogenation on the other nitrogen to form∗NH-NH will proceed after overcoming a positive free energy change of 0.35 eV.In the subsequent steps,the intermediates∗NH-NH2and∗NH2-NH2are formed with the free energy change values of −0.19 and 0.06 eV,respectively.A negative free energy change of −1.71 eV is obtained with the formation of the first NH3.Finally,the second NH3molecule can be formed with a downhill step of −0.20 eV.Therefore,the formation of∗N2H species is the PDS due to the maximumΔGvalues(0.62 eV) among all the elementary steps for CrN4/MnN4-C.WhenU=−0.62 V is applied,all elementary reactions become downhill,and the whole electrochemical NRR processes turn to be spontaneous.
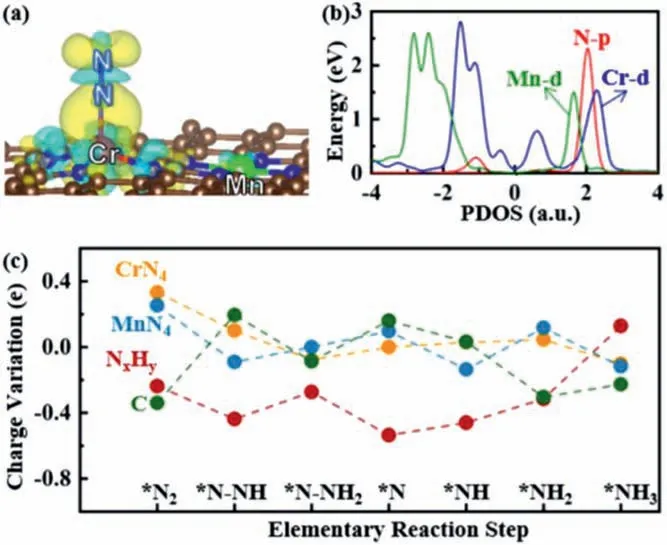
Fig.4.(a) Charge density difference maps of N2 adsorption on CrN4/MnN4-C,where the isosurface value is set to be 0.005 e/˚A3.The positive and negative charges are shown in cyan and yellow,respectively.(b) Partial density of states (PDOS) of N2 adsorption on CrN4/MnN4-C.(c) The Bader charge variation of the CrN4/MnN4-C along distal pathway.
According to the detailedΔGvalues of CrN4/CrN4-C,CrN4/FeN4-C,CrN4/CuN4-C and CrN4/ZnN4-C in Table S3 (Supporting information),we can find that the free energy change of PDS for these catalysts are 0.63,0.63,0.64 and 0.63 eV,respectively.Hereby,the computedULfor 5 CrN4/M’N4-C is from−0.64 V to −0.62 V,which are much lower than that for recently reported ruthenium SAC Ru1-N3(−0.73 V) with the yield rate of 120.9 μgNH3mgcat.−1h−1[47].TheULresults also indicate the better NRR activity of 5 CrN4/M’N4-C by comparing with the single atom catalysts with the same metal atom such as Cr anchored on defective graphene (−0.98 V) [44],single Mn-N4sites anchored on porous carbon (−1.04 V) [48],Fe-N4/graphene (−1.35 V) [49],Cu on N-doped carbon (−1.85 V) [50].Besides,considering that NRR is conducted in aqueous electrolytes,the solvation effect has been also studied on CrN4/M’N4-C (M’=Cr,Mn,Fe,Cu and Zn) along distal pathway.As shown in Fig.S1 (Supporting information),the solvation effect could reduce the limiting potential in the range of−0.49 eV to −0.37 V.Here,CrN4/M’N4-C (M’=Cr,Mn,Fe,Cu and Zn) is expected as promising candidates for electrochemical NRR.
To clarify the origin of the catalytic activity of the 5 CrN4/M’N4-Cs (M’=Cr,Mn,Fe,Cu and Zn),we first analyzed corresponding charge density difference (Fig.4a) and partial density of states(Fig.4b) of the adsorbed N2molecule on CrN4/MnN4-C as an example.The charge density difference results confirmed that both positive and negative charges accumulate around the adsorbed N2molecule and Cr atom of CrN4/MnN4-C,which is beneficial to promote the “acceptance–donation” process [5,51]that metal atom donates electrons to the antibonding orbitals of N2and accepts lone-pair electrons of N2.In addition,it is noted that a small part of positive charge accumulated on Mn atom,which suggests that Mn atom probably plays a role in electron transfer to Cr atom due to the so-called modulation effect [38]and promotes the N2activation on Cr active site.The modulation effect of CrN4/MnN4-C,where Cr atom is the only active center,while Mn atom is considered as the modulator,is completely different from the previously reported synergetic effect on double-atom catalysts (DACs) such as (Fe,Co)/CNT [52]and Ni/Fe-N-C [53],in which binary metal is demonstrated as one active center to catalyze reactions.Additionally,the computed partial density of states (PDOS) of N2adsorption on CrN4/MnN4-C showed that the hybridization mainly occurred between Cr d-orbital and N2p-orbital,and there was a small part of orbital overlap between Mn d-orbital and N2p-orbital.These results clarified the origin of the activation of N2molecule on CrN4/MnN4-C.To deeply understand the catalytic mechanism,the charge transfer variations on the basis of Bader charge differences of all reaction intermediates NxHyadsorbed on CrN4/MnN4-Cviathe distal pathway are summarized in Fig.4c.Obviously,the charge was transferred from CrN4to the adsorbed NxHyspecies for most of the elementary reaction steps.Even though the NxHyintermediates were mainly located at the CrN4active site,the neighbored MnN4acted as a reservoir to store or release electrons when needed.Consequently,the modulation effect between the dual metal single atoms is effective in electron transfer,leading to the activation of N2and then promotes the subsequent hydrogenation step of NRR.
In conclusion,a series of energetically stable adjacent dual metal single atom supported on N-doped graphene (MN4/M’N4-C) are designed for electrochemical NRR by means of DFT calculations.Based on the results of N2adsorption free energy,and Gibbs free energy change of NRR,it was predicted that 5 CrN4/M’N4-C(M’=Cr,Mn,Fe,Cu and Zn) exhibited promising NRR activity with the limiting potential of −0.64 V to −0.62 V.The modulation effect is observed in the adjacent dual metal single atom catalysts based on the analysis of charge distribution and PDOS.This work not only provides new insight for developing neighboring dual single atom catalysts at atomic level,but also highlights the importance of the modulation effect between the multi-active centers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the open project of State Key Laboratory of Advanced Welding and Joining,Harbin Institute of Technology (No.AWJ-19M07) and the National Natural Science Foundation of China (No.U2067216).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.102.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
