Constructing a novel Ag nanowire@CeVO4 heterostructure photocatalyst for promoting charge separation and sunlight driven photodegradation of organic pollutants
Yn Song,Rn Wng,Xiuyun Li,Biqi Sho,Hongpeng You,Chozheng He,∗
a Institute of Environmental and Energy Catalysis,School of Materials Science and Chemical Engineering,Xi’an Technological University,Xi’an 710021,China
b State Key Laboratory of Rare Earth Resource Utilization,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
c University of Science and Technology of China,Hefei 230026,China
Keywords:Cerium vanadate Photocatalyst Heterojunction Degradation mechanism First-principles
ABSTRACT Exploiting efficient and recyclable photocatalysts is a vital matter for environmental purification.Herein,cerium vanadate (CeVO4) sub-microspheres and silver nanowire (AgNW)@CeVO4 with core-shell architecture as photocatalysts are rationally constructed by hydrothermal approach.The AgNW@CeVO4 photocatalyst obtained by depositing CeVO4 on the surface of Ag NWs possess one dimensional continuous structure,which expand the optical absorption range and reduce the band gap of CeVO4 photocatalyst.Moreover,the resultant AgNW@CeVO4 photocatalyst demonstrates superior photocatalytic performance in the degradation of rhodamine B,methylene blue,and 4-nitrophenol pollutants upon solar light irradiation,compared with pure CeVO4.The excellent photocatalytic activity can be ascribed to the introduction of Ag NWs,which afford rapid charge transport channels and reservoir for the electrons in the AgNW@CeVO4 heterostructure to promote separation of electron–hole pairs.The first-principles investigations reveal increase of adsorption energy of oxygen molecules on the CeVO4 surface with the presence of Ag.Meanwhile,Ag NWs can further improve the photocatalytic efficiency of the AgNW@CeVO4 based on the plasmonic effect.More importantly,the good structural stability and recyclability of AgNW@CeVO4 are observed due to the strong synergistic effect,which ensures long-term usability of photocatalyst and great promise in water purification.This work can offer valuable reference into designs and construction of Ce-based heterojunction photocatalysts for environmental remediation.
Demand for catalyst progressive increase year by year,in order to mitigate global energy shortage crisis and environmental deterioration problems.As a significant category,photocatalyst exhibit fascinating superiority and potency in the field of energy and environment,which can be used to accomplish water splitting to produce hydrogen and oxygen,carbon dioxide reducing,and organic pollutants degrading [1–5].In allusion to photodegradation of organic dyes,abundant semiconductor based photocatalysis have been exploited and investigated [6–8].However,the narrow light absorption band will impede the holistic photocatalytic efficiency,which stimulates researchers to exploit novel photocatalyst and innovate picturesque configuration.Among the numerous classes of emerging photocatalysts,rare earth compounds with 4f configurations have revealed remarkable luminescent properties and improved photocatalytic activity due to the strong interaction with organic compounds [9–12].Remarkably,cerium vanadate(CeVO4) has been developed as a newfangled photocatalyst based on cerium with plentiful energy level and the VO43−activated centers with valid light absorption in the crystalline structure [13–17].
In previous studies,the photocatalysts with nanoscale emerge superior catalytic efficiency by virtue of exposure of abundant active sites and sufficient contact area with the organic pollutants [18–20].Nevertheless,collection of the nanoparticles for recycling is intrinsic imperfection,which severely restrict practical applications of the photocatalysts for water decontamination.Therefore,the exploitation of individual photocatalysts with nanocrystal assembled and hollow configuration is extremely significative [21,22].Despite exposure of abundant active sites,the mono-component semiconductor materials with hollow configuration have intrinsic restrict in practical application on account of the rapid recombination of photogenerated electrons and holes.In an effort to overcome these issues,the semiconductor is incorporated with noble metals,which is a meaningful and efficient strategy [23–25].The noble metals as electron sinks will effectively improve the integral photocatalytic performance through transferring the photogenerated electrons and efficiently reducing the recombination of the photogenerated electron–hole pairs [26–28].
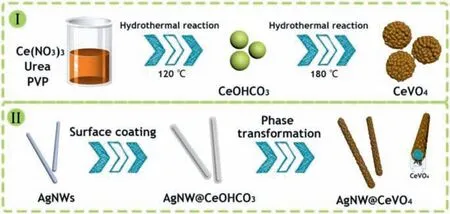
Fig.1.Conceptual scheme of synthesis route for (I) CeVO4 hollow spheres and (II)AgNW@CeVO4.
