Four-step spin-crossover in an oxamide-decorated metal-organic framework
Siguo Wu,Sukhen Bala,Zeyu Ruan,Guozhang Huang,Zhaoping Ni,Mingliang Tong
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education,School of Chemistry,Sun Yat-Sen University,Guangzhou 510275,China
Keywords:Spin crossover Oxamide Metal-organic framework Hofmann-type Host-guest interaction
ABSTRACT Spin-crossover (SCO) complexes with multiple spin states are promising candidates for high-order magnetic storage and multiple switches.Here,by employing the N,Nʹ-4-dipyridyloxalamide (dpo) ligand,we synthesize two Hofmann-type metal-organic frameworks (MOFs) [Fe(dpo){Ag(CN)2}2]·3DMF(1) and [Fe(dpo){Ag(CN)2}2]·0.5MeCN·2DEF (2),which exhibit guest dependent four-step SCO behaviors with the sequences of LS → ∼LS2/3HS1/3 → LS1/2HS1/2 → ∼LS3/10HS7/10 → HS and LS → ∼LS2/3HS1/3 → LS1/2HS1/2 → ∼LS1/4HS3/4 → HS,respectively.Therefore,the incorporation of hydrogen-donating/hydrogen-accepting groups into the Hofmann-type MOFs may effectively explore the multi-step SCO materials by tuning hydrogen-bonding interactions.
Multi-stable materials have aroused significant interests in scientific community for their potential applications in informatics.Spin-crossover (SCO) complexes,especially for iron(II) species whose spin states can be manipulated between diamagnetic lowspin (LS,t2g6eg0) state and paramagnetic high-spin (HS,t2g4eg2)state via external stimuli (such as temperature,pressure,light irradiation and guest molecules),are conceivable elements for memory devices and opto-magnetic switches [1-5].When the competitions of long-range ferro- and antiferro-elastic interactions occur between SCO units,elastic frustrations may lead to multi-step SCO behaviors,which can act as the multi-stable materials with the potential applications in high-order data storage and multi-switches[6-8].
Hofmann-type clathrates,consist of iron-cyanometalate meshes and pillar ligands,are promising candidates for constructing high-performance SCO materials [9-11].Extensive efforts have been devoted to promote the SCO cooperativityviafine-tuning the hydrogen-bonding interactions [12-14]andπ-πinteractions[15,16].By means of guest exchange,the implementations of guest programmable multistep switches,gate-opening with multiplex responses and bidirectional chemo-switching have been achieved in porous 2D/3D Hofmann-type frameworks [17-20].However,the synergetic effects of host-guest interactions are complicated since SCO dynamics are influenced by the size,distribution and geometry of the guests,the porous capacity and characteristic of the host as well as the direction and strength of host-guest interactions[11].Integration of hydrogen-donating/hydrogen-accepting groups(imide [21],amide [22],amino [18],hydroxyl [14,23],urea [24],etc.) into pillar ligands would be a judicious choice for achieving strong host-guest interactions in Hofmann-type metal-organic frameworks (MOFs).
Since hydrogen-bonding interactions play an important role in multi-step SCO behaviors [12-14,18],an oxamide-decorated ligandN,Nʹ-4-dipyridyloxalamide (dpo,Scheme 1) is firstly employed to construct two SCO Hofmann-type MOFs [Fe(dpo){Ag(CN)2}2]·3DMF(1) and [Fe(dpo){Ag(CN)2}2]·0.5MeCN·2DEF (2),in which the oxamide unit can act as hydrogen bond donor as well as acceptor.Hence,four-step SCO behaviors with two new sequences are observed,although the reported four-step spin transition properties are extremely rare so far [12,14,16,18,22,25-27].Moreover,spin transition temperatures and thermal hysteresis loops are altered by different solvent guests.

Scheme 1.Representation of the dpo ligand.
Complexes 1 and 2 were prepared by slow diffusion methods and isolated as the yellow block crystals.The crystalline samples were assessed by elemental analysis,powder X-ray diffraction (Figs.S1 and S2 in Supporting information),thermogravimetric (TG) measurements (Figs.S3 and S4 in Supporting information)and infrared spectra (Figs.S5 and S6 in Supporting information).TG analyses reveal that the guest molecules in 1 and 2 can be completely removed upon heating to around 435 and 473 K,respectively.
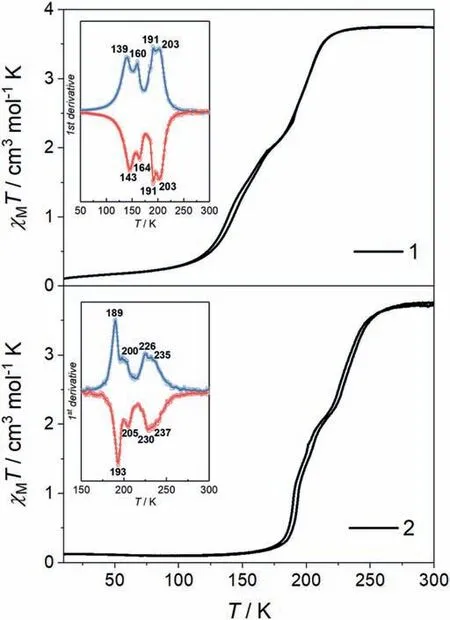
Fig.1.Variable-temperature magnetic susceptibility for 1 and 2 with a sweep rate of 2 K/min.Inset: 1st derivative plots of χMT curves.The blue and red lines represent for the cooling and warming modes,respectively.
