Silicotungstic acid-derived WO3 composited with ZrO2 supported on SBA-15 as a highly efficient mesoporous solid acid catalyst for the alkenylation of p-xylene with phenylacetylene
Xueting Bai,Yongle Guo,Zhongkui Zhao
State Key Laboratory of Fine Chemicals,Department of Catalysis Chemistry and Engineering,School of Chemical Engineering,Dalian University of Technology,Dalian 116024,China
Keywords:Alkenylation SBA-15 Zirconium dioxide Tungsten trioxide Silicotungstic acid Solid acid
ABSTRACT Highly dispersed silicotungstic acid-derived WO3 composited with ZrO2 supported on SBA-15 (WZ/SBA-15) as an ordered mesoporous solid acid catalyst was prepared via a facile incipient wetness impregnation (IWI) method that active ingredients,ZrO2 and WO3,were impregnated into the channels of SBA-15 simultaneously with a subsequent calcination process.The relationship between catalyst nature and performance was explored by high resolution transmission electron microscopy (HRTEM),high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM),FT-IR,X-ray photoelectron spectroscopy (XPS),X-ray diffraction (XRD),N2 adsorption-desorption,NH3 temperature-programmed desorption (NH3-TPD),and FT-IR of pyridine adsorption (Py-IR) characterization techniques.The catalytic performance of W12Z15/SBA-15 is not only greater than that of single component solid acid catalysts,WO3/SBA-15 and ZrO2/SBA-15,but also W12/Z15/SBA-15 prepared by impregnating active ingredients,ZrO2 and WO3,into SBA-15 in sequence.The outstanding performance of W12Z15/SBA-15 is derived from the strong interaction between ZrO2 and WO3,which results in more acid sites,and relatively high specific surface area,large pore volume,and ordered mesoporous structure of SBA-15.The characterization and reaction results clearly demonstrate that the synergy of ZrO2 and WO3 has a clear boost for the alkenylation.The optimized W12Z15/SBA-15-500 achieves a 99.4% conversion of phenylacetylene and a 92.3% selectivity of main product α-arylstyrene for the alkenylation of p-xylene with phenylacetylene,with very low level of oligomers producing at the same time.Moreover,W12Z15/SBA-15-500 shows excellent catalytic stability and regeneration.Therefore,W12Z15/SBA-15-500 is a promising solid acid catalyst for the alkenylation.
Alkenyl aromatics,synthesized by acid-catalyzed alkenylation of aromatic compounds with alkynes,especially solid acid catalysts in terms of its features of high efficiency,low-cost,clean,renewability,easy-separation,and applicability toward large-scale industrial continuous production [1–3],are widely applied to diverse fields such as flavors,dyes,natural products,pharmaceuticals,and agrochemicals [4–6].Due to oligomerization of alkynes as the result of the poor stability of vinyl cation species [7],alkenylation remains a serious challenge to overcome.Compared with homogeneous catalysts,which leads to heavy pollution and equipment corrosion issues,heterogeneous catalysts,especially solid acid catalysts,show greater application potential for the alkenylation,whose acidity and pore structure have decisive effect on the catalytic activation,selectivity,and coke resistance.Therefore,it is urgent to develop highly efficient solid acid catalysts for alkenylation [8].
Some pioneering work has been done by Sartori who used HSZ-360 to catalyze the alkenylation of aromatics,which gave an unsatisfactory result owing to the existing irreconcilable contradiction between catalytic activity and selectivity.Besides,the use of HY zeolite to alkenylation contributed to a low catalytic efficiency,ascribed that the reaction only took place on the external surface of the catalyst as a result of the narrow pore channels within the HY zeolite [9].Consequently,the mesoporous solid acid catalyst may be a wise choice for alkenylation.In our previous reports,a series of supported phosphotungstic acid mesoporous solid acid catalysts have been developed for alkenylation,but inevitable use of volatile organic solvent for the recovery of spent catalysts results in economic and environmental issues [10–12].Besides,a series of sulphated La-mediated ZrO2-based solid acid catalysts have been developed for the alkenylation [13–15],considering the superacidic properties and good thermal stability of sulphated zirconia [16–18],but SO42−tends to leach in the reaction,resulting in the deactivation of catalysts.Moreover,hierarchical Hβzeolite and ceriamodified hierarchical Hβzeolite have been developed as highly efficient solid acid catalysts for alkenylation due to their high thermal stability,while coke is easy to form due to the existence of micropores [19,20].Therefore,the further work on developing robust alkenylation solid acid catalysts is required.

