Achieving simultaneous Cu particles anchoring in meso-porous TiO2 nanofabrication for enhancing photo-catalytic CO2 reduction through rapid charge separation
Jinyn Xiong,Mengmeng Zhng,Mengjie Lu,Ki Zho,Cho Hn,Gng Cheng,Zhipn Wen,∗
a College of Chemistry and Chemical Engineering,Hubei Key Laboratory of Biomass Fibers and Ecodyeing &Finishing,Wuhan Textile University,Wuhan 430200,China
b School of Chemistry and Environmental Engineering,Wuhan Institute of Technology,Wuhan 430205,China
c School of Materials Science and Energy Engineering,Foshan University,Foshan 528000,China
d Australia School of Civil and Environmental Engineering,Faculty of Engineering and Information Technology,University of Technology Sydney,Sydney,NSW 2007,Australia
Keywords:TiO2 Copper Photo-catalytic CO2 reduction Photo-catalysis Charge separation Schottky junction
ABSTRACT A facile solvo-thermal approach was successfully employed to prepare titanium oxide (TiO2) nanoaggregates with simultaneous copper particles anchoring.The as-synthesized composite could convert CO2 into CH4 and CO products under simulated solar irradiation.The impact of copper loading amounts on the photo-reduction capability was evaluated.It was found proper amount of Cu loading could enhance the activity of CO2 photo-reduction.As a result,the optimal composite (TiO2-Cu-5%) consisting of TiO2 supported with 5% (mole ratio) Cu exhibits 2.2 times higher CH4 yield and 3 times higher CO yield compared with pure TiO2.Conduction band calculated from the band gap and valence X-ray photoelectron spectroscopy (XPS) indicated TiO2 nano-aggregates have suitable band edge alignment with respect to the CO2/CH4 and CO2/CO redox potential.Furthermore,with involving of Cu particles,an efficient separation of photo-generated charges was achieved on the basis of photocurrent response and photoluminescence spectra results,which contributed to the improved photo-catalytic performance.The present work suggested that the Cu-decorated TiO2 could serve as an efficient photo-catalyst for solar-driven CO2 photo-reduction.
Overcoming the current challenge of energy crisis and climate change resulting from excessive fossil fuels combustion along with CO2emissions has attracted great attention.Semiconductor photo-catalysis,especially solar-driven photo-catalytic CO2reduction,was regarded as one of promising approaches for environmentally friendly converting CO2into hydrocarbon fuels.To achieve such an artificial photosynthesis conversion,the development of a high-active photo-catalyst towards CO2photo-reduction reaction is a prerequisite issue.
Since Inoueet al.[1]reported the conversion of CO2to small amounts of hydrocarbon fuels in the presence of photosensitive semiconductor powders suspended in water as catalysts,various semiconductor photo-catalysts,including oxides [2,3],sulfides[4,5],perovskite [6],carbon nitride [7],metal/covalent organic framework [8–10],etc.,had been widely employed to study for the CO2photo-reduction.Among those semiconductor photo-catalysts,TiO2-based materials are still at the center of attention due to their remarkable stability and suitable band structure [11].However,pure TiO2materials usually suffered from the drawback of the rapid recombination of photo-induced electron-hole pairs,which prohibited the transfer of charge carriers and accordingly slowed the photo-catalytic CO2reduction reaction down,resulting in a low photo-catalytic activity.
As a matter of fact,the CO2photo-reduction reaction contains three main processes.Firstly,the semiconductor photo-catalysts absorb the light and produce electron-pairs.Then,the electrons and holes would be separated and transfer to the surface of the semiconductor.Lastly,CO2reduction and H2O oxidation reactions occur with involving of electrons and holes,respectively.In other words,under the condition of thermodynamic equilibrium,the photo-catalytic CO2reduction performance is determined by the kinetics of the above three processes.[11]Therefore,it is reasonable to promote the capability of CO2photo-reduction through enhancing the efficiency of one or more of the above three processes during the photo-catalysis reaction.

Fig.1.Illustration for the fabrication process and formation mechanism of TiO2-Cu hybrids.
