Enhanced photocatalytic hydrogen production under visible light of an organic-inorganic hybrid material based on enzo[1,2-b:4,5-b’]dithiophene polymer and TiO2
Fng Jing,Ynmeng Guo,Bo Li,Yi-Fn Chen,∗,Chunmn Ji,∗,Jinwei Li,b,∗∗
a Hainan Provincial Key Laboratory of Fine Chemicals,Advanced Materials of Tropical Island Resources of Ministry of Education,School of Chemical Engineering and Technology,Hainan University,Haikou 570228,China
b MediCity Research Laboratory,University of Turku,Tykistökatu 6,Turku 20520,Finland
Keywords:Photocatalysis TiO2 Benzo[1,2-b:4,5-b’]dithiophene polythiophene Organic-inorganic hybrid materials Sol-gel process
ABSTRACT Titanium dioxide (TiO2) has been limited in photocatalysis due to its wide band gap (3.2 eV) and limited absorption in the ultraviolet range.Therefore,organic components have been introduced to hybrid with TiO2 for enhanced photocatalytic efficiency under visible light.Here,we report that benzo[1,2-b:4,5-b’]dithiophene polymer was an ideal organic material for the preparation of a hybrid material with TiO2.The energy band gap of the resulting hybrid material decreased to 2.9 eV and the photocatalytic hydrogen production performance reached 745.0 μmol g−1 h−1 under visible light irradiation.Meanwhile,the material still maintained the stability of hydrogen production performance after 40 h of photocatalytic cycles.The analysis of the transient current response and electrochemical impedance revealed that the main reasons for the enhanced water splitting of the hybrid materials were the faster separation of electron hole pairs and the lower recombination of photocarrier ions.Our findings suggest that polythiophene is a promising organic material for exploring hybrid materials with enhanced photocatalytic hydrogen production.
Energy shortage and environmental pollution have always been problems that all human beings have to solve.The most reliable and environmentally friendly way to solve the problems is to use the renewable and low-cost characteristics of solar energy to achieve heterogeneous catalysis [1–6].Among them,the production of hydrogen by splitting water through solar energy is regarded as a significant method of solar energy conversion [7–8].Titanium dioxide (TiO2) is widely used in the photocatalyst market due to its non-toxic,stable chemical properties,and low cost.However,there are still many shortcomings such as low solar energy utilization and serious photo-generated charge recombination.To enhance the photocatalytic performance of TiO2,it has been modified by means of metal and non-metal ion doping [9–12],dye sensitization [13–14],and narrow band gap semiconductor compounding [15–16].
In recent years,the research of organic polymer regarded as semiconductors in the field of photocatalysis has made certain progress.Organic polymer semiconductors have flexible composition and structures and can bring functional groups into them to adjust energy position and band gap,thereby achieving different photocatalytic reaction and improving the photocatalytic activity [17–19].Among them,polythiophene semiconductors based on thiophene derivatives has been extensively studied,because the delocalization of holes on the oligothiophene moieties of the polymer may increase their photo and chemical stability and have a narrow band gap to exhibit the ability of photo splitting water [20–23].For example,a side-chain-extended conjugation strategy has been utilized to design and synthesize a conjugated polymer based on benzodithiophene- S,S-dioxide decorated with two additional thiophenes,exhibiting an excellent performance of hydrogen evolution reaction of 20.31 mmol g−1h−1and a record apparent quantum yield of 7.04% at 500 nm.Liet al.