Fig.1.HRTEM images of (a) W12Z15/SBA-15,and (b) W12/Z15/SBA-15.HAADF-STEM images of (c) W12Z15/SBA-15 and (d) W12/Z15/SBA-15.
Tungstated zirconia catalysts have attracted considerable attention in the fields of academia and industry owing to their enhanced regeneration ability and good thermal stability [21],and have been applied to diverse reactions [22–28],but rare reports on alkenylation.Here,we developed highly dispersed silicotungstic acid (STA)-derived WO3composited with ZrO2supported on SBA-15 (called as WZ/SBA-15) as mesoporous solid acid catalyst prepared by incipient wetness impregnation (IWI) method,that the active components,WO3and ZrO2,were impregnated into the channels of SBA-15 simultaneously to enhance the interaction among them,with a subsequent calcination process for the alkenylation.Besides,alkenylation ofp-xylene with phenylacetylene was adopted as a model reaction to test the catalytic properties of asprepared solid acid catalysts.In addition,the effects of the molar ratio of ZrO2to WO3(n(ZrO2/WO3)) and calcination temperature (T) on the catalysts performance were investigated as well.High resolution transmission electron microscopy (HRTEM),highangle annular dark-field scanning transmission electron microscopy(HAADF-STEM),N2adsorption-desorption,FT-IR,X-ray diffraction(XRD),X-ray photoelectron spectroscopy (XPS),NH3temperatureprogrammed desorption (NH3-TPD),and FT-IR of pyridine adsorption (Py-IR) characterization techniques were employed to reveal the relationship between the catalysts’nature and catalytic properties.
The SBA-15 material was prepared according to the procedure described by Zhao [29].Solid acid catalysts W12ZX/SBA-15-T(X is defined as the molar content of ZrO2in contrast to WO3,X=12,15,and 24;Tstands for the calcination temperature) were prepared by IWI method by using the mixed aqueous solution containing zirconium nitrate pentahydrate and STA as an impregnant with a subsequent calcination process at a certain temperature for 3 h,and details of preparation process are described in Supporting information.The WO3-ZrO2composite oxide structure of the obtained product is proved by XRD and XPS.Without specific emphasis,W12ZX/SBA-15 stands for the samples calcined at 550 °C.The preparation methods of ZrO2/SBA-15,WO3/SBA-15,and W12/Z15/SBA-15 are similar to W12Z15/SBA-15 with some changes.Related characterization parameters and details of catalytic performance tests are showed in Supporting information.
Fig.1 shows the HRTEM and HAADF-STEM images of W12Z15/SBA-15 and W12/Z15/SBA-15.Obviously,the ordered mesoporous structure of SBA-15,proved by XRD and N2adsorptiondesorption,can be seen in both samples after the loading of active ingredients,ZrO2and WO3.Besides,it is clear that no obvious accumulation of large particles can be observed in W12Z15/SBA-15,while the phenomenon is serious in W12/Z15/SBA-15.The results obtained above implies the enhanced interaction between ZrO2and WO3in W12Z15/SBA-15,which is in favor of the dispersion of WO3in ZrO2to produce more acid sites,supported by other characterization results.