It is interesting to note that noble metals with one dimensional nanostructure reveal effortless electrons transference,which will play an important role in the heterostructure photocatalysts for improving photocatalytic activity [29–31].Under optical radiation,the noble metals provide rapid electronic channels and reservoirs for accelerating the transfer of photo-generated electrons.As an excellent electron mediator,Ag with nanostructure is successfully used for impeding rapid recombination of electron and the hole and elevating photocatalytic disinfection activity of individual photocatalyst [32–34].Therefore,exploiting original photocatalyst and designing particular morphology of heterostructures have always been of great interest and critical importance on behalf of investigation and optimization their catalytic activity [35–38].
In this work,we demonstrate a facile and scalable strategy to prepare CeVO4photocatalyst with hollow submicrospheres.In addition,the combination of Ag nanowires (AgNWs) and CeVO4is rationally designed to construct core shell architecture,which is expected to show advantages over the single component of catalyst.Benefiting from the electron transfer and boosted optical absorption in the presence of the AgNWs,the resultant AgNW@ CeVO4reveals prominent photocatalytic activity towards contaminants of rhodamine B (RhB),methylene blue (MB) and 4-nitrophenol(4-NP).Under full spectrum light irradiation.Significantly,the good structural stability and recyclability of AgNW@CeVO4are observed,which ensure long-term usability of photocatalyst and great promise in water purification.
The fabrication strategies of the CeVO4hollow spheres and AgNW@CeVO4nanowires are schematically depicted in Fig.1.This strategy as for the synthesis of CeVO4hollow spheres involves in the construction of highly uniform spherical CeOHCO3precursor and transformation of CeVO4hollow spheres through ions exchange process based on Kirkendall diffusion mechanism.Firstly,introduction of PVP as surfactant provides new interfaces action,which plays a crucial role in controlling of size and shape and favors the formation of highly uniform spherical CeOHCO3precursor during the hydrothermal process.For such strategies without PVP the crystalline octahedron emerged in the initial reaction stage (Fig.S1 in Supporting information),as a result,the hollow structure and coating layer are strictly limited in generalization.Subsequently,the hollow construction of CeVO4can be accomplished due to the difference of diffusion effect for cationic and anionic species during chemical transformation.Based on the above conceptual innovation,the uniform CeOHCO3precursors are deposited on the surface of a robust AgNWs substrate to form AgNW@CeOHCO3,and then AgNW@CeVO4were successfully synthesized by way of ion exchange process.
The X-ray diffraction (XRD) patterns of CeOHCO3precursors and CeVO4hollow spheres obtained in the presence of PVP are revealed in Fig.S2a (Supporting information).It can be seen that no obvious diffraction peaks are observed in the XRD pattern of CeOHCO3,signifying the sample exhibits an amorphous character.In the XRD pattern of CeVO4hollow spheres,the typical diffraction peaks can be readily indexed with the tetragonal structured CeVO4(JCPDS card No.84-1457),which manifests that the single phase CeVO4is synthesized.Subsequently,the morphology and microstructure of the products in different stages were indagated by Field-emission scanning electron microscope (FE-SEM)and transmission electron microscope (TEM) analyses.Fig.S2b(Supporting information) exhibits that the CeOHCO3precursors possess a monodisperse characteristic and highly homogeneous spherical morphology with a diameter of approximate 200 nm and a relatively smooth surface.After undergoing ion exchange process,the spherical morphology of samples (with an average diameter of 200 nm) is maintained.Nevertheless,high-magnification FE-SEM image (the inset in Fig.S2c in Supporting information) reveals that the as-prepared CeVO4presents a hollow feature and a rough surface texture,which is composed