Light-mediated CO2-responsiveness of metallopolymer microgels
Xiofei Wng,Xuezhen Lin,Huijun Qiu,Jind Xie,Zhengyu Lu,Yusong Wng,Weiti Wu,d,∗
a State Key Laboratory for Physical Chemistry of Solid Surfaces,Collaborative Innovation Center of Chemistry for Energy Materials,The Key Laboratory for Chemical Biology of Fujian Province,and Department of Chemistry,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen 361005,China
b School of Materials Science and Engineering,Xiamen University of Technology,Xiamen 361024,China
c Hefei National Laboratory for Physical Sciences at the Microscale,University of Science and Technology of China,Hefei 230026,China
d School of Chemistry and Chemical Engineering,Ningxia University,Yinchuan 750021,China
Keywords:Stimuli-responsive Metallopolymers Light Dilute CO2 Reversible uptake-release
ABSTRACT Here,we report a finding on light-mediated CO2-responsiveness.It is found on the microgels that are made of side-chain type metallopolymers containing metalla-aromatics.Turbidity and laser light scattering studies on dilute aqueous dispersion of these microgels in dark indicate high CO2-responsivity,but poor reversibility upon N2 purge,which can be improved by exposing to light.This light-mediated CO2-responsiveness can be elucidated by the loss of aromaticity from initial photoexcitation and concurrent formation of a less reactive,antiaromatic excited state of relatively low CO2 binding affinity,and by subsequent relief of antiaromaticity that can enhance the CO2 removal.The finding is also checked by CO2 uptake-release experiments on the microgels,which enables both CO2 capture of high capacity and CO2 removal of good reversibility under a mild condition,allowing effective and reversible response to dilute CO2.
Stimuli-responsive polymeric materials that can reversibly respond to CO2provides great opportunities [1–6].Besides the prospect of CO2capture and removal in easing the world’s energy and environmental problems,with CO2as a stimulus to induce polymer phase transitions,it is able to regulate properties of CO2-responsive polymeric materials for cell mimics,drug delivery,and many other applications [6–12].
Because of the prospective applications,efforts have been made to explore fundamental principles of polymer design to enable CO2-responsiveness,including the choice of CO2-responsive moieties and how effective CO2-responsiveness can be expected to be under the conditions of interest [11,12].Up to now,CO2-responsive polymeric materials are principally synthesized by the functionalization of polymer chains with basic groups,which can react with acidic CO2in water based on acid–base pair theory [7–11].Owing to reversible nature of the acid–base equilibrium,CO2can be removed by bubbling with inert gases (e.g.,N2gas) under a mild condition.This offers an advantage of free of contamination by accumulated chemicals;yet,it also makes the materials barely respond to dilute CO2,due to low capacity of the CO2-responsive polymeric materials for CO2capture from dilute CO2sources,which undisputedly is a critical drawback [1–12].As a theoretical possibility,enhancing CO2binding affinity might allow CO2capture of high capacity [13–16],which paves the way to address this problem [11]and thus have recently received attention in CO2-responsiveness design [17,18].For instance,efforts are made by exploiting frustrated Lewis pair (FLP) [19],and use CO2to bridge FLPcontaining polymersviadynamic covalent bonding [17,18].However,high CO2binding affinity in turn leads to difficulty in CO2removal without heating (≥60 °C typically).The challenge of seeking stimuli-responsive polymeric materials of a distinct mechanism to allow both CO2capture of high capacity and CO2removal of good reversibility under a mild condition remains to be solved.
In this work,we would like to report a concept of how to tune CO2binding affinity to mediate CO2-responsiveness by using sidechain type metallopolymers containing metalla-aromatics as CO2-responsive moieties on microgels.Unlike bases or FLP groups reported in previous articles that give rise to intra-/intermolecular acid-base activation of CO2viaionic or dynamic covalent bonding[1–14],metallopolymers (or organometallic polymers) with metal functionalities display typically high CO2binding affinityviastrong interactions (e.g.,metal complexes) [20–22],which however are rarely used for harnessing CO2-responsiveness.Here our intention to use those metallopolymers containing metalla-aromatics is because most metalla-aromatics are thermodynamic stable and possess properties of both organometallics and aromatics [23,24].An alteration in (anti)aromaticity is known to efficiently tune reactivity [25].For instance,(anti)aromaticity can promote reactivity of FLP groups with small molecules like CO2[26,27].Interestingly,light exposure could lead to a change in (anti)aromaticity,offering a facile way to vary reactivity [28–30].This tunning in reactivity may depend on a combination of properties: the number of electrons,the orbital topology,and the electronic state,in the sense that a change in each of these factors lead to a reversal in the allowedness and forbiddenness;in particular,the results pointed to a reversal of (anti)aromaticity following photoexcitation [28–30].Inspired by these,one wonders if it is possible to obtain polymeric materials,microgels [3,31–33]here of metallopolymers containing metalla-aromatics,of effective and reversible CO2-responsiveness by utilizing light as a mediator (Scheme 1).