[25]designed a copolymer photocatalyst (Co-PBDT-bpy) based on benzodiazepine (BDT) and the ligand bipyridine (bpy) and subsequently chelating with cobalt.The photocatalytic hydrogen production performance of the Co-PBDT-bpy material was 140 μmol g−1h−1.However,owing to the high hydrophobicity and low dispersity,almost all organic polymer photocatalysts perform the photocatalytic reaction with the organic solvents like dimethylformamide (DMF) [24,25]and methanol [24],which decreases their stability and increases their cost.The organic-inorganic semiconductors hybrid is a structure and function optimization strategy for exploring artificial photocatalytic systems.Intrinsically,the hybrid material is endowed with a synergistic effect on the organic-inorganic semiconductor ingredients.The composite material shows a wide absorption range in the ultraviolet and visible regions of the solar spectrum and a matched energy position relationship to facilitate the charge and carrier separation efficiency at the semiconductor interface,which achieves the efficient heterogeneous photocatalysis.For example,Baiet al.[26]designed and constructed a Z-scheme system composed of P10 (homopolymer of dibenzo[b,d]thiophene sulfone) and BiVO4for overall water splitting under visible light irradiation.Houet al.[27]fabricated a composite material through an in-situ polycondensation procedure of 4,7-dibromobenzo[c][1,2,5]thiadiazole (BBT) and 1,3,5-triethynylbenzene in the presence of commercial TiO2(P25).The composite material exhibited dramatically enhanced visible-lightresponsive photocatalytic activities (18.0 times higher activity for H2evolution) as compared BBT alone.In the polymer/inorganic composite system,the introduction of inorganic semiconductors extremely improves the dispersity and hydrophilicity of polythiophene semiconductors in water to upgrade the photocatalytic activity of heterogeneous photocatalysts.However,the research of polythiophene/TiO2composites for photocatalysis is rare.This may be due to the poor stability of the composites arising from the weak binding between polythiophene and TiO2.
Here,we tackle these challenges in the design and synthesis of polythiophene/TiO2hybrid materials using two strategies.Firstly,we selected a benzo[1,2-b:4,5-b’]dithiophene polymer (herein collectively referred to as P42) showed in Scheme 1 as the organic semiconductor.Benzo[1,2-b:4,5-b’]dithiophene (BDT) was introduced into the polythiophene semiconductor.The BDT unit is consistent with two thiophene rings with a benzene core and is as an electron donor building block for conjugated polymers because of its outstanding properties,which includes a wide absorption with high absorptive coefficients,excellent thermal and chemical stability,and facile structural modification [28–29].In addition,the 4,8-positions of the BDT units are functionalized by two 2-(2-ethylhexyl) thiophene groups to realize the following two molecular engineering strategies: (1) the thiophene groups are employed to obtain a larger conjugation and thus lead to a wide light absorption ability;(2) the 2-ethylhexyl alkyl chains on the thiophene groups were introduced to increase the solubility and suppress the aggregation of polymer molecules.Secondly,we utilized an in-situ preparation strategy to obtain the organic-inorganic composite.The P42 was introduced into the preparation process of TiO2by the convenient sol-gel method,which not only enhanced the stability of the hybrid,but also facilitated the separation and transmission of photogenerated electrons,thereby improving the charge and carrier separation efficiency at the semiconductor interface.As a result,the composite material had porous channel structures with a large surface area,which was beneficial for the photocatalytic reaction.The preparation process has a low cost,easy-to-control conditions,simple operation,and excellent photocatalytic stability.All these advantages suggest that the design and synthesis of the P42-TiO2hybrid material provides an excellent example for the development of efficient polymer/TiO2photocatalysts.
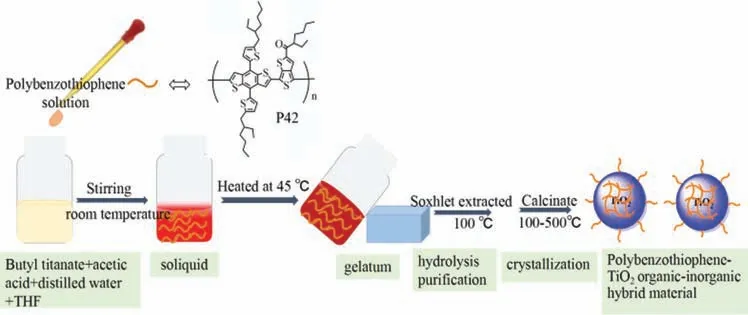
Scheme 1.The illustration of the preparation process of P42-TiO2 organic-inorganic hybrid material.