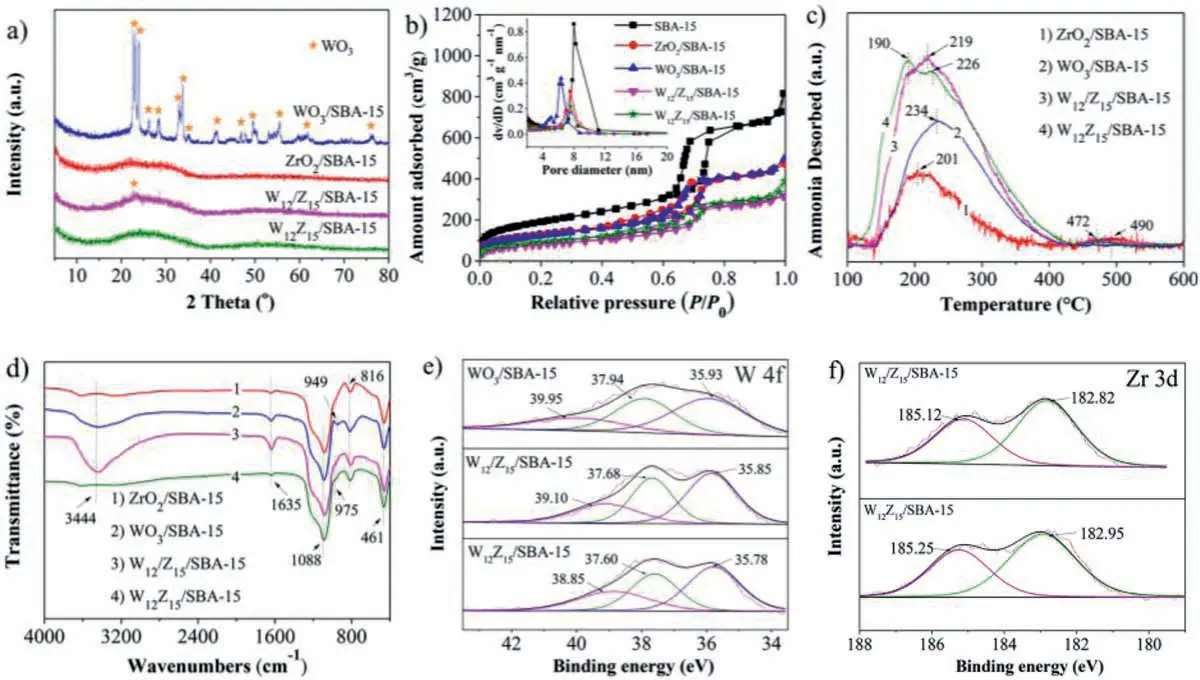
Fig.2.(a) Wide angle XRD patterns.(b) N2 adsorption-desorption isotherms (insert: pore size distributions).(c) NH3-TPD profiles.(d) FT-IR spectra.(e) W 4f and (f) Zr 3d XPS profiles of the prepared catalysts with diverse preparation methods.
Fig.2a shows the wide XRD patterns of WO3/SBA-15,ZrO2/SBA-15,W12/Z15/SBA-15,and W12Z15/SBA-15.Obviously,a broad peak at around 2θ=20o-35o,indicating the amorphous phase of SBA-15 [30],can be observed in all samples.For WO3/SBA-15,typical diffraction peaks of WO3can be observed,meaning that the STA has decomposed into WO3due to the high calcination temperature [31–34].Moreover,no typical diffraction peaks of ZrO2in ZrO2/SBA-15 can be observed,suggesting that ZrO2uniformly disperses inside the channels of SBA-15 according to previous reports [35,36].Noticeably,no obvious peaks attributed to WO3and ZrO2can be observed in W12Z15/SBA-15,evidencing that the impregnation method,that active ingredients,ZrO2and WO3,are impregnated into the channels of SBA-15 simultaneously,achieves the uniform dispersion of WO3in ZrO2,which is derived from the strong interaction in the formed composite oxide WO3-ZrO2.Nevertheless,some peaks assigned to WO3can be observed in W12/Z15/SBA-15 clearly,and the large grains of WO3suggest the uneven dispersion of WO3due to the relatively weak interaction between ZrO2and WO3in contrast to W12Z15/SBA-15.The analysis results of XRD are consistent with HRTEM and HAADF-STEM.From the small angle XRD results shown in Fig.S1 (Supporting information),the typical diffraction peaks at 2θ=0.5o-2oare attributed to the diffraction of the (100),(110) and (200) planes of SBA-15[37],associated withP6mmhexagonal symmetry of SBA-15,which can be observed in W12Z15/SBA-15,indicating the preservation of ordered mesoporous structure,being consistent with the analysis results above.N2adsorption-desorption isotherms and pore size distributions of the as-prepared catalysts are presented in Fig.2b,and SBA-15 is introduced as comparison.The specific surface area(SBET) and pore volume (Vtotal) are listed in Table S1 (Supporting information).From Fig.2b,the characteristic isotherms suggest the mesoporous structure of all the samples.However,the loading of active ingredients results in the remarkable decreases ofSBET,Vtotaland pore size in all samples,especially W12Z15/SBA-15 and W12/Z15/SBA-15,as shown in Table S1 and Fig 2b.Noticeably,the sharply reduced pore size of WO3/SBA-15 relative to others indicates the STA tends to aggregate into large particles and evolves into WO3after the calcination process at high temperature,resulting in the blockage of channels,which is harmful for mass transfer.Consequently,the increased pore sizes of W12Z15/SBA-15 and W12/Z15/SBA-15,especially the former one,in contrast to WO3/SBA-15,evidences the effect of ZrO2on the dispersion of WO3.Moreover,compared with W12/Z15/SBA-15,W12Z15/SBA-15 has largerSBET,Vtotaland pore size,which is favorable for the dispersion of acidic sites and mass transfer.The acidic properties are the crucial factor for the catalytic performance.Thus,NH3-TPD method was applied to uncover the acid sites nature of the prepared catalysts.NH3-TPD profiles are presented in Fig.2c,and the amount of acid