Compared effects of “solid-based” hydrogen peroxide pretreatment on disintegration and properties of waste activated sludge
Hai-Chao Luo,Wan-Qian Guo,Qi Zhao,Hua-Zhe Wang,Nan-Qi Ren
State Key Laboratory of Urban Water Resource and Environment,Harbin Institute of Technology,Harbin 150090,China
Keywords:Waste activated sludge“Solid-based” hydrogen peroxide Oxygen reactive species Sludge disintegration Nutrients release
ABSTRACT The effects of two solid-based hydrogen peroxides sodium percarbonate (SPC) and calcium peroxide (CP)on waste activated sludge (WAS) disintegration were investigated.Both oxidants achieved efficient WAS disintegration for the synergistic effect of alkaline and oxidation.The strong alkaline condition led to the leakage of ammonia and the existence of abundant calcium ions accelerated the fixation of phosphorus via precipitation in CP WAS disintegration process.However,the spongy-like layer and low pH condition retarded the release of gaseous ammonia in SPC group.Hydroxyl radical was the main oxygen reactive species in SPC approaches which were more intense than CP by electron spin resonance (ESR) analysis.CP treated WAS contented more small particle size matter and total suspended solids (TSS) increased dramatically.In conclusion,CP pretreated sludge was more suitable for fertilization,while SPC was in favor of anaerobic digestion.This study clarified the differences between these two oxidants and their intermediates on nutrients release in sludge disintegration.
Biological technologies have been applied widely in both municipal and industrial sewage treatment Wastewater treatment plants (WWTPs) all over the world.Contaminants were removed by the metabolic activities of microorganisms (microbial dissimilation for organic substance degradation and assimilation for biological proliferation) involved in these technologies.The refractory substances,the particulate organic matter which is not completely degraded,and the proliferating microbial cells enter the sludge.Thus,huge amounts of waste activated sludge (WAS) were produced in turn,which was the main drawback and current burden of WWTPs.The WAS disposal expense accounted for 20%-60% of the total operational cost of WWTPs [1].Moreover,WAS contained considerable organic and nutrients (nitrogen and phosphorus) resources.For example,anaerobic digestion (AD) was applied or investigated generally for methane,hydrogen,and volatile fatty acids production with WAS as substrate [2].However,disruption of extracellular polymeric substances (EPS),biomass cell for the release of available organic matters,and sequencing dissolution of particulate organic matter involved in the hydrolysis stage were the ratedetermining step for the AD process [3].WAS disintegration was approved as an effective approach to overcome these obstacles and to accelerate the AD process which had received extensive attention from researchers and industry practitioners.
Multiple WAS disintegration approaches such as thermal,acid/alkaline,ultrasonic,ozone,microwaves,advanced oxidation processes (AOPs),and combined technologies were proposed and developed in the past few decades [4–6].AOPs based on strong oxidizing of high valence state metals [7]and free radical species formed during a series of reactions [8,9]were particularly concerned for numerous advantages (such as high efficient,energysaving,potential for recalcitrant compounds removal) [10].Several oxidantse.g.,hydrogen peroxide,persulfate,peroxymonosulfate,ferrate and permanganate were widely used in these AOPs[11-14].Some of them were directly used as additives to accelerated the release of organic constituents which strengthen the WAS disintegration or AD process [2,15].However,some residual inorganic ions (e.g.,manganese ion) or their transformation products(e.g.,hydrogen sulfide and other sulfur-containing odorous substances) might cause second environmental pollution.
Recently,sodium percarbonate (SPC) and calcium peroxide(CP) were widely used as substitutes of hydrogen peroxide in Fenton/Fenton-like reaction for eliminating contaminants [16,17].Both these two oxidants are known as “solid-based” hydrogen peroxide (HP) were more stable than hydrogen peroxide and easier to transport [16,18].Both SPC and CP were used to remove refractory contaminants because of their own strong oxidizing properties[19,20].Meanwhile,intermediate products and residual ions of SPC(sodium carbonate,HP,HCO3−/CO32−) and CP (Ca(OH)2,HP,oxygen,Ca2+) are clean and environment-friendly.Both SPC and CP can make the pH rise to an alkaline condition which was beneficial for AD processes [21].However,there was still a good deal of discrepancy between them.The dissolution rate of SPC was much higher than CP.CP can slowly release oxygen at a “control” rate(Eq.1) and form HP and Ca(OH)2(Eq.2) [22]when contacts with hydrous media and dissolves in water,respectively.The maximum 0.47 g HP/g CP can be liberated based on the stoichiometric ratio [23].While SPC is directly dissolved in water to form HP and Na2CO3 (Eq.3).