The preparation process of benzo[1,2-b:4,5-b’]dithiophene polymer-TiO2organic-inorganic hybrid material (denoted as P42-TiO2) is shown in Scheme 1.The hybrid material was prepared by a sol-gel method (Fig.S1 in Supporting information).Tetrabutyl titanate (1 mL) was added to a well stirred solution containing 1 mL tetrahydrofuran (THF),168 μL acetic acid and 106 μL water.Then,the different amount percentage of the benzodithiophene polymer (0/0.43/0.86/1.7/2.5 wt%) was dispersed in 1 mL tetrahydrofuran solution and was added to the above mixture.A gel was formed and heated at 45 °C overnight.The material was extracted using a Soxhlet extractor for two days and then calcined at 200 °C in a tube furnace using a temperature-programmed method (2.25 °C /min) from the room temperature for 120 min.To elaborate the formation process of the hybrid P42-TiO2materials,the intermediate products at various reaction time were collected to characterize their crystalline states by checking their XRD patterns (Fig.S2 in Supporting information).In the soliquid process,butyltitanate conducted a shrinkage reaction with P42 producing a uniform and transparent soliquid.The amorphous crystal phase diffraction peak at 21° (denoted as sol-20 min) was attributed to the close contact between the polymer P42 and inorganic butyltitanate phase.After further hydrolysis and polycondensation for butyltitanate,a more broaden amorphous crystal diffraction peaks at 21° (denoted as gel-10 h) was observed,suggesting the gelation process.In this process,P42 was embedded into the phase of the inorganic TiO2.The hybridization process of the organic and inorganic phase resulted in the broaden amorphous crystal diffraction peaks.Then,the precursor conducted a violent hydrolysis reaction to form anatase TiO2by Soxhlet apparatus with distilled water for 24 h (denoted as sox-24 h).The amorphous crystal phase diffraction peaks at the low angles were very weak,which indicated that P42 was inserted and embedded into the phase of inorganic TiO2.Thus,the organic and inorganic phase were robustly combined.Finally,the hybrid material was calcined at 200 °C (denoted as cal-200) to remove impurity.The hybrid materials calcined at 200 °C above with various P42 amounts were denoted as TiO2-200,0.43%P42-TiO2-200,0.86%P42-TiO2-200,1.7%P42-TiO2-200 and 2.5%P42-TiO2-200.
The hybrid material above (20 mg) was dispersed in a photocatalytic reaction cell containing 4 mL H2O and 10 mL methanol.K2PtCl4aqueous solution (1 mmol/L) was added under stirring and the resulting mixture was stirred another 60 min under the irradiation by a 300 W xenon lamp.After the illumination,the material was centrifugally washed with water and methanol,and then dried in vacuum.Pt loading (1.0 wt%) was tested by inductively coupled plasma atomic emission spectrometry (ICP-AES).
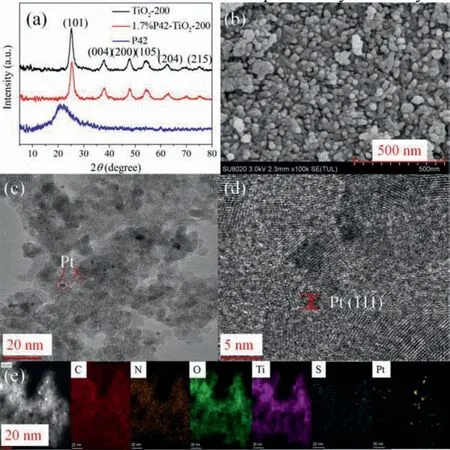
Fig.1.(a).The XRD patterns of TiO2-200,1.7%P42-TiO2-200 and P42;(b) SEM images of 1.7%P42-TiO2-200;(c) TEM,(d) HR-TEM,and (e) the elemental mappings image of Pt@1.7%P42-TiO2-200.