Temperature-dependent magnetic susceptibility measurements for 1 and 2 were performed over the range of 10−300 K with a sweep rate of 2 K/min (Fig.1).At room temperature,theχMTvalue of 1 is close to 3.75 cm3K/mol,indicating a pure HS state.It slightly decreases to 3.65 cm3K/mol at 230 K and then declines gradually.An inclined plateau is observed in the middle of SCO curve,corresponding to a HS1/2LS1/2intermediate state.Upon further cooling,theχMTvalue reaches to 0.11 cm3K/mol at 10 K,which is consistent with a LS state.The 1stderivative plot of magnetic data suggests four-step SCO behavior with a sequence of LS→∼LS2/3HS1/3→LS1/2HS1/2→∼LS3/10HS7/10→HS,although their magnetic plateaus are not obvious.The critical temperatures for the peaks/valleys are at 139/160/191/203 K and 143/164/191/203 K in the cooling/warming modes,respectively.Therefore,the largest width of thermal hysteresis (ΔT) loop is only∼4 K.
For 2,the cooling and warming SCO curves with hysteresis loop shift to the higher temperature region.A gradual four-step SCO behavior with the sequence of LS → ∼LS2/3HS1/3→ LS1/2HS1/2→ ∼LS1/4HS3/4→ HS can be inferred from the differential magnetic curve,although no obvious magnetic plateaus are observed in 2.The related 1stderivative peaks/valleys locate at 189/200/226/235 K and 193/205/230/237 K in the cooling/warming modes,indicating the thermal hysteresis width is up to 5 K.The four-step SCO behaviors of 1 and 2 are further confirmed by two cycles of magnetization measurements(Figs.S7 and S8 in Supporting information).
To corroborate the multi-step SCO behaviors,DSC measurements were carried out with a sweeping rate of 10 K/min.As shown in Fig.S9 (Supporting information),four pairs of exothermic/endothermic peaks locate at 136/149/186/195 K and 144/158/189/199 K in the cooling/warming modes,respectively,which confirms the four-step SCO behavior in 1.These DSC peaks slightly deviate from theTcpeaks from the magnetic data,which should be due to the different scanning rates.Analogously,four sets of exothermic (193/201/226/235 K) and endothermic(195/204/228/238 K) peaks are found in the cooling and warming modes,confirming the four-step SCO behavior in 2 (Fig.S10 in Supporting information).The total enthalpy changes (ΔH) of 1 and 2 are around 13.21 and 9.84 kJ/mol,which are in the normal range for SCO FeIIcompounds [28].
Complex 1 crystalizes in triclinic space group and retains the geometric symmetry at 120,182 and 298 K (Table S1 in Supporting information).The asymmetry unit consists of a FeIIion,a dpo pillar ligand,two [Ag(CN)2]−linkers and three DMF molecules (Fig.2a).Each FeIIcenter adopts a distorted octahedral geometric fashion with [FeN6]coordination environment.The equatorial positions are occupied by CN groups of linear [Ag(CN)2]−ligands,giving rise to the [Fe{Ag(CN)2}2]∞network.The FeIIions between the adjacent layers are axially linked by the bidentate dpo ligands,which results a two-fold interpenetration framework (Fig.2c).Permanent 1D channels along the diagonal direction of theaandbaxis are found,wherein three DMF guests are housed (Fig.S11 in Supporting information).Two DMF molecules are involved into the strong N−H···O hydrogen bonds on either side of the dpo ligand (green dotted lines in Fig.2a).Meanwhile,the remaining DMF molecule is engaged in the weak intermolecular interactions with the neighboring DMF guest (purple dotted line in Fig.2b) and dpo ligand.
The detailed structural parameters of 1 can give a clue of the stepped SCO behavior,although only one unique FeIIion is solved in the intermediate state (Table S3 in Supporting information).At 120 and 298 K,the average Fe−N bond length is 1.963 and 2.178 ˚A,in line with the LS and HS FeIIions,respectively.In contrast,the
Complex 2 possesses a similar two-fold interpenetration framework with 1D channel along thebaxis (Fig.S12 in Supporting information) but crystalizes in orthorhombic space groupPca21at 120,215 and 300 K.Unlike 1,the asymmetric unit in 2 contains two crystallographically inequivalent FeIIions,two dpo ligands as well as four [Ag(CN)2]−linkers belonging to two sets of 3D frameworks.Meanwhile,one MeCN and four DEF serve as the guest molecules (Fig.3).Most importantly,one DEF form a bifurcated H-bond (N3−H3···O7···H10−N10) with two dpo ligands from two 3D frameworks,which may contribute to the asymmetry of twofold interpenetration framework and improve their effective transmission via host-guest interactions.The remaining amide groups in two dpo ligands separately generate the strong hydrogenbonding interactions (N2−H2···O5 and N11−H11···O8) with two DEF molecules.The remaining DEF and MeCN molecules are only involved into the van der Waals interactions with the neighboring DEF guests.