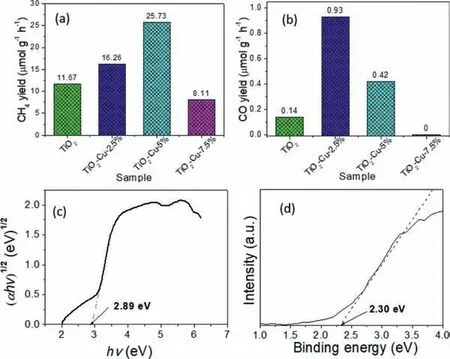
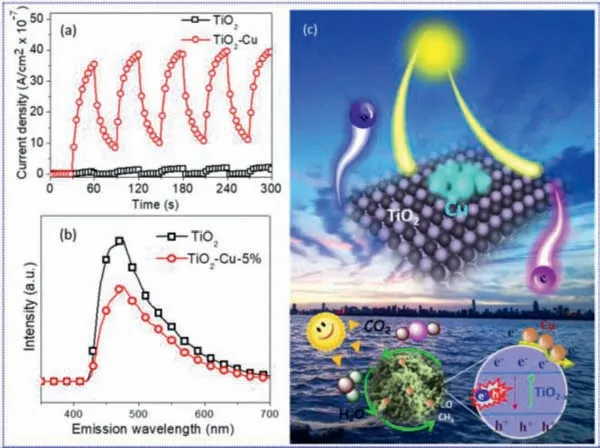
The past few years,a tremendous flurry of research interest have been devoted onto the surface,interface,and composition engineering of TiO2-based photo-catalysts for enhancing their relatively photo-catalytic activity [12,13].Especially,continued breakthroughs have been made in the co-catalyst effect [14–17],oxygen vacancy involving [18-22],hetero-junction construction [23–28],etc.Among them,coupling with metal co-catalyst [29,30],as one of promising approaches,has attracted more interest thus it can enhance the photo-catalytic performance through promoting the electron–hole separation and migration.For example,Xieet al.[31]examined the effect of noble metal co-catalysts and found that the rate of CH4formation increased in the sequence of Ag Based on the above backgrounds and inspired by the challenges,the present study focuses on the solar-driven CO2photo-reduction activity of nonprecious Cu particle anchored TiO2.Combining with previous work on preparation of hetero-phase TiO2[36]and Cu-MOx(M=W,Ti and Ce) [37]nano-composites,a facile onepot polyol-mediated solvo-thermal approach was employed to successfully prepare TiO2-Cu nano-hybrids.The corresponding photocatalytic performance was evaluated through CO2reduction under simulated sunlight irradiation.The contribution of the Cu particles anchored on the TiO2nano-aggregates to its superior photocatalytic reduction capability was also studied. Fig.1 shows the preparation process of TiO2-Cu composites,which contains the formation of mesoporous TiO2nanostructure and simultaneous decoration of Cu particle by polyol reduction strategy.The X-ray diffraction (XRD) patterns of the as-synthesized products were displayed in Fig.2a.It can be found the diffraction peak of the material synthesized without involving of Cu2+precursor could be well indexed with the standard anatase phase TiO2(JCPDS No.1-562).After introducing the Cu2+precursor into the reaction system,it was found the as prepared materials were composed of TiO2and Cu from the XRD diffraction peaks,which correspond well with standard patterns of JCPDS No.1-562 and 1-1242.Furthermore,with increasing of Cu2+precursor concentration from TiO2-Cu-2.5% to TiO2-Cu-7.5%,the diffraction peak of Cu became stronger.This result suggested that the TiO2-Cu nanohybrids had been successfully synthesized.As shown in the UV–vis diffuse reflectance spectrum (UV-DRS) of the as-prepared materials (Fig.2b),an obvious absorption tail could be detected in the visible light region,suggesting that the involving of copper could tailor the light absorption of the materials.As shown in Fig.S1a(Supporting information),when further increased the copper loading amounts,the XRD diffraction peaks of copper in the composites continue growing.At the meantime,as shown in Fig.S1b (Supporting information),the absorption tail of those composites obviously shifted to the visible light region,but the absorption peak intensity below 350 nm become weaker due to the decreased TiO2amount. X-ray photoelectron spectroscopy (XPS) was further used to confirm the intrinsic characteristics and chemical states of the asprepared materials.Take TiO2-Cu-5% as an example,the survey XPS spectrum in Fig.2c suggests the sample consists of the elements of Ti,O,and Cu.Figs.2d-f show high-resolution XPS spectra of Ti 2p,O 1s,and Cu 2p,respectively.The binding energy peaks located at 458.5,459.5,and 464.2 eV belong to the valence states of Ti 2p.The peaks at 529.7 and 531.2 eV of O 1s spectrum (Fig.2e) are attributed to the formation of Ti-O bonds in TiO2.As displayed in Fig.2f,the two main peaks at 932.5 and 952.4 eV correspond to the metallic Cu(0) 2p3/2and Cu 2p1/2state [38],respectively. The morphology and structure of the as-obtained TiO2and TiO2-Cu-5% products were shown in Fig.3a.The TiO2sample shows morphology of aggregated spherical nanoparticles.With involving of Cu precursor