Di-4-ANEPPDHQ probes the response of lipid packing to the membrane tension change in living cells
Nn Li,Weifei Zhng,Hifeng Lin,Jin-Ming Lin,∗
a Beijing Key Laboratory of Microanalytical Methods and Instrumentation,MOE Key Laboratory of Bioorganic Phosphorus Chemistry &Chemical Biology,Department of Chemistry,Tsinghua University,Beijing 100084,China
b Division of Chemical Metrology and Analytical Science,National Institute of Metrology,Beijing 100029,China
Keywords:Lipid packing Membrane tension Osmotic shocks Polarity-sensitive probe Phase separation
ABSTRACT Membrane tension plays a significant role in many cellular processes including cell adhesion,migration and spreading.Despite the importance of membrane tension,it remains difficult to measure in vivo.Recently,the development of non-invasive fluorescent probes have made great progress,especially excitedstate deplanarization in molecular rotors has been applied to image membrane tension in living cells.Nevertheless,an intrinsic limitation of such kind of probe is that they depend on the lipid packing,and how the lipid packing responds to the membrane tension change remains unclear.Therefore,in this work,we used a polarity-sensitive membrane probe to investigate the possible response mechanism of lipid packing to the change of membrane tension that was regulated by osmotic shocks.The results showed that an increase in membrane tension could stretch the lipids apart with large displacements,and this change was not homogeneous on the whole membrane,instead,increase of membrane tension induced phase separation.
At the whole cell level,plasma membrane tension is tightly regulated during cell adhesion [1,2],cell migration [3-5]and cell spreading [6].For example,membrane tension can acts as a key mechanical regulator of actin polymerization to create cell polarity during cell migration [7,8].Besides,membrane tension also regulates the life activity at the subcellular level.Increasing membrane tension is able to inhibit endocytosis by counteracting clathrin polymerization and cause membrane fission [9].As a consequence of its multiple roles,the study of membrane tension has attracted much attention.
Although membrane tension plays a significant part in modulating various cellular behaviors,it is extremely difficult for researchers to measure it.Over the past decade,extraction of small membrane tubes from the plasma membrane has been regarded as the most attractive technique to characterize the membrane tension [10].However,the measure process requires peeling the membrane off the actin cortex,which is not suitable for observing the real time and dynamic life activity on living cells.Thus it is important to develop some non-invasive fluorescent probes [11,12].Recently,excited-state deplanarization in molecular rotors has made great progress in imaging membrane tension [13,14].Whereas an intrinsic limitation of such kind of probe is that they depend on the lipid packing,and the reported results are the combination of both membrane tension and lipid packing.
Lipid packing is defined as the density of lipid acyl chains:tighter and more ordered acyl chains indicate higher lipid packing,while more loosened and more disordered acyl chains suggest lower lipid packing [15].Lipid packing has been considered to be closely relevant to the plasma membrane tension.As membranes are not able to bear large strains,they require surface area regulation to accommodate tension changes in case of plasma membrane lysis.Theoretically,an increase in membrane tension is expected to cause cell swelling,which involves an increase in surface area.Lipid packing can reflect the extent of plasma membrane stretch.Intuitively,an increase of membrane area can stretch the lipids apart with large displacements between lipid acyl chains,thus causing lipid packing loosening.However,there is also one possibility,since tilted lipid tails can be a way to adapt the rapid increase of surface area in response to increasing membrane tension [16],consequently the lateral compression of the acyl chains will appear owing to the lipid tilting.Therefore,the response of lipid packing to the tension change remains unclear.

Fig.1.Illustration of lipid-packing response to the membrane tension change under different osmolarity of solutions.(a) The possible response of lipid packing to the change of membrane tension in different osmolarity of solutions.(b) The chemical structure of di-4-ANEPPDHQ probe.
To investigate the possible response mechanism of lipid packing to the change of membrane tension (Fig.1a),we used a polaritysensitive membrane probe (di-4-ANEPPDHQ) to indicate lipid packing (Fig.1b).This di-4-ANEPPDHQ probe has two positive charges on its headgroup,making it possible to have a good water solubility.This characteristic allows the probe to directly load into the cells without the need of vehicles,such as Pluronic F127 orγcyclodextrin that may disturb cell membranes.Besides,the double positive charges of the probe retard the rate of flipping from the outer leaflet of the bilayer to the inner leaflet,largely decreasing the background interference.The interactions between di-4-ANEPPDHQ and lipid bilayers have been modeled using a series of atomistic molecular dynamics simulations [17].The hydrophilic region of this probe is mostly located in the interface of the membranes in both the liquid disordered phase and liquid ordered phase with an average insertion depth ranging from+2.5 ˚A to −3 ˚A.The hydrophobic part of this probe is localized deeper in membranes with an average insertion depth between −4 ˚A and −5 ˚A in the liquid disordered membrane and around −12.5 ˚A in the liquid ordered membrane.This probe is solvatochromic after excitation due to a large charge shift,in which the positive charge is shifted from pyridinium nitrogen towards amino-naphthylstyryl nitrogen of the molecule,and the charge shift can induce a 60 nm red shift in the emission maxima when embedded in a more-polar membrane environment [18,19].The shift in emission profile between liquid-disordered and liquid-ordered phases was imaged using confocal laser scanning microscopy (CLSM).A quantitative assessment of membrane lipid packing,defined as a generalized polarization (GP) value,was calculated by a ratiometric measurement of the fluorescence intensity recorded in two spectral channels using ImageJ software with a custom-written macro [20].Decrease in GP values suggested the lipid packing loosening.For the change of membrane tension,a common approach was applied by regulating the osmolarity of solutions surrounding cells.As the fragile membrane structures need to maintain their integrity,they have to sustain large fluctuations in the different osmolarity of solution,which can cause pressure differences between the cytosol and the external environments,thus changing the membrane tension.In turn,the tension change forces the membrane structures to quickly adapt in case of damages.When cells are treated with a hypotonic shock,they extend their surface area and increase their membrane tension.During this process,the lipid packing on membrane is supposed to vary in response to the tension change,but how the lipid packing will respond remains unclear.In this work,di-4-ANEPPDHQ probe was used to indicate the variations of lipid packing,and different osmotic shocks were applied to regulate the membrane tension.