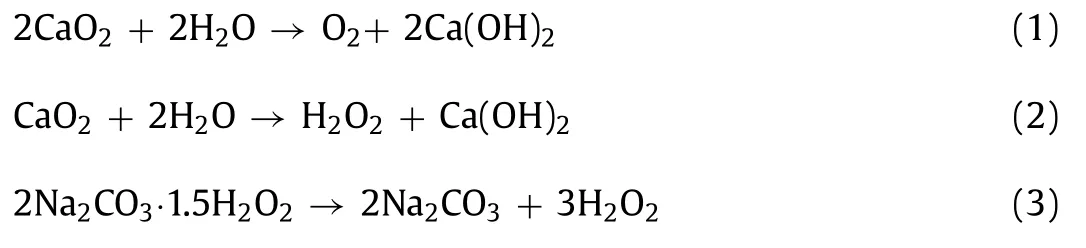
Although both SPC and CP can make the pH rise to alkaline conditions,the detailed characteristics of Ca(OH)2and Na2CO3were distinct.Recently,researchers indicated that pretreatment approach with CP as a direct additive can remove endocrine-disrupting chemicals in WAS,promote sludge solubilization [24]and improve the quantity and quality of VFAs in the AD process [25].Both SPC and CP were employed to enhance WAS dewaterability [8,26].However,few investigations focused on the effects of SPC and CP on inorganic nutrients release (ammonia and phosphorus) during the sludge disintegration process.Solo and combined effects of intermediates of these two oxidants on organic substances also were limited report.
Considering the advantages,similarities,and differences of these two “solid-based” hydrogen peroxide,the main subjects of this study were to investigate the effect of these two oxidants on WAS disintegration;to measure the nutrients release and dissolution in WAS disintegration process;to analyze the roles of intermediate products and their combined effect involved in these processes;to determine the WAS properties variation before and after pretreatment by these two oxidants.This study aimed to clarify the differences between these two oxidants and their intermediates in the sludge disintegration process,which contributes to deepening the application of these two oxidants in WAS treatment during subsequent laboratory and practical research.
WAS used for the disintegration process was collected from the Wenchang wastewater treatment plant (Harbin,Heilongjiang Province,China) with a configuration of typical enhanced biological phosphorus removal (EBPR) processes.The collected sludge was settled for 24 h in 4 °C and then pass through 20 mesh sieves to remove large particles.The main characteristics of WAS were TSS 15380 ± 280 mg/L,VSS 9955 ± 300 mg/L,TCOD 11350 ± 283 mg/L,SCOD 71 ± 16 mg/L and pH 7.2 ± 0.1,respectively.
All experiments were conducted in 500 mL glass beakers with 250 mL WAS.A magnetic stirrer was applied to keep the suspension of the mixture (WAS ®ents) at 400 rpm/min.The initial pH was unadjusted for all experiments.The glass beakers with neither SPC nor CP dosage were the control group.A certain dosing of SPC or CP was added to the glass beakers according to the predetermined dosage (0.05,0.1,0.3,0.5 g/g volatile suspended solids(VSS)) for experimental groups.10 mL disintegrated WAS samples collected from each group were centrifuged at 4000 g for 10 min(4 °C) at experiment intervals.The supernatant was collected and filtered by the 0.45 μm polyether sulfone (PES) membrane syringe filter and stored at 4 °C for the analysis of soluble substances.