into the reaction system,the obtained TiO2-Cu-5% sample keeps the same morphology (Fig.3b).However,the energy dispersive X-ray spectroscopy (EDX)-mapping in Figs.3c-f suggests the material contains the elements of Ti,O,and Cu.TEM images in Figs.3g-h indicate that both the TiO2and TiO2-Cu-5% samples comprise many small nano-crystals,leading to apseudo-porous structure.As shown in HRTEM image in Fig.3i,the lattice fringes of the nanoparticles can be easily identified to be 0.35 and 0.21 nm apart,which is in good agreement with the(101) plane of TiO2and the (111) plane of Cu,respectively.The above results confirm the successful decoration of Cu nanoparticles on the surfaces of the TiO2nano-aggregates.The N2adsorption–desorption isotherms (Fig.S2 in Supporting information) of the TiO2-Cu-5% sample display a distinct Type II hysteresis loop and reveal the typical characteristics of porous materials.The calculated BET surface area is 159.9 m2/g,which corresponds to the average pore sizes of the 9.1 nm. The photo-catalytic activities of the as-synthesized TiO2and TiO2-Cu nano-composites were evaluated through solar-driven CO2reduction in the presence of H2O vapor under continuous artificial sunlight irradiation.Figs.4a and b show the CO2photo-reduction activity and the corresponding evolution rates of the above materials.It was found pure TiO2could convert CO2into CH4and CO products under light irradiation.Furthermore,the involving of proper amounts of copper could enhance the evolution rates of CH4and CO.When the TiO2-Cu-5% was used as the catalyst,the evolution rates of CH4and CO were 25.73 and 0.42 μmol g−1h−1,respectively,which were significantly promoted comparing with that of pure TiO2(11.67 and 0.14 μmol g−1h−1for CH4and CO,respectively).It is clear that the TiO2-Cu-5% present a 2.2 times higher yield of CH4and 3 times higher CO yield compared with pure TiO2.When further increase the Cu content (TiO2-Cu-7.5%),the CO2photo-reduction activity decreased and it was attributed to the decrease of TiO2in such hybrid (Fig.S3 in Supporting information).As shown in Fig.S3,when the loading amount of Cu was 50%,no CO was produced and the CH4yield of TiO2-Cu-50% was only one tenth of pure TiO2.This is due to that too much Cu could shield light absorption of TiO2(Fig.S1b).In addition,the XRD pattern of the sample after CO2photo-reduction was also collected and displayed in Fig.S4 (Supporting information).It was found the composites still showed the main constitute of TiO2and Cu.But the diffraction peak of Cu2O also appeared due to the oxidation of Cu by the generated O2during the photo-catalysis reaction. Fig.2.(a) XRD patterns and (b) UV-DRS spectra of TiO2 and TiO2-Cu products.(c) Whole survey,(d) Ti 2p,(e) O 1s and (f) Cu 2p XPS spectra of TiO2-Cu-5%. Fig.3.SEM images of TiO2 (a) and TiO2-Cu-5% (b).(c-f) EDX elemental mapping images of TiO2-Cu-5%.TEM image of TiO2 (g).TEM (h) and HRTEM (i) images of TiO2-Cu-5%. It is well-known that proper matching of valence band (EVB)and conduction band (ECB) sites is important for CO2photoreduction.As shown in Fig.4c,the band gap (Eg) of TiO2was calculated to be 2.89 eV from the Kubelka-Munk function.At the same time,the valence band extreme of TiO2on the basis of Valence band XPS spectra in Fig.4d was 2.30 eV.Then,theECBlevel from(EVB−Eg) was −0.59 eV.Previous studies have pointed out that the potential for reducing CO2to CH4and CO in water at a pH value of 7 is −0.24 V (ECO2/CH4=−0.24 Vvs.NHE) and −0.53 V(ECO2/CO=−0.53 Vvs.NHE) [39–40],respectively.In this work,theECBvalue of TiO2(corresponding to −0.59 V) was more negative than those values.This indicates that CH4and CO could be the preferred product. To better understand the improvement of photo-catalytic activities for the TiO2-Cu nano-composites,the transient photocurrent responses of the as-prepared products were performed to characterize the generation,migration,and recombination of photoinduced electrons and