sites (Na) of the as-prepared catalysts is listed in Table S1.The amount of acidic sites in single component solid acid catalysts ZrO2/SBA-15 is 71.22 μmol/g and WO3/SBA-15 possesses a few more acid sites,reaching 136.53 μmol/g.For W12/Z15/SBA-15,the amount of acid sites is basically equal to the sum of ZrO2/SBA-15 and WO3/SBA-15.Surprisingly,the amount of acid sites increased sharply in W12Z15SBA-15,reaching to 232.46 μmol/g due to the formation of more composite oxide WO3-ZrO2originated from the special impregnation way of active species described above,which is conductive to enhance solid acidity proved by previous reports [21,38].The large number of acid sites in W12Z15SBA-15 evidences the strong interaction of WO3and ZrO2.Moreover,the acidic properties of W12Z15/SBA-15 and W12/Z15/SBA-15 solid acid catalysts were also tested by Py-IR at 150 °C desorption temperature,which can be assigned to total acid sites.As shown in Fig.S2 (Supporting information),the vibration bands at 1540 cm−1and 1450 cm−1are ascribed to the B and L acid sites,respectively.Obviously,both B and L acidic centers can be observed on the two samples,and the L/B acid site on W12Z15/SBA-15 is much larger than that on W12/Z15/SBA-15.Combined with NH3-TPD results,the increased acid sites in W12Z15/SBA-15 relative to W12/Z15/SBA-15 are attributed to L acid sites.Both B and L acid sites can promote this reaction according to previous reports [1–3,11,13].Fig.2d shows the framework FT-IR spectra of as-prepared solid acid catalysts.All samples display three absorption peaks at 1088,816 and 461 cm−1,which are attributed to Si-O-Si stretching,Si-O stretching,and Si-O-Si bending modes of vibration in SBA-15,respectively [39–41].These bands,together with the band at about 3458 cm−1and the band at 1635 cm−1assigned to vibration of the -OH stretching vibration mode and adsorbed water respectively [40,42],can be observed in all samples.In the spectra of ZrO2/SBA-15,no obvious vibration mode of ZrO2can be observed due to overlapping with SBA-15 [43,44].And for WO3/SBA-15,the band at 949 cm−1is assigned to the Si-O-W linkage [45–47].Obviously,the peak intensity of the band reduces sharply and shifts to 975 cm−1in W12Z15/SBA-15 and W12/Z15/SBA-15 after the introduction of ZrO2,evidencing the strong interaction between WO3and ZrO2.Noticeably,the strong absorption peaks of −OH stretching vibration mode and adsorbed water can be observed in WO3/SBA-15 and W12/Z15/SBA-15,meaning the existence of a considerable amount of water,which is harmful for alkenylation,because the acid sites are easily covered by the water molecules from the competitive adsorption of aromatic compound,resulting in a situation that the aromatic compound are hindered to approach the active centers [48].The electronic environment of active sites can affect the adsorption properties of reactant [49–52],so the prepared catalysts were investigated by XPS.Fig.2e shows the XPS spectra of the W 4f region.The peaks at binding energies of 35.93 eV and 37.94 eV in WO3/SBA-15 can be assigned to W6+species [53–56].A small peak at ∼40 eV is due to W 5P3/2[53].Clearly,these peaks shift to lower region in W12/Z15/SBA-15 and W12Z15/SBA-15,implying the strong interaction between WO3and ZrO2,which is further confirmed