Photocatalytic hydrogen production was carried out in a glass reaction tank.A quartz cover was connected with a closed gas circulation,and it was swept with high purity Ar2before lighting.A 20 mg of photocatalyst was dispersed in 20 mL of 10 vol% triethanolamine (TEOA) aqueous solution.Then the mixture was exposed to a 300 W xenon lamp equipped with aλ >420 nm filter.The reaction solution was continuously stirred and cooled to 5 °C through cooling water circulation.The amounts of hydrogen production were measured using an on-line gas chromatography at intervals of 1 h.
After we obtained the materials,the crystal structure,morphology,optical property and visible-light photocatalytic activity were analyzed using powder X-ray diffraction (PXRD),scanning electron microscopy (SEM),transmission electron microscopy (TEM),X-ray photoelectron spectroscopy (XPS) and UV–vis diffuse reflectance spectra (UV–vis DRS).The PXRD analysis of TiO2-200,1.7%P42-TiO2-200 and P42 is shown in Fig.1a.The TiO2-200 material presented an anatase crystal phase,with characteristic diffraction peaks at 2θ=25.2o,37.9o,47.8o,54.3o,62.5o.The 1.7%P42-TiO2-200 showed the same XRD pattern as the TiO2-200,indicating that the doping process did not disrupt the anatase crystal form of TiO2.However,no obvious diffraction peaks of P42 were observed in the XRD pattern of the 1.7%P42-TiO2-200,which may be due to the low loading and high dispersibility of P42 in the TiO2-200.The thermogravimetric analysis in air atmosphere of P42 indicated that the pyrolysis temperature of P42 was 350 °C.Moreover,the thermogravimetric analysis of TiO2and 1.7%P42-TiO2material in air atmosphere showed that the proportion of P42 in the 1.7%P42-TiO2composite was about 1.4%,which approximately equals to the calculated proportion 1.7%.
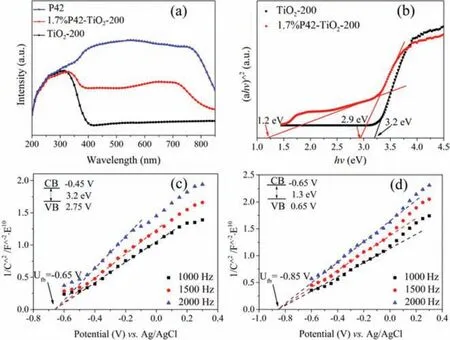
Fig.2.(a) The UV–vis DRS of TiO2-200,1.7%P42-TiO2-200 and P42.(b) The Tauc plot of TiO2-200 and 1.7%P42-TiO2-200 and the Mott-Schottky plot of (c) TiO2-200 and (d) P42.
The SEM image of the 1.7%P42-TiO2-200 nanocomposite material showed that the composite aggregates were stacked by nanospheres with an average diameter around 15 nm (Fig.1b).Platinum was supported on the surface of the composite material as a co-catalyst to give rise to more hydrogen.The TEM image of the TiO2containing 1.7%P42 calcinated at 200 °C loaded with 1.0 wt% Pt (denoted as Pt@1.7%P42-TiO2-200) showed that the size of the platinum particles was about 3 nm (Fig.1c).The highresolution TEM (HR-TEM) image (Fig.1d) shows that the distance between two adjacent lattice planes of platinum nanoparticles was about 0.229 nm,which is consistent with the distance between the platinum (111) planes.In addition,the corresponding element map(Fig.1e) and energy spectrum (Fig.S4,and Table S1 in Supporting information) of the material show that carbon (C),oxygen (O),sulfur (S),titanium (Ti) and platinum (Pt) were evenly distributed throughout the hybrid material.
In general,the surface area of catalysts plays a significant role in the performance of photocatalytic hydrogen production.Thus,we assessed the change of the surface area of TiO2without and with the hybrid of P42 by detecting the nitrogen adsorption-desorption isotherms of the as-prepared materials at 77 K.The surface area of the 1.7%P42-TiO2-200 was determined as 175.95 m2/g which was approximately equal to that of the TiO2-200 197.63 m2/g (Fig.S5 and Table S2 in Supporting information).These results revealed that the surface area of the hybrid would not be decisive on the photocatalytic activity.