holes.It was clearly observed in Fig.5a that the photocurrent density of the TiO2-Cu-5% sample electrode was much higher than that of pure TiO2,suggesting a higher separation and lower recombination rate of photo-generated electronhole pairs in such a hybrid during the photo-catalysis process [41–45].Photoluminescence (PL) spectra were further used to study the electron-hole separation of the photo-catalyst.As shown in Fig.5b,the TiO2-Cu-5% sample displayed a lower fluorescence intensity than that of pure TiO2,which indicates a higher separation rate of photo-generated electron and hole pairs during CO2photoreduction.Considering the Cu has higher work function than that of TiO2[14,46,47],the photo-induced electrons in TiO2would be transferred to the Cu.Meanwhile,the formation of Schottky barrier between TiO2and Cu resulted from the strong interfacial interaction in the TiO2-Cu nano-composite could promote the transfer and separation of photo-generated electrons. Fig.4.Photo-reduction activity towards the conversion of CO2 into CH4 (a) and CO(b) upon TiO2 and TiO2-Cu nano-composites under simulated solar light irradiation for 4 h.(c) Calculated band gap from UV-DRS spectra of TiO2.(d) Valence band XPS spectra of TiO2. Fig.5.(a) Photocurrent response and (b) PL spectra of TiO2 and the TiO2-Cu-5%nano-composites.(c) Schematic illustration of the charge transfer paths in the TiO2-Cu nano-composite towards CO2 photo-reduction. On the basis of the above analysis,the impact of the Cu particles anchoring on the photo-catalytic CO2reduction in the TiO2material was illustrated in Fig.5c.As mentioned in the photoreduction test,the photo-catalysts firstly would adsorb a certain amount of CO2molecules before initiated the Xe lamp,as the adsorption is a prerequisite for the occurrence of a photo-catalysis reaction [48–49].Upon the light irradiation,the TiO2could absorb the light photons to generate photo-induced electrons and holes.Then,the electrons in the conduction band of TiO2would be transferred to the surface to engage in the photo-catalytic CO2reduction for yielding products.In particular,with involving of Cu particles,it would accept the photo-generated electrons from TiO2and accelerate the rapid charge separation and transfer.As a result,more effi-cient electron-hole separation in the TiO2-Cu nano-composite was achieved.Finally,the photo-catalytic CO2reduction to CH4and CO is significantly enhanced by more photo-generated electrons participating in the photo-catalysis process. In summary,the TiO2-Cu nano-composite was successfully prepared by a simple polyol approach,which combines the formation of TiO2nano-aggregates with reduction of Cu2+to Cu.The UV–vis diffuse reflectance and Valence band XPS spectra suggested the prepared TiO2nano-aggregates had suitable band edge alignment with respect to the CO2/CH4and CO2/CO redox potential.Under simulated sunlight irradiation,an enhanced CH4and CO yield was achieved in the photo-reduction of CO2using the TiO2-Cu nanocomposite.The TiO2-Cu-5% sample exhibits 2.2 times higher CH4yield and 3 times higher CO yield compared with pure TiO2.This performance enhancement is realized because efficient separation of photo-generated charges was achieved with involving of Cu particles into the TiO2nano-aggregates.It is expected this work could provide a rational reference for designing efficient and low cost photo-catalysts towards CO2reduction. Declaration of competing interest The authors declared that they have no conflicts of interest to this work. Acknowledgments This work was supported by the National Natural Science Foundation of China (No.22102122),the Hubei Provincial Natural Science Foundation (No.2019CFB386) and the Central Committee Guides Local Science and Technology Development Special Project of Hubei Province (No.2019ZYYD073). Supplementary materials Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.07.052.

 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