by the analysis of Zr 3d spectra.The much lower binding energies of W6+species in W12Z15/SBA-15 suggest the enhanced interaction between WO3and ZrO2,resulting in the formation of more composite oxide WO3-ZrO2.Fig.2f shows the XPS spectra of Zr 3d region.The binding energies of 182.82 eV and 185.12 eV are assigned to Zr4+species in W12/Z15/SBA-15 [53],which shift to higher region in W12Z15/SBA-15 due to the high electron attractor effect of the neighboring W atoms [57],which demonstrates the enhanced interaction between WO3and ZrO2in contrast to W12/Z15/SBA-15 again.According to the analysis results above,W12Z15/SBA-15 prepared by the special impregnation method mentioned above possesses more acid sites,relatively highSBET,largeVtotaland moderate pore size due to the uniform dispersion of WO3in ZrO2originated from the strong interaction among them,which is in favor of alkenylation.
In order to verify the effect of synergy of WO3with ZrO2on the boost for the alkenylation ofp-xylene with phenylacetylene,the catalytic properties of prepared catalysts were investigated and the reaction results are depicted in Table 1.The alkenylation reaction ofp-xylene with phenylacetylene is a quite complex competition process,and the mixture consists of main productα-(2,5-dimethylphenyl) styrene (I),also called asα-arylstyrene,and sideproducts acetophenone (II),α-(2,5-dimethylphenyl)ethylbenzene(III),β-(2,5-dimethylphenyl)styrene (IV),and oligomers (V).From Table 1,W12Z15/SBA-15 exhibits the best catalytic performance due to more acid sites,relatively highSBET,largeVtotaland suitable pore size.The conversion of phenylacetylene can reach 94.7% with aconsiderable selectivity ofα-arylstyrene,reaching 88.4%.Besides,fewer oligomers (V),being easy to evolve into coke during the reaction,can be attained over W12Z15/SBA-15 compared with the other three samples,evidencing that the oligomerization is limited and therefore coke reduces,preventing the deactivation of catalysts consequently.However,W12/Z15/SBA-15 presents poorer catalytic properties compared with W12Z15/SBA-15,both the conversion of phenylacetylene and the selectivity ofα-arylstyrene,due to the relatively weak interaction between WO3and ZrO2.Moreover,the specific activity results shown in Table S2 (Supporting information) suggest that the excellent performance of W12Z15/SBA-15 is attributed to the increase of number of active sites and the enhanced intrinsic activity of acid sites,evidencing the superiority of W12Z15/SBA-15 again.Not surprisingly,it is anticipated that both ZrO2/SBA-15 and WO3/SBA-15 show bad results for the alkenylation,even though ZrO2/SBA-15 presents considerableα-arylstyrene selectivity,reaching 93.3%.Combined with characterization and reaction results,the enhanced interaction of WO3with ZrO2in W12Z15/SBA-15,contributing to the formation of more composite oxide WO3-ZrO2resulting from the special impregnation way of active component,has a decisive effect on the boost for alkenylation ofp-xylene with phenylacetylene.Clearly,W12Z15/SBA-15 prepared by the method described above is more conducive for alkenylation.Consequently,the composition of active species and the calcination temperature of the solid acid catalysts were optimized to obtain the optimal catalyst.