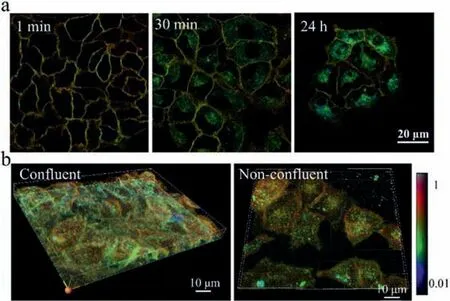
Fig.2.The GP images in living cells.(a) Labelling of A549 cells with time.Probes mostly stayed in the plasma membrane in the beginning,but after 30 min of incubation,bright spots with low GP values were seen inside the cells,even after 24 h,this probe signal could be visualized.(b) Both confluent and non-confluent A549 cells exhibited higher GP values on the apical side than on the basolateral part.The color bar corresponded to the GP values.
To verify the feasibility of this probe,we firstly evaluated if this probe could indicate phase differences in living cells.To better display the image data,the grayscale images were pseudo-colored,and color range was adjusted to maximize contrast.The images provided both GP value and structural information.When micromolar amounts of this probe were added to the medium of A549 cells,plasma membrane staining appeared in less than 1 min,with enough signal to be visualized.This probe remained stable in the plasma membrane,accompanied by the appearance of some endosomal labelling after 30-min incubation.We did not notice any cell growth defects in up to 24-h culture in the presence of this probe (Fig.2a).Further,the GP values of membrane on the apical side (towards the medium) were found to be higher than those on the basolateral in both confluent and non-confluent cells (Fig.2b,Figs.S1 and S2 in Supporting informatioon).This result was consistent with the previous report,that the apical membrane can form a large liquid-ordered phase owing to the enrichment in sphingolipids and cholesterol,while the basolateral membrane was composed of a less ordered lipid phase [21].This finding clearly showed that di-4-ANEPPDHQ probe could indicate lipid packing differences in different compartments of living cells.
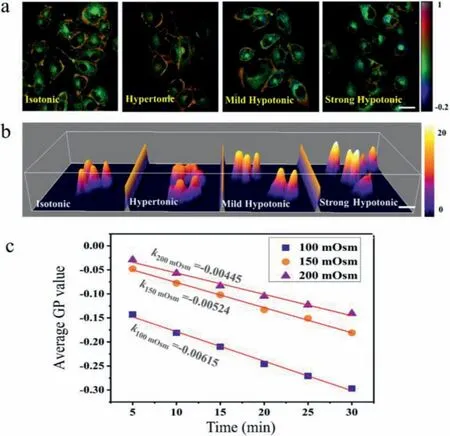
Fig.3.Response of GP values to osmotic shocks on living cells.(a) GP images of A549 cells in isotonic,hypertonic,mild hypotonic and strong hypotonic buffer.The color bar corresponded to GP values.Scale bar: 10 μm.(b) The height of A549 cells in isotonic,hypertonic,mild hypotonic and strong hypotonic buffer.The color bar corresponded to the height in μm.(c) The change of GP values with time increasing in different hypoosmotic shocks (200 mOsm/L,150 mOsm/L,and 100 mOsm/L).