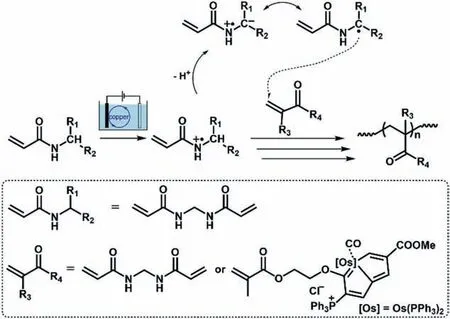
Scheme 1.Synthesis of metallopolymer microgels.
The microgels were synthesized through electrochemical polymerization [34]of a Craig-type coplanar Möbius metalla-aromatic compound (OsHEMA;2.0 × 10−4mol/L) [35]with a crosslinkerN,N’-methylenebisacrylamide (MBAAm;2.0 × 10−6mol/L),and with copper nanoparticles (36 ± 5 nm;see Fig.S1 in Supporting information for TEM,XRD and UV-vis absorption results) as mediators for electron transfer,so as to avoid direct electrolysis of OsHEMA on the electrode that might lead to disproportional reactions [36].On an anodic scan,an oxidation starts to develop on copper nanoparticles at a low potential ofca.+0.2 VversusAg/AgCl (Fig.S2 in Supporting information),leading to formation of strong oxidizing copper species [36,37],which can attack MBAAm to form N-localized radicals [34,36].These N-localized radicals could trigger a cascade of reactions that typically involve the loss of a proton and formation of C-localized radicals,which then initiate polymerization on attacking vinyl groups of the monomer/crosslinker,making them enter into free radical polymerization [36]and particle growth processes in the electrochemical systems [34,38].In this circumstance,the synthesis should proceed upon applying a suitable potential of+0.3 V that is above the oxidation wave of copper nanoparticles,but below those of Os-HEMA (starting atca.+0.6 VversusAg/AgCl) and MBAAm (starting at+1.6 VversusAg/AgCl) (Fig.S3 in Supporting information),resulting in toroidal-like microgels (collected after reacting for 4 h at 25.0 °C,and purified before characterization;Fig.1a).The mean diameter of freeze-dried toroids is 1.46 ± 0.21 μm,and the width is 69 ± 23 nm;the outline is barely sharp due to the existence of corona [34,39]on surface that may be indistinctly seen in Fig.1b.Energy dispersive X-ray (EDX) elemental mapping results indicated
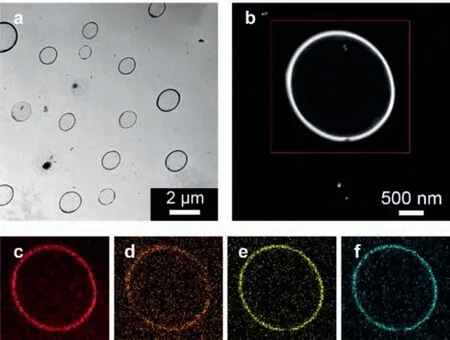
Fig.1.(a,b) TEM images of the microgels.(c–f) Typical EDX elemental mapping of(c) carbon,(d) oxygen,(e) phosphorus,and (f) osmium on the single gel particle shown in (b).
the existence of carbon,oxygen,phosphorus and osmium in an individual toroid;no copper salts or other inorganics were detected(Figs.1c-f).The edge of EDX elemental maps matches well that in annular dark-field TEM image (Fig.1b),indicating that those elements and corresponding moieties are distributed throughout microgels.31P NMR spectrum of the microgels (Fig.S4 in Supporting information) showed two signals of the metalla-aromatic moiety atca.13.0 (for CPPh3) and 3.6 ppm (for OsPPh3),and in1H NMR spectrum (Fig.S5 in Supporting information) atca.12.2 (for OsCH)and 7.1-8.2 ppm (for other aromatic protons) [35],indicating the integration of the metalla-aromatic moiety to the microgels.The weight percentage of the metalla-aromatic moiety was estimated to beca.95.4 wt%,viaanalyzing the percentage of osmium metal by inductively coupled plasma mass spectrometry [35],which was close to the feeding for microgel synthesis (ca.99.9 wt%).The microgels were hydrophilic (Fig.S6 in Supporting information) and kept stable in water (Fig.S7 in Supporting information).To verify the shape and composition of microgels (Table S1 in Supporting information),over five samples were repeated,and ten samples were prepared at 20–30 °C,which displays negligible influence on synthesis.The microgels were reproducible from batch to batch,with an average yield of ≥80%.