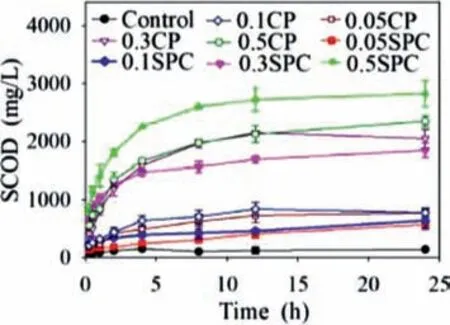
Fig.1.Effect of SPC &CP dosages on WAS disintegration.
To investigate the WAS disintegration mechanism of SPC and CP meanwhile to distinguish the differences between these solidbased HP,several experiments were conducted under the same condition as described former except for the dosing of reagents.The solo and synergetic effects of intermediate products were also studied.WAS without reagent was the control group.Experimental groups included SPC (0.5 g/g VSS),sodium carbonate (SC,0.34 g/g VSS),SC+HP (0.34 g/g VSS+0.16 g/g VSS),CP (0.5g/g VSS),CaO(0.39 g/g VSS),CaO+HP (0.39 g/g VSS+0.24 g/g VSS),HP (0.24 g/g VSS).The dosage of SC,HP,and CaO was calculated according to the stoichiometry of SPC and CP,respectively.All experiments were performed in duplicate at room temperature and the mean values were reported.
Chemical oxygen demand (COD),NH4+-N,PO43−-P,TSS,VSS were measured following the standard methods.Protein,carbohydrates were analyzed by the modified Lowry method and phenolsulfuric method,respectively.Bovine serum albumin and glucose were acted as standard substances for corresponding methods,respectively [27].Sludge particle size was measured by Mastersizer 2000 analyzer (Malvern Instrument,Ltd.,UK).5,5-Dimethyl-1-pyrrolineN-oxide was utilized as the spin-trapping agent to determine the generated free radicals by electron spin resonance(ESR) [28].DI water and WAS supernatant were utilized as reaction mediums to identify the free radicals generated in SPC and CP disintegration processes in this study.Certain mass SPC &CP that equal to 100 mL WAS with SPC and CP dosage at 0.5 g/g VSS was added in 100 mL Milli-Q water (DI water) or WAS supernatant,respectively.Then 1 mL reaction samples were transferred into a 10 mL centrifuge tube containing DMPO.Then the mixed samples were quickly inserted into the cavity of the ESR spectrometer (Bruker A200,United States) by 100 μL capillary tube.
Sludge disintegration degree (SDD) was calculated according to Eq.4.
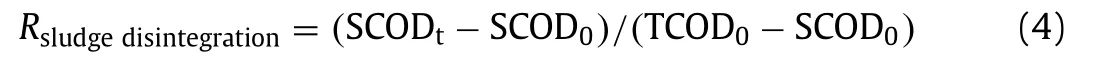
SCODtand SCOD0were soluble COD in the supernatant of disintegrated WAS at timetand initial soluble COD in raw sludge,TCOD was the total COD in raw sludge.
Results showed that both “soli-based” hydrogen peroxide SPC and CP accelerated the disintegration of WAS shown in Fig.1.Soluble COD (SCOD) released by disintegrated WAS with SPC and CP pretreatment increased dramatically in the first 2 h especially when dosages were greater than 0.3 g/g VSS.CP reacted with water which released HP and formed Ca(OH)2during the initial period according to Eq.2 [23],while SPC directly released HP and formed SC once contacted with water Eq.3 [29].Reactive oxygen species (e.g.•OH,•O2−,HCO4−) might be generated in series reaction Eqs.5-12 [20,29].SC and Ca(OH)2were also formed in the meantime which shifted the pH to an alkaline condition.Previous study by Liet al.indicated that Ca(OH)2alone can significantly improve the solubility of WAS [25].Thus,the synergistic effect of reactive oxygen species and alkaline accelerated disruption of floc structure and cell walls and improved solubilization of WAS which caused the significant increase of SCOD in the initial 2 h.

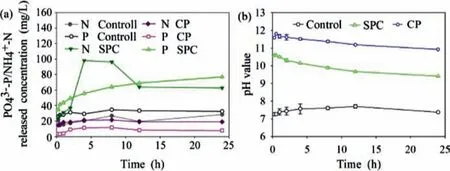
Fig.2.(a) Nutrients release and (b) pH variation during CP &SPC treatment processes.