Subsequently,we performed the XPS measurement of the 1.7%P42-TiO2-200 to confirm that P42 was successfully dispersed on the TiO2-200.A composition of 20.9% Ti,52.48% O,0.43% S and 26.19% C in the 1.7%P42-TiO2-200 was obtained (Fig.S6 in Supporting information),corresponding to the composition of 23.44% Ti,54.53% O,and 22.03% C in the TiO2-200 °C (Fig.S7 in Supporting information).The O 1s,Ti 2p,N 1s,C 1s and Pt 4f spectra of the Pt@1.7%P42-TiO2-200 is shown in Fig.S8.The XPS spectrum of platinum shows mainly the signals at Pt 4f7/2(70.21 eV) and Pt 4f5/2(74.42 eV),which were attributed to Pt(0) (see Table S3).These results demonstrated that the Pt element was reduced and successfully loaded on the surface of the hybrid material.
To understand the effect of photophysical and photochemical properties of the composites,The UV–vis solid-state diffraction spectra of P42,1.7%P42-TiO2-200 and TiO2-200 were showed in Fig.2a.The TiO2-200 had an intense peak at 325 nm with an absorption edge of 400 nm,corresponding to a 3.2 eV band gap(Fig.2b),which was consistent with previous reports [30].The polymer P42 had a very wide range of light absorption at 400–800 nm owing to theπ-π∗transition with the charge transfer character.After hybridization of P42 with TiO2,a typical absorption band of the 1.7%P42-TiO2-200 was significantly broadened from 325 nm to 800 nm with two intense absorption range at 325 nm and 600–750 nm,which could be attributed to the robust linkage of organic-inorganic hybrid materials on the molecular or atomic levels.Differing from the TiO2-200,with only a band gap in the Tauc plots,the 1.7%P42-TiO2-200 showed two clear band gaps: A narrow band gap of 1.2 eV and a wide band gap of 2.9 eV (Fig.2b),which could be ascribed to P42 and TiO2-200,respectively.Compared with the band gap (1.3 eV) of the pure P42 (Fig.S9 in Supporting information),The narrow band gap (1.2 eV) of P42 doped in the P42-TiO2-200 indicated that the robust linkage existed between P42 and TiO2in the hybrid P42-TiO2-200 materials.
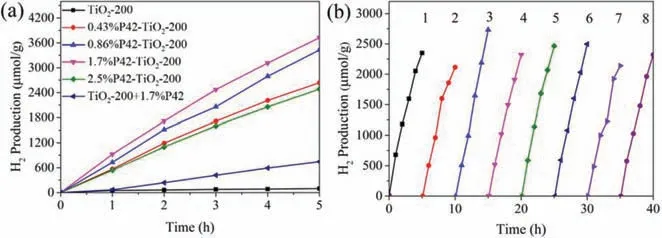
Fig.3.(a) Effect of the amount of polymer doped on the catalyst on the performance of P42-TiO2-200 photocatalytic hydrogen production,(b) Photocatalytic H2 production over the recyclability of 1.7%P42-TiO2-200 in 20 mL H2O/TEOA (9:1,v/v)with 1.0 wt% Pt loading under visible light irradiation (λ > 420 nm).
To clarify the possibility for subsequent photocatalytic hydrogen production from water,Mott-Schottky measurements of TiO2-200 and P42 were monitored at frequencies of 1000,1500 and 2000 Hz,respectively (Figs.2c and d).The positive slope was consistent with ann-type semiconductor.The flat band positions of TiO2-200 and P42 relative to the Ag/AgCl electrode were −0.65 eV and −0.85 eV,respectively,which was more negative than the redox potential of H+/H2.Since it is known that the conduction band(CB) potential in n-type semiconductors is approximately equal to the Vfb[13].Consequently,the CB of the TiO2-200 and P42 should be −0.45 eV and −0.65 eV (vs.Ag/AgCl),showing more negative values than the reduction potential of H+/H2.Based on the band gap diagrams in Fig.2b and Fig.S9,the valence bands of TiO2-200 and P42 were calculated as 2.75 eV and 0.65 eV,respectively(vs.Ag/AgCl).The conduction band value of P42 was more negative than the conduction band edge of TiO2-200,thus offering a thermodynamic driving for an efficient electron injection from P42 to TiO2-200.Moreover,the VB potential of P42 was more positive than the redox couple of TEOA as sacrificial agents,which would promote polymer regeneration and suppress the recapture of the injected electrons.