of small nanoparticles with an average diameter of 25 nm.To further confirm the hollow structure of CeVO4,TEM analyses are performed,as shown in Fig.S2d(Supporting information).It can be seen clearly that the samples present hollow and porous architecture,which will be conducive to improve the degradation property of samples for organic contaminants by virtue of sufficient contact between catalysts and contaminants.Additionally,from the high-resolution TEM image of CeVO4hollow spheres (Fig.S2e in Supporting information),clear lattice fringes are observed and the average lattice space of 0.369 nm is obtained,corresponding to the (202) planes of tetragonal structured CeVO4.The distinct dot array (Fig.S2f in Supporting information) in the fast Fourier transfer (FFT) diffraction pattern collected from the high−resolution TEM image presents the single crystallinity for CeVO4.These results confirm
In the cases of AgNW@CeVO4,the XRD pattern (Fig.2a) exhibits that the main diffraction peaks can be assigned to the tetragonal structured CeVO4(JCPDS card No.84-1457) and the extra peaks located at 38.2°,44.4° and 64.6° match well with correspond to (111),(200) and (220) of Ag (JCPDS card No.87-0720),respectively,confirming the coexistence of Ag and CeVO4.FE-SEM and TEM analyses are applied to verify the morphology and microstructures of AgNWs and AgNW@CeVO4samples.Fig.2b reveals the microstructure of the AgNWs,which presents homogeneous one-dimensional nanowires structure with an average diameter of around 90 nm and smooth surface [39].By contrast,the AgNW@CeVO4samples maintain the one-dimensional structure and exhibit a binary hierarchical architecture,including the Ag-NWs core layer and in situ grown CeVO4shell layer with a rough surface,as shown in Fig.2c.Additionally,the low-magnification TEM image (Fig.2d) of single AgNW@CeVO4confirms the ore-shell structure with an obvious shell thickness of approximately 40 nm and the corresponding high-magnification TEM image displays legible crystalline phase with a d-spacing of 0.369 nm,which is assigned to the (202) interplane spacing of CeVO4.The EDS elemental mapping analysis is employed to examine the element distribution information of samples,as revealed in Figs.2e–h.It is clear that Ag element is uniformly distributed in the core region,while homogenous Ce and V elements are distributed in the shell region,further confirming the formation of AgNW@CeVO4with core-shell structure.
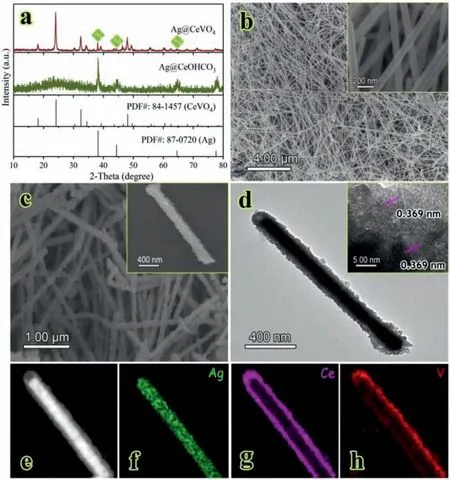
Fig.2.(a) XRD patterns of AgNW@CeOHCO3 and AgNW@CeVO4,SEM images of (b)AgNWs and (c) AgNW@CeVO4 samples,(d) TEM images of the AgNW@CeVO4,(e–h)STEM image and the corresponding EDS elemental mapping of the AgNW@CeVO4 pure phase and high crystallinity of the resulting CeVO4 hollow spheres.
The bandgaps of semiconductor photocatalysts possess crucial effect for their catalytic performance and result in the absorption range of light during the photocatalytic process.The UV–vis-NIR absorption spectra are employed to survey investigate the optical absorption range and determine the bandgaps of the CeVO4and AgNW@CeVO4samples.The absorption curves of as-prepared samples are presented in Fig.S3a (Supporting information).It is noted that the optical absorption range is broadened after introducing AgNWs to CeVO4photocatalyst,which will lead to shrink the bandgap of AgNW@CeVO4sample and aggrandize the utilization efficiency of visible light.The bandgaps of the CeVO4and AgNW@CeVO4samples are calculated based on the following equation (Kubelka−Munk formula) [40,41]:

whereαdenotes absorption coefficient,νcorresponds to light frequency,his Planck constant,and Eg represents the bandgap of sample.Fig.S3b,c (Supporting information) show the curves of(αhν)2versus hν,deriving from the corresponding absorption spectra.The bandgaps of the CeVO4and AgNW@CeVO4are determined to be 2.76 and 2.12 eV,respectively.The diminution of bandgaps for AgNW@CeVO4indicates that the incorporation of CeVO4with AgNWs is beneficial to aggrandize the utilization efficiency of visible light and improve the photocatalytic activity.