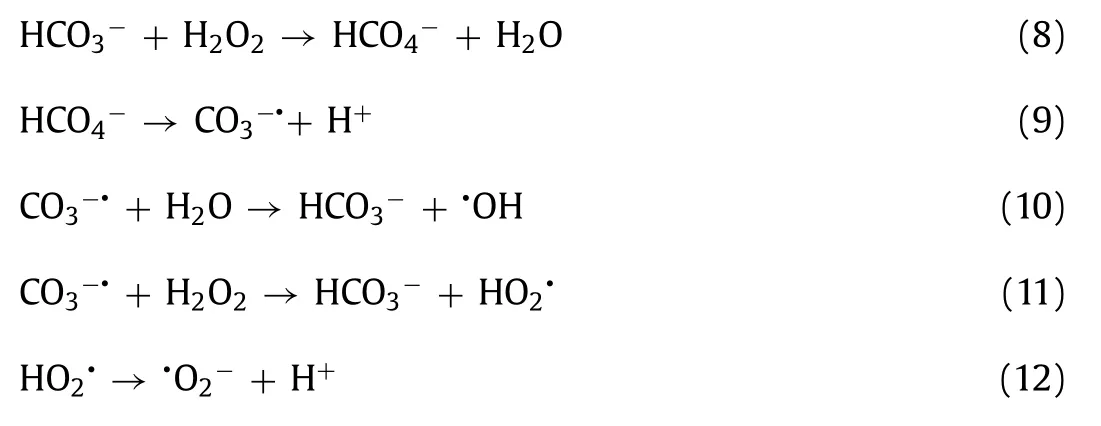
However,the SCOD concentration in the SPC groups was remarkably higher than CP groups in this reaction duration.Because the SPC group provided much more HP for reactive oxygen species production than the CP groups.HP is unstable under strongly alkaline conditions and releases oxygen gas due to the disproportionation reaction [30].The shear-force of quickly formed oxygen gas released by HP which might accelerate the peeling of organic particles from sludge flocs was beneficial for WAS disintegration.The sludge released organic particles and floc structure and cell wall from sludge were further hydrolyzed under alkaline conditions,which led to the rapid release of SCOD in subsequent reactions.The generation of reactive oxygen species slowed down and even directly released oxygen in the CP group due to the low solubility of CP.This results in a great reduction of SCOD release in the CP group.The SCOD increased or even decreased slightly in the remaining 16 h due to the effect of oxidation decreased due to the consumption of HP in SPC groups.Maet al.indicated that the pollutant oxidation capability of decomposed SPC was lower [31].Thus,the optimal disintegration duration of both SPC and CP was 8 h.
Generally,SCOD of both SPC and CP WAS disintegration experiments increased with increasing dosages of both oxidants.SCOD of WAS disintegrated by CP did not increase anymore when the dosage was greater than 0.3 g/g VSS.However,the SCOD of disintegrated WAS in 0.5 g/g VSS CP group was a bit higher in the first 4 h than 0.3 g/g VSS CP group and increased constantly throughout the reaction process but it decreased slightly in the remained last 12 h.The low solubility and reaction rate of CP with water accounts for this situation.Taking slightly increased WAS disintegration and economic feasibility into account,0.3 g/g VSS was considered to be the optimal dosage for the CP process.Therefore,0.3 g/g VSS and 0.5 g/g VSS were considered as the optimal dosage for CP and SPC in WAS disintegration process,respectively.The SCOD increased to 2595 ± 7.0 mg/L and 1960 ± 70.7 mg/L in optimal dosage SPC and CP group after 8 h reaction which was about 26.0 and 19.6 times higher than the control group,respectively.WAS disintegration rate of control,SPC and CP group at 8 h were 0.2%,22.4% and 16.7%,respectively.