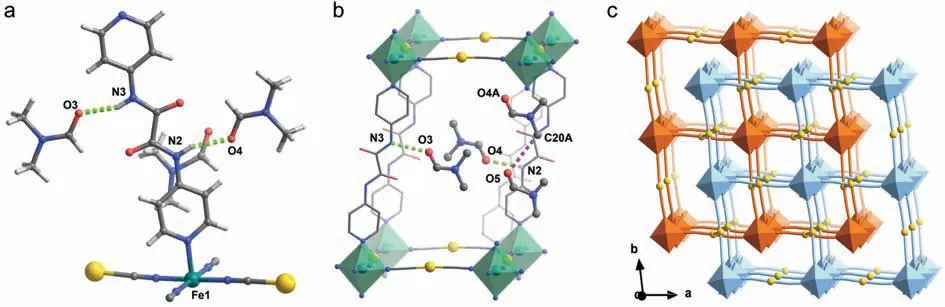
Fig.2.(a) The asymmetric unit,(b) hydrogen-bonding interactions and (c) two-fold interpenetration framework in 1.Color code: FeII,green;Ag,golden;O,red;N,blue;C,grey,H,off-white.Green/purple dotted lines represent for the strong host-guest H-bonds/the weak guest-guest H-bonds.
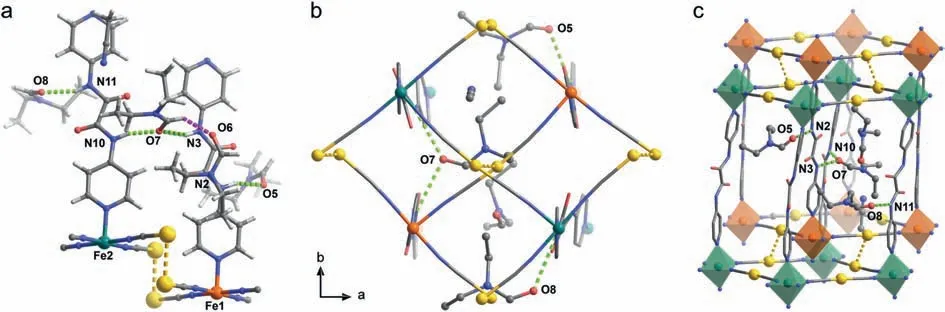
Fig.3.(a) The asymmetric unit,(b) hydrogen-bonding interactions viewed along c axis and (c) two-fold interpenetration framework in 2.Color code: FeII,orange/green;Ag,golden;O,red;N,blue;C,grey,H,off-white.Green/purple/golden dotted lines represent for host-guest H-bonds/guest-guest H-bonds/argentophilic interactions.
After carefully checking the single-crystal diffraction patterns,no superstructures [30]are found in 2 amid spin transition.The structural parameters of two inequivalent FeIIsites in 2 are further explored in detail.The
By introducing different guest molecules,complexes 1 and 2 exhibit four-step SCO behaviors with different spin transition temperatures and thermal hysteresis loops.To explore the magnetostructural relationship between 1 and 2,the structural data in the HS state are compared in detail.1 and 2 possess the same structural components and topological network for the twofoldinterpenetrated frameworks and the similar
In summary,we successfully constructed two Hofmanntype SCO complexes [Fe(dpo){Ag(CN)2}2]·3DMF (1) and[Fe(dpo){Ag(CN)2}2]·0.5MeCN·2DEF (2) by utilizing oxamidefunctionalized ligand,which provided a platform to tune SCO behaviorviahydrogen-bonding interactions.Accordingly,the framework adapted to the different guests and then exhibited different SCO behaviors.The magnetic data displayed four-step SCO behaviors with the sequence of LS → ∼LS2/3HS1/3→ LS1/2HS1/2→ ∼LS3/10HS7/10→ HS and LS →∼LS2/3HS1/3→LS1/2HS1/2→∼LS1/4HS3/4→HS for 1 and 2 respectively.The higherTcin 2 resulted from the competitive contributions of guest size effect and the larger Fe−N≡C angle.Meanwhile,the improved SCO cooperativity in 2 resulted from the contributions of the argentophilic interactions and bifurcated H-bond.In the future,this oxamide-decorated ligand can extend to other Hofmann-type system by altering the cyanometallate,which may yield fruitful SCO behaviorsviahydrogen-bonding interactions.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgment
This work was supported by the National Key Research and Development Program of China (No.2018YFA0306001),the National Natural Science Foundation of China (Nos.21950410521,21771200 and 21773316),and the Pearl River Talent Plan of Guangdong (No.2017BT01C161).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.012.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