The extended absorption and reduced band gap of the hybrid material P42-TiO2encouraged us to evaluate its hydrogen production performance under visible light using TEOA as a sacrificial agent and the material loaded with 1% Pt.Firstly,we explored the effect of P42 doping amounts screened between 0.43 and 2.5 wt%P42-TiO2-200 on the hydrogen production performance of the entire catalytic system,and the results are presented in Fig.3a.The photoactivity increased with the increasing polymerdoped amounts,reaching a maximum for the 1.7% mass fraction of P42.The H2generation rate could reach 745.0 μmol g−1h−1and was 38 times than that of pure TiO2-200 material,and it was 5 times the hydrogen production of the material mechanically mixed by TiO2-200 with 1.7% (wt%) of P42 (denoted as TiO2-200+1.7%P42).However,further increase in the percentage to 2.5 wt% resulted in the activity decrease.In addition,we also optimized the calcination temperature on the performance of photocatalytic hydrogen production (Fig.S10 in Supporting information).The results show that the catalyst calcined at 200 °C exhibited the best photocatalytic performance.A cycle stability experiment was carried out on the 1.7%P42-TiO2-200 material to evaluate the stability and recyclability of the prepared catalyst material.As shown in Fig.3b,the photocatalytic hydrogen production rate of the 1.7%P42-TiO2-200 material hardly changed after 40 h of photoreaction.Moreover,the XRD analysis of 1.7%P42-TiO2-200 before and after photocatalytic reaction was showed in Fig.S11 (Supporting information).Both patterns mainly presented the anatase TiO2crystal phase characteristic diffraction peaks and weak amorphous crystal phase diffraction peaks for P42,indicating that the prepared organic-inorganic hybrid material had remarkable stability under light irradiation.
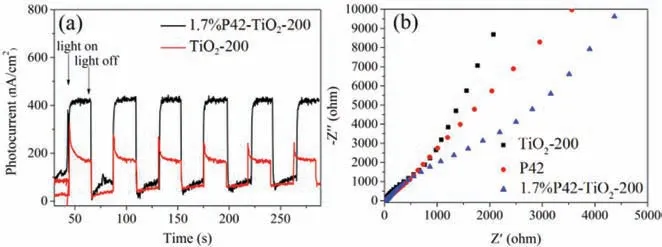
Fig.4.(a) The photocurrent intensity of the 1.7%P42-TiO2-200 (black curve) and TiO2-200 (red curve) and (b) EIS spectra of the 1.7%P42-TiO2-200 (blue triangles),TiO2-200 (black squares) and P42 (red dots).(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.).
To further confirm the enhanced photo-response,the photocurrent-time (i-t) curves of the TiO2-200 and the 1.7%P42-TiO2-200 electrode have been detected under illumination with several on/off switches (Fig.4a).The 1.7%P42-TiO2-200 electrode showed significantly higher photocurrent than TiO2-200.The photocurrent response of the 1.7%P42-TiO2-200 under visible light was much higher than that of TiO2-200 (see Fig.4a).Once the catalytic material was excited by visible light,the photogenerated charge separation efficiency of the 1.7%P42-TiO2-200 was significantly improved.These results indicated that more effective interfacial electron transfer between polymer semiconductor and TiO2in the P42-TiO2-200 system,which was beneficial to improve the photocatalytic activity.In addition,to gain insight into the charge-carrier transfer resistance and the photocatalytic activity of the hybrid material,electrochemical impedance spectra (EIS) of TiO2-200 and 1.7%P42-TiO2-200 were obtained using a three-electrode cell system under illumination (Fig.4b).1.7%P42-TiO2-200 had a smaller arc radius than TiO2,indicating that the lower charge-carrier transfer resistance and faster interface charge-carrier migration at the polymer/TiO2/electrolyte interface.