Table 1 Reaction results for the alkenylation of p-xylene with phenylacetylene over prepared catalysts with diverse preparation methods.a
According to results above,the introduction of ZrO2is favorable for the dispersion of WO3,and the formation of composite oxide WO3-ZrO2can produce more acid sites.Therefore,a series of W12ZX/SBA-15 solid acid catalysts with variousn(ZrO2/WO3) were prepared.The effect ofn(ZrO2/WO3) on the catalyst structure was investigated by XRD experiments,and the characterization results are shown in Fig.S3 (Supporting information).Obviously,two weak peaks assigned to WO3can be observed in W12Z12/SBA-15,not seen in W12Z15/SBA-15 and W12Z24/SBA-15.Therefore,it is clear that the high loading of ZrO2helps to disperse WO3through the strong interaction among them.Figs.S4a and b (Supporting information) present N2adsorption-desorption isotherms and pore size distributions of the as-prepared W12ZX/SBA-15 catalysts.TheSBET,andVtotalare listed in Table S3 (Supporting information).With the rise ofn(ZrO2/WO3) from 12/12 to 24/12,theSBETdecreases monotonically due to the high loading of active ingredients.Moreover,Vtotaland pore size increase whenn(ZrO2/WO3)increases from 12/12 to 15/12 due to the more uniform dispersion of WO3in ZrO2reducing the blockage of channels,resulting from the strong interaction among them.However,smallerVtotaland pore size can be obtained in W12Z24/SBA-15 due to excessive addition of ZrO2compared with W12Z15/SBA-15,which is harmful for mass transfer.According to the results above,the conclusion can be obtained that the appropriate addition of ZrO2is beneficial to the dispersion of WO3to form composite oxide WO3-ZrO2,which is conductive to produce more accessible acid sites.Nevertheless,excessive addition of ZrO2results in the reducing of exposure of composite oxide WO3-ZrO2to surface,fewer accessible acid sites producing consequently confirmed by NH3-TPD results.Fig.S5 (Supporting information) shows the NH3-TPD profiles of the as-prepared catalysts,and the number of acid sites is listed in Table S3 (Supporting information).Clearly,W12Z15/SBA-15 has the acidest sites due to the appropriate loading of ZrO2,being consistent with analysis results above.Fig.3a and Table S4 (Supporting information) shows the catalytic performance of as-prepared W12ZX/SBA-15 catalysts with diversen(ZrO2/WO3).Based on more acid sites,largerVtotaland pore size compared with the other two samples,W12Z15/SBA-15 shows best catalytic performance for alkenylation.Due to the suitable pore size,the least oligomers(V) can be obtained over W12Z15/SBA-15.According to the results above,W12Z15/SBA-15 stands for the optimum composition of ZrO2and WO3.
Subsequently,a series of solid acid catalysts calcined at variousTwere prepared.The textural properties of the as-prepared catalysts were investigated by N2adsorption-desorption experiment,and relevant results are illustrated in Figs.S6a and b (Supporting information).SBETandVtotalare listed in Table S5 (Supporting Information).It is obvious that theSBETdecreases monotonically with the rise ofT.Moreover,Vtotalpresents a completely opposite tendency in contrast toSBET.The pore size increases whenTrises from 450 °C to 500 °C,and it decreases whenTfurther increases from 500 °C to 550 °C.Fig.S7 (Supporting information) shows the NH3-TPD profiles of the as-prepared solid acid catalysts calcined at diverse temperature,and the amount of acid sits of relevant catalysts is listed in Table S5.Clearly,W12Z15/SBA-15-500 has the acidest sites due to suitableT,reaching 234.75 μmol/g,which is greater than W12Z15/SBA-15-550.Fig.S8 (Supporting information)shows the FT-IR spectra of the as-prepared catalysts,and ZrO2/SBA-15 and WO3/SBA-15 are introduced as reference.Obviously,for W12Z15/SBA-15-450,the intensity of bands at around 1635 cm−1and 3444 cm−1are obviously stronger compared with the other samples,meaning the existence of quantity of crystalline water,which is harmful to alkenylation.Combined with the results above,appropriateTof solid acid catalyst can produce more acid sites,and largerVtotal,which is favorable for alkenylation.As is shown in Fig.3b and Table S6 (Supporting information),W12Z15/SBA-15-500 shows the best catalytic performance with a 99.4% conversion of phenylacetylene and a 92.3% selectivity ofα-arylstyrene due to the acidic sites,largeVtotal.Moreover,very low level of oligomers (V)can be obtained at the same time,which is attributed that suitable pore size reduces the formation of coke and avoids the blockage of channels,being conductive to mass transfer.Besides,W12Z15/SBA-15-450 shows obviously worse catalytic properties compared with the other three samples,resulting from the overlay of active sites by the considerable crystalline water proved by FT-IR.Therefore,the calcination temperature of 500 °C is most favorable for obtaining optimized solid acid catalyst for alkenylation.