We then wondered if this probe could indicate the membrane tension-induced change of lipid packing.To test this possibility,membrane tension was perturbed by osmotic treatments.In hypertonic shocks,the culture medium containing 100 mmol/L sucrose was incubated with cells.In mild hypotonic shocks,the buffer composed of 25% 1 mmol/L CaCl2aqueous solution and 75% cell culture medium was added to cells,while in strong hypotonic shocks,the buffer was composed of 50% 1 mmol/L CaCl2aqueous solution and 50% cell culture medium.During a 10-min hypertonic shock,the height of cells slightly decreased,but GP values remained nearly consistent with the isotonic group.In contrast,when the cells were treated with hypotonic shocks for 10 min,especially strong hypoosmotic treatment,the height of cells significantly increased,and the average GP values on cytomembrane have a significant decrease (the area of red part shrank) (Figs.3a and b).The decreased GP value after hypotonic shocks suggested that an increase in membrane tension could lead to a more polar membrane environment.That is to say,an increase of membrane tension averagely stretched the lipids apart with large displacements between lipid acyl chains.To further verify this phenomenon,different hypoosmotic shocks (200 mOsm/L,150 mOsm/L,and 100 mOsm/L) were applied to regulate the membrane tension,and the change of GP values with time increasing was monitored.The results showed that the GP values reduced linearly with the incubation time increasing,and the slopes were different in various hypoosmotic shocks (200 mOsm/L: −0.00445 ± 2.21 × 10−4;150 mOsm/L: −0.00524 ± 1.51 × 10−4;100 mOsm/L: −0.00615 ±2.21 × 10−4) (Fig.3c).Besides,there was significant cell swelling in hypotonic buffers,and an increase in the rate of exocytosis was observed to adapt an increase in membrane area (Fig.S3 in Supporting information).
Further,a lipid phase separation was found in strong hypotonic shocks,accompanied by the appearance of inhomogeneous liquid-order and liquid-disorder phase on membrane.In the first 5 min,the membrane thickness and GP values were relatively homogeneous.5 min later,the membrane thickness began to change,some parts became thick,and some parts became thin.It is worth noting that the lipid packing in thick parts firstly became loosening,and the loosening area became larger and larger as hypotonicity continued.As we can see,the newly formed liquid-disordered phase supported most of the stretch,while liquid-ordered phase clustered at the regions of intercellular junction that cannot be stretched easily (Fig.4a).This phenomenon was attributed to the inhomogeneities of the lipid composition induced by the phase separation.The GP histograms,which reflected the number of pixels at each GP value,exhibited two prominent peaks after 30-min hyperosmotic shock,which also suggested that increasing in membrane tension could cause a lipid phase separation (Fig.4b).These results were consistent with the previous reports that an increase in membrane tension could cause a lipid phase separation to accommodate the area change [22,23].Further,we also investigated the changes of GP values in the membrane homogeneity under hypertonic shocks (400 mOsm/L).As shown in Fig.S4 (Supporting information),although the cell volume dwindled with time increasing,the GP values on cytomembrane remained nearly consistent for 30-min treatment,and there was no appearance of lipid phase separation in the whole process.Besides,an increase in endocytosis was observed after hypertonic treatment due to the decrease in membrane tension.
In conclusion,we reported using di-4-ANEPPDHQ as a fluorescence probe to investigate the response of lipid packing to the change of membrane tension in living cells.The results showed that an increase in membrane tension could stretch the lipids apart with large displacements on average,rather than causing lipid tilting.Besides,it should be noted,the decrease of GP values in the membrane under hypotonic shocks was not homogeneous.Instead,increase of membrane tension induced phase separation,in which the liquid-disordered phase supported most of membrane stretch and liquid-ordered phase was mainly located at the junctions between cells.These results enabled us to provide an explicit response mechanism of lipid packing to the change of membrane tension.
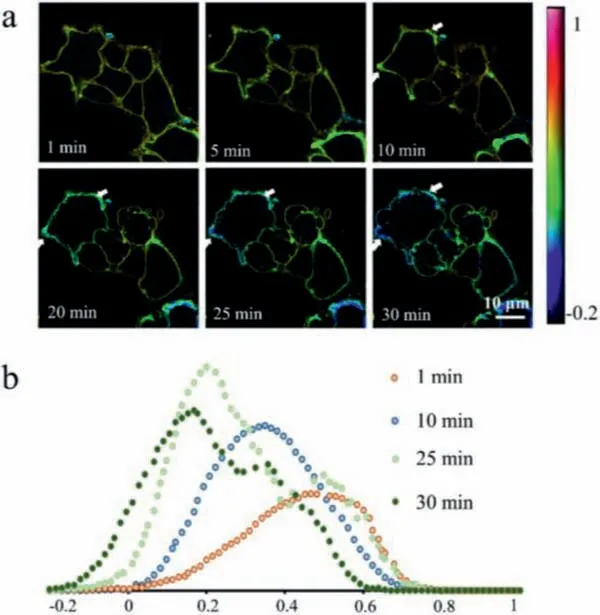
Fig.4.Lipid phase separation appeared in response to the increase of membrane tension.(a) Confocal microscopy images of membrane lipid packing indicated by fluorescence probe di-4-ANEPPDHQ when A549 cells were treated with strong hypotonic shocks for 30 min.The color bar corresponded to GP values.(b) Histogram of the GP values at different time,which showed the number of pixels in each GP value.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was financially supported by Research and Development Program in Key Areas of Guangdong Province,China (No.2019B020209009),National Natural Science Foundation of China(Nos.21727814,22034005,81872829) and the China Postdoctoral Science Foundation (No.2020M680502).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.060.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