Phase diagrams for aqueous microgel dispersion (0.015 wt%)were studied by UV-vis spectroscopy at a wavelengthλ=700 nm(of incident light intensityI0),where absorption is negligible (Fig.S8 in Supporting information).The decrease in transmitted light intensity (IT) can be associated with scattering,to give the normalized attenuation coefficientδλas [34]:

where the light attenuationDλ=lg(I0/IT),cfor the microgel concentration (wt%),anddfor the cuvette thickness (1 cm).The tests were made at a slow temperature ramp (3 °C/h;using a temperature controller,±0.1 °C) followed by a long waiting (30 min,after temperature reaching equilibrium) and a rapid measurement time (<1 s),to ensure in the equilibrium state of microgels at each measurement.The dispersion was bubbled with N2or CO2gas (20.0 mL/min under 1 atm in dark) and equilibrated in a cuvette for tests.Upon purge with CO2at a set temperature in the range of 20–80 °C,theδ700changed immediately and reached equilibrium within 30 min of CO2purge (Fig.S9 in Supporting information),and theδ700(equilibrated;the same below) increased with the amount of CO2going through the dispersionviaadjusting purge time (Fig.2a).The increase in theδ700is possibly associated with the binding of CO2to the metalla-aromatic moiety[20–22],and ether oxygens as well [33],which could reduce hydrogen bonding of polymers with water and thus the hydrophilicity that lead to the drop of transmittance [34].Since this subtle variation can also disrupt counterbalance of hydrophilic and hydrophobic forces in microgels,which is a key factor responsible for thermo-responsiveness [39,40],then,temperature-dependence on theδ700was studied.Results indeed show an increasedδ700at elevated temperatures (Fig.2a),representing a possible transition atca.54.6 °C upon N2purge (Fig.S10 in Supporting information);the transition temperature drops toca.47.5 °C,43.3 °C and 41.8 °C,respectively,after a 30 s,5 min and 30 min of CO2purge (Figs.S10 and S11 in Supporting information).Those results demonstrate that the microgels are CO2-responsive.In further cyclic tests (Fig.2b and Fig.S9),purge again with N2following the CO2purge,theδ700cannot fully recover.The recovery ratioRδisca.5% at 20.0 °C,as estimated byRδ=(δCO2-δN2)/(δCO2-δ0),in whichδ0is the initialδ700,andδCO2is theδ700after a CO2purge andδN2is that after one cycle of CO2/N2purge.A largerRδappears at the higher temperature (Fig.2b and Fig.S12 in Supporting information).However,unsatisfying recovery ratios were recorded over the range of temperatures for turbidity analysis (20–80 °C;e.g.,Rδ≈45% at 80.0 °C),demonstrating a poor reversibility.
The reversibility of CO2-responsiveness can be improved by irradiation to an ultraviolet lamp (offering 30.0 W/cm2area light of a wavelength 365 nm) (Fig.2b;keeping the temperature at the set value while turning off gas purge and the lamp for rapid measurements,the same below).This seems to be realized by altering the variations on theδ700under CO2/N2purge.Compared with results measured in dark,when the light was applied,the rising extent of theδ700upon purge with CO2became lower,whereas the reducing extent of theδ700became more significant upon purge again with N2.Taken together the two variation manners,light-mediated CO2-responsiveness could be achieved and theRδapproached 100% at 20.0 °C,indicating good reversibility under a mild condition.Similar results were obtained at other temperatures in the range of 20–80 °C (e.g.,at 80.0 °C,Fig.2b and Fig.S12).