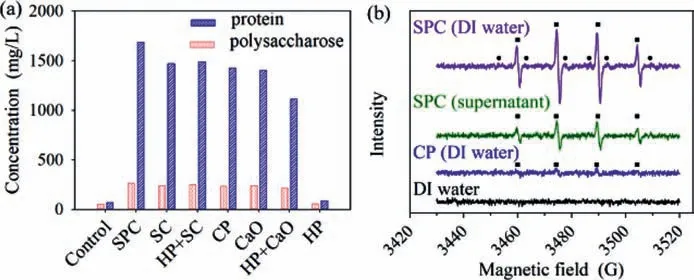
Fig.3.(a) Effect of intermediates on organic substances release after 8 h treatment and (b) ESR identified free radical involved in SPC and CP disintegration processes(■represented the signal of •OH;●represented the signal of •O2−).
There were remarkable inconsistencies of inorganic nutrients released performance of WAS during SPC and CP disintegration processes Fig.2a.Although both SPC and CP effectively disintegrated WAS,NH4+-N and PO43−-P concentrations in the SPC group were significantly higher than the CP group.The release of NH4+-N and PO43−-P in WAS disintegration process was mainly through two pathways: disruption of WAS floc structure and cell walls leading to intracellular substances leakage [32]or further hydrolysis of particles organic matters and macromolecule substances such as protein and polyphosphate [33].NH4+tends to be converted to ammonia by reacting with hydroxide anions under strongly alkaline conditions Eq.13.

The pH value in CP WAS disintegration process was higher than 11 throughout the whole reaction durations Fig.2b.Thus,the lower NH4+-N in the CP group mainly because of the ammonia leakage.SPC also shifted pH to an alkaline condition.The pH value in the SPC disintegration process was lower than CP group,but NH4+-N concentration decreased significantly when the spongylike layer formed due to sharply released HP in initial reaction duration was disrupted during 8-12 h.However,the NH4+-N concentration was still significantly higher than the CP group.Thus,the primary cause of the significantly higher NH4+-N in the SPC group was that the spongy-like layer blocked the contact between WAS and air which retarded the release of ammonia.In conclusion,it was suggested that an ammonia removal system for tail gas was necessary for both SPC and CP disintegration process in practical application.
The alkaline pH conditions in both the SPC and the CP groups were in favor of the phosphate precipitation formation (e.g.,struvite,calcium phosphate,ferric phosphate).However,the CP group provided more additional cations to form phosphate precipitation because Ca(OH)2was an intermediate product of CP in Eqs.1 and 2.CaO and calcium salts were the commonly used and efficient reagents in the phosphorus recovery field [12,34].Thus,the lower PO43−-P in the CP group was mainly because of precipitation formation in the reaction between Ca2+and PO43−.It was suggested that WAS disintegrated by CP was more suitable for agricultural use given that most of the released PO43−-P was converted into calcium phosphate and low solubility of calcium phosphate might ensure the sustainable supply of phosphorus fertilizer [35].
Solo and combined effects of these selected oxidants and corresponding intermediates on organic substances release were conducted in this study Fig.3a.Protein and polysaccharose were the main components of organic substances contained in WAS [36].HP increased the organic substance more slightly when compared with alkaline intermediates.Protein and polysaccharose released significantly in both SC and CaO groups which confirmed that alkaline intermediates were the dominant factors in SPC and CP WAS disintegration processes.Meanwhile,SC treatment resulted in higher protein release than CaO.SC was employed in structural extracellular polymeric substance extraction processes in the research of [37].It was notable that the combination of SC and HP made organic substances release increased slightly compared with solo SC treatment which was consistent with the report that bicarbonate can activate HP [38].While the combination of CaO and HP made the opposite effect.This is due to the acceleration of HP decomposition under strongly alkaline conditions created by CaO.
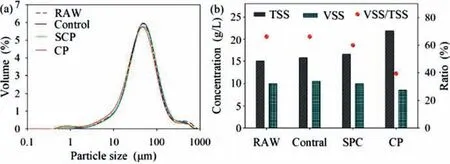
Fig.4.(a) WAS particle size distributions variation with SPC &CP treatment and(b) TSS and VSS/TSS variation after SPC &CP disintegration processes.