In addition,the stability and regeneration of the obtained optimized solid acid catalyst W12Z15/SBA-15-500 was investigated,and the conversion and selectivity as a function of time on steam for the alkenylation ofp-xylene with phenylacetylene over the fresh and regenerated W12Z15/SBA-15-500 is shown in Figs.3c and d,with WO3/SBA-15 introduced as comparison.At 300 min,W12Z15/SBA-15-500 presents the highest conversion of phenylacetylene with the highest selectivity ofα-arylstyrene,reaching 99.4% and 92.9% respectively.77.9% conversion can be maintained for up to 480 min of time on stream over W12Z15/SBA-15-500 solid acid catalyst with excellent 91.3% selectivity ofα-arylstyrene.Moreover,the selectivity ofα-arylstyrene still reaches 87.8% at 720 min with a poor catalytic activity,implying the deactivation of part of catalysts.As for regenerated one,recovered from spent W12Z15/SBA-15-500 by a simple calcination process,the activity and selectivity ofα-arylstyrene are just a little less than the fresh one with the same variation tendency.It is evident that the deactivation of catalyst is derived from carbon deposition,which can be removed by a simple calcination process.However,the catalytic performance of WO3/SBA-15 is bad due to more oligomers producing during the reaction,which will evolve into coke to overlay the active sites,resulting in the rapid deactivation of catalyst.According to the evidence described above,W12Z15/SBA-15-500 possesses excellent long-time stability and renewability.

Fig.3.(a) Reaction results for the alkenylation of p-xylene with phenylacetylene over W12ZX/SBA-15 catalysts with diverse n(ZrO2/WO3) and (b) Reaction results for the alkenylation of p-xylene with phenylacetylene over W12Z15/SBA-15-T catalysts with diverse calcination temperature;Reaction conditions: np-xylene/Phen=25:1,Tr=150 °C,Ps=1.0 MPa,mcat.=0.8 g,VHSV=7.5 mL h−1 gcat.−1,TOS=6 h.(c,d) The catalytic stability and regeneration performance of the optimized W12Z15/SBA-15-500 solid acid catalyst for the alkenylation of p-xylene with phenylacetylene.WO3/SBA-15 was introduced as comparison.
In conclusion,the developed ordered mesoporous solid acid catalyst W12Z15/SBA-15-500 prepared by IWI method with a subsequent calcination process presents excellent catalytic performance for the alkenylation ofp-xylene with phenylacetylene,with a 99.4% conversion of phenylacetylene and a 92.3% selectivity of main product (I)α-arylstyrene,which is attributed that the special preparation method that active components,WO3and ZrO2,are impregnated into the channels of SBA-15 simultaneously achieves the uniform dispersion of WO3in ZrO2,resulting in the formation of more composite oxide WO3-ZrO2,more accessible acid sites producing consequently.Besides,the relatively high specific surface area,large pore volume and ordered-mesoporous structure of SBA-15 are favorable for mass transfer.Moreover,the regenerated catalyst obtained by a simple calcination process shows comparable catalytic properties with the fresh one,meaning the excellent regeneration of the developed solid acid catalyst.Consequently,W12Z15/SBA-15-500 can be regarded as an outstanding candidate for the alkenylation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (No.21276041) and by the Chinese Ministry of Educationviathe Program for New Century Excellent Talents in University (No.NCET-12-0079).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.07.071.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