An examination on the observedδ700change may raise a few questions.The first possible question associates with aggregation:may the recordedδ700relate to aggregation of microgels? It has been reported that macromolecular clews in solutions below a critical concentration (ca.0.1 wt%) can conserve individuality [41].Given that theδ700was recorded on dispersions of a much lower concentration (0.015 wt%),aggregation of microgels may be excluded.This hypothesis is supported by particle dynamics,through characterizing particle diffusion using an exponentPthat is obtained by diffusing wave spectroscopy.If aggregation occurred,a change on particle dynamics can be reflected in a change from freely diffusive (P≈ 1) to subdiffusive motion (P <0.8) [42].Here,a constantPofca.1 is obtained in the temperature range of 20–80 °C (Fig.S13 in Supporting information),confirming that the microgels did not lose individuality.Theδ700change reflects transitions of individual microgels.To provide a direct evidence,dynamic light scattering (DLS) was used to monitor the average hydrodynamic radius
The second possible question relates to the observation of lightmediated CO2-responsiveness of the microgels.The characteristics of the change inδ700and
Given that the difference in CO2binding affinity can lead to a change in CO2uptake/release capacity [13–17],single-gas uptakerelease experiments were performed.Fig.3a indicates a larger CO2uptake capacity of the microgels in dark than that acquired in light at the same temperature of 20.0 °C,in agreement with the variation in theδ700and
Further optimizing the experimentsviaa combination of CO2capture in dark and CO2removal in light,the former can enable a large CO2uptake capacity ofca.4.2 mmol/g under 1 atm CO2pressure at 20.0 °C,and the latter allows partial release (by keeping CO2purge while exposing to light) or full release (by N2purge in light) of CO2under ambient pressure at 20.0 °C (Fig.4a).This finding is curious,since both CO2capture of high capacity and CO2removal of good reversibility are realized under a mild condition,without large temperature swings (Fig.S19 in Supporting information) that is required for CO2uptake/release reported previously (Table S2 in Supporting information) [1–18].It is the change in the heat of uptake that produces large differences in binding of CO2to polymers: CO2capture in dark favors the formation of strong interactions (theQappabove 60 kJ/mol;e.g.,metal complexes),and light exposure facilitates weak interactions (theQappbelow 60 kJ/mol;e.g.,quadrupole-π) coming to dominate[20,51,52].As strong interactions favor CO2capture from dilute sources,one may wonder how low a CO2level can induce an effective CO2-responsiveness.To this end,the dispersion was bubbled with CO2/N2mixed-gases (Fig.4b).Distinctly,the microgels could respond to CO2at low CO2levels,as evidenced by attainment of a plateau of CO2uptake capacity ≥3.9 mmol/g even with CO2level lower down to 30 vol% in dark (Fig.4c);correspondingly,the microgels deswelled,with
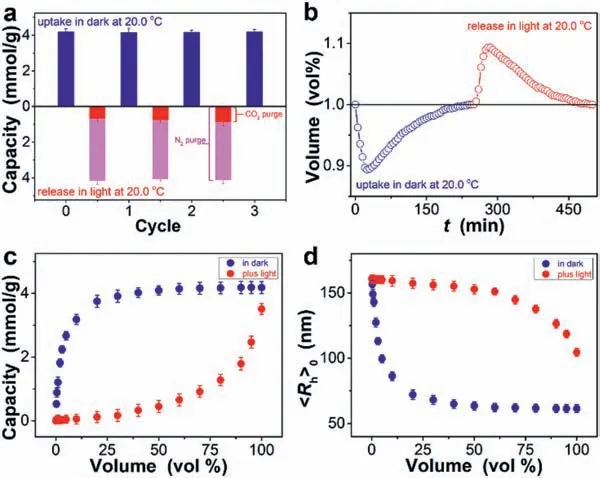
Fig.4.(a) CO2 capacity of uptake in dark upon CO2 purge,and that release in light upon CO2 purge or N2 purge.(b) Time-domain volume of CO2 in the gas flow,upon purge with 1 vol% CO2 gas.(c) CO2 uptake capacity in dark and in light,upon purge with CO2/N2 mixed-gases,and (d) changes in the
Light-mediated CO2-responsiveness was also observed on spherical metallopolymer microgels (Fig.S21 in Supporting information).These spherical microgels could also exhibit both CO2capture of high capacity (ca.3.9 mmol/g under 1 atm at 20.0 °C in dark) and CO2removal of good reversibility (by exposing to light)under a mild condition (Fig.S22 in Supporting information).
In summary,we demonstrate the concept of mediating CO2-responsiveness through tuning CO2binding affinity by using sidechain type metallopolymers containing metalla-aromatics as CO2-responsive moieties on microgels.Studies on the dilute aqueous dispersions of these microgels in dark indicate high CO2-responsivity,but poor reversibility upon N2purge,which can be improved by applying light exposure.This light-mediated CO2-responsiveness can be elucidated by the loss of aromaticity from initial photoexcitation and concurrent formation of a less reactive,antiaromatic excited state of relatively low CO2binding affinity,and by subsequent relief of antiaromaticity that can enhance CO2removal.Our results underscore the vast potential of bridging metalla-aromatic chemistry and polymer science to enable both CO2capture of high capacity and CO2removal of good reversibility under a mild condition,providing a novel strategy for the design of valuable materials that can effectively,reversibly respond to dilute CO2.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by National Natural Science Foundation of China (Nos.21774105,21805164,20923004),Chuying Plan Youth Top-notch Talents of Fujian Province,and National Fund for Fostering Talents of Basic Science (No.J1310024).
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