To elaborate the photocatalytic mechanism of the hybrid Pt@P42-TiO2-200 catalysts,steady-state solid photoluminescence(PL) spectra were measured to monitor the electron transfer from excited P42∗to TiO2for other comparable systems as control experiments (Fig.S12 in Supporting information).The P42 showed an intensive emission peak centered at 814 nm,which was attributable to its strong recombination of excited charge pairs by visible light at 700 nm.For comparison,the PL spectra of the other systems under the same conditions showed a decrease in fluorescence intensity,following the order: P42>physical-mixing gel-TiO2/P42>P42-TiO2-200.This indicated that the improved excitation dissociation and more efficient charges transport occurred at the interface between the P42 and TiO2in the P42-TiO2-200 hybrid material than that in the physical-mixing system and the pure P42.Moreover,the averaged decay time of the samples confirmed the above PL quenching results (Fig.S13 in Supporting information).The P42 exhibited the longest averaged decay time of 669.4 ps.With the incorporation of TiO2,the averaged decay time was shortened to 664.2 ps for the physical mixture and 67.49 ps for P42-TiO2-200,respectively.Therefore,it is reasonable to assume that the electron can transfer from excited P42∗to TiO2in the hybrid,and finally to the surface of the Pt nanoparticles to reduce H+to H2.Meanwhile,P42∗became oxidized P42+and then was reduced back to P42 by triethanolamine as the sacrificial reagent.Moreover,holes in TiO2could travel from the more positive VB of TiO2to P42+,which leaded to an all-round efficient charge separation and enhanced photocatalytic activity (Fig.5).
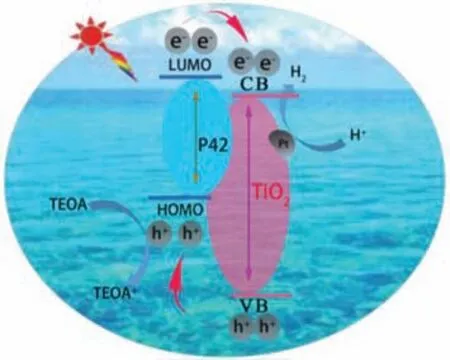
Fig.5.The mechanism for the photocatalytic hydrogen production of the P42-TiO2 system.
In summary,we have successfully prepared organic-inorganic hybrid materials from a benzodithiophene polymer and TiO2using a simple sol-gel method.Under visible light irradiation,the 1.7%P42-TiO2-200 material exhibited a high photocatalytic hydrogen production efficiency,reaching 745 μmol g−1h−1,which was far higher than the photocatalytic hydrogen production performance of TiO2.The analysis of UV–vis DRS and XPS suggested that the polymer was successfully doped in the material.The amounts of doping of the polymer were very small,but the photocatalytic performance was remarkably improved.The improved photocatalytic activity of the P42-TiO2hybrid material was attributed to the strong absorption of the polymer in the visible light range,the faster charge separation and transfer of the hybrid material,and the synergistic relationship between P42 and TiO2.Polythiophene based polymers have been widely used to degrade dyes.However,it has been rarely reported for the applications in photocatalytic hydrogen production.Our findings have demonstrated that the benzo[1,2-b:4,5-b’]dithiophene polymer is a promising material for hydrogen evolution and environmental remediation,providing potential ideals for the design of component or multi-component photocatalyst.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful for the financial support from Hainan Province Natural Science Foundation of China (No.219QN151),the National Natural Science Foundation of China (21801052),Hainan University Start-up Fund (No.KYQD(ZR)1852) and the Construction Program of Research Platform in Hainan University (No.ZY2019HN09).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.07.056.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