ESR measurements with DMPO as reactive oxygen species scavengers were conducted to identify and distinguish the functional free radicals involved in SPC and CP sludge disintegration processes.DMPO can trap•OH and•O2−to produce DMPO-OH and DMPO-OOH spin adduct,respectively.Neither buffer reagents dosage [24]nor pH adjust [8]was conducted before ESR measurements or reactors operation.DMPO-OH that processes four split lines with a 1:2:2:1 height ratio was detected in both DI water and WAS supernatant reaction medium with SPC dosage,but only in DI water reaction medium with CP dosage Fig.3b.Maet al.confirmed that•OH was generated from SPC decay [29].Stronger intensity signal of•OH that was detected in SPC reactions indicated more•OH were generated,which was consistent with the higher SCOD release in SPC WAS disintegration.The organic substances in the supernatant might act as•OH scavengers that compete with DMPO for•OH capture.Thus,the intensity signal of•OH in the supernatant medium was weaker than the DI water medium.•O2−was another reactive oxygen species for both SPC and CP.However,DMPO-OOH consisting of a sextet ESR signal was not detected in any reaction medium with oxidants dosage in this study.DMPOOOH is unstable and can decompose to DMPO-OH [25].Thus,•OH was the dominant reactive oxygen species in both SPC and CP WAS disintegration processes.
Particle size distributions for RAW and control group were similar to each other Fig.4a.Both SPC and CP treatments affected the particle size distribution due to efficient WAS disintegration.Meanwhile,both approaches disrupted and hydrolyzed large particles size organic matter (300-1000 μm) into smaller ones and simultaneously decreased the content of medium particle size matters(30-100 μm).The content of particle matter decreased in all particle size ranges except for those less than 30 μm in the CP group.The bridging effect of calcium iron on released carbohydrates and proteins [39]became negligible after WAS was severely disintegrated [26].The residual CP particles and calcium phosphate precipitation formation also accounted for higher small particle size(<30 μm) matter content in the CP group.The d(0.5) for SPC treatment was not significantly different from the control (44.1 μmvs.44.4 μm).Although cells in sludge flocs were disrupted leading to the leakage of intracellular substances in SPC treatment,the relatively weaker alkaline condition could not destroy sludge flocs completely in the SPC group and EPS could serve as a “flexible skeleton” to keep the stabilization of sludge flocs structure[40].
TSS,VSS,and VSS/TSS were measured to estimate sludge organics reduction in this study.Shown as in Fig.4b,due to residual inorganic ions and their crystallized products or precipitate,TSS of disintegrated WAS increased in both SPC and CP.TSS increased by 45.4% with an efficient reduction of VSS (13.7%) in the CP group.The significant increase of TSS was in line with the P released performance that indicated fixation of P and formation of precipitation in the CP group.It was noticeable that VSS/TSS dramatically decreased to lower than 40% in the CP group.However,VSS only slightly decreased in the SPC group.This phenomenon was inconsistent with its higher WAS disintegration rate.HCO3−inevitably exists in SPC disintegrated WAS resulted in the over-estimate of VSS value.Meanwhile,it should be pointed out that the existence of HCO3−/CO32−(natural buffering system) would maintain the alkaline condition which benefits the AD process [41].
Effects of SPC and CP on WAS disintegration were conducted and compared in this study.Both SPC and CP enhanced WAS disintegration significantly,and the optimal dosage was 0.5 g/g VSS and 0.3 g/g VSS,respectively.Although both oxidants result in alkaline conditions,the concentration of NH4+-N in SPC treated WAS was higher than CP treated WAS attributed to the spongy-like covering formed accompany with initial SPC reaction duration.The alkaline condition accelerated ammonia leakage but favored the reaction of phosphorus and calcium that release during CP reaction to form precipitation.•OH was identified as the dominant reactive oxygen species,while alkaline intermediates account for the dominant effect on organic substances release in WAS disintegration processes.Dramatically,TSS increased with VSS/TSS ratio decreased oppositely after CP treatment.This study compared the effects of SPC and CP as well as their corresponding intermediates on WAS disintegration,which contributes to the further application of these two oxidants in WAS disposal.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.51978201) and the State Key Laboratory of Urban Water Resource and Environment (No.2020DX08).
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