In order to evaluate the separation efficiency of photogenerated charge carriers in the as-prepared photocatalysts,the photocurrent variations are monitored according toI–tcurve tests with several on-off cycles of full spectrum light irradiation.Fig.S4a (Supporting information) reveals the photocurrent density of CeVO4and AgNW@CeVO4photocatalysts under intermittent light illumination.Obviously,both of the photocatalysts exhibit fast and reproducible light response,and AgNW@CeVO4photocatalyst possesses clearly stronger photocurrent intensity than pure CeVO4,which indirectly reflects that the introduction of AgNWs promotes the generation of charge carriers and separation efficiency of the photogenerated electron–hole pairs [42,43].Such a highly sensitive photocurrent response and separation efficiency of carriers will make AgNW@CeVO4photocatalyst to achieve more excellent photocatalytic activity.As can be seen from the PL spectra (Fig.S4b in Supporting information),high PL intensity of CeVO4suggests fast recombination of photogenerated electron-hole pairs [44].Interestingly,the PL spectrum of AgNW@CeVO4reveals weaker emission intensity.The results further confirm that the introduction of Ag-NWs is conducive to separation and transfer of electron-hole pairs.
The catalytic activity of the as-prepared CeVO4and AgNW@CeVO4photocatalysts was evaluatedviaphotodegradation of RhB under simulated sunlight irradiation.Fig.3a reveals the degradation efficiency of photocatalysts for RhB at different irradiation time.The direct photolysis behavior of RhB as a blank experiment is compared.Before photodegradation,the photocatalysts are dispersed in RhB solution for accomplishing an adsorption-desorption equilibrium under a dark condition for 60 min.It can be seen that the CeVO4and AgNW@CeVO4photocatalysts exhibit a slight adsorption effect,which suggests the photolysis of RhB is chiefly attributed to catalytic function of photocatalysts.For the CeVO4,the degradation of RhB gradually increases with the prolongation of irradiation time,and 64% of RhB is degraded within 120 min under simulated sunlight illumination.Accompany with the introduction of AgNWs,the obtained AgNW@CeVO4sample reveals a considerably higher photocatalytic activity.As can be noticed,the photodegradation rate of RhB reaches up to 94% within 120 min of full spectrum light irradiation.Furthermore,based on the Langmuir Hinshelwood (L-H)kinetic model,the photocatalysis degradation results conform topseudo-first-order photocatalysis kinetics,and the corresponding reaction rate constants (k) of the different photocatalysts are calculated from the following equation [45,46]:

whereC0corresponds to the initial concentration of RhB solution,Cis the concentration of RhB solution attmin,and thetrepresents the irradiation time.Significantly,as presented in Fig.3b,AgNW@CeVO4photocatalyst possesses higherkvalue(0.02216 min−1) than CeVO4hollow sphere,which demonstrates AgNWs contribute to optimizing photocatalytic activity of CeVO4for RhB.The phenomenon can be explained that the AgNWs with excellent electronic transmission capability are beneficial to transfer the photogenerated electrons,inducing reducing the combination of electron–hole pairs.
The stability and recyclability of AgNW@CeVO4photocatalyst are also crucial parameters for cyclic utilization in practical applications.In order to evaluate the recycling performance of AgNW@CeVO4,the cycling tests of photodegrading RhB are performed under full spectrum light irradiation,as shown in Fig.S6(Supporting information).The photodegradation efficiency of photocatalysts for RhB nearly constant during five sequential cycles,manifesting AgNW@CeVO4presents great recycling stability during the photocatalytic reactions.In addition,we also evaluated the photodegradation property under solar irradiation by using MB and 4-NP as contaminants.Obviously,AgNW@CeVO4exhibited the high catalytic activity for degradation of MB and 4-NP due to the fact that the AgNW@CeVO4can provide active sites for adsorption and catalytic reduction of contaminants.Fig.S7 (Supporting information) presents the XRD pattern and SEM images of AgNW@CeVO4photocatalysts after photodegradation cycling tests,which reveal that the photocatalyst maintains the original crystal structure and morphology features.The results further determine a good stability of AgNW@CeVO4photocatalyst.

Fig.3.(a) Photocatalytic degradation of RhB under full spectrum light irradiation as a function of the irradiation time without catalyst,and over the as-prepared CeVO4 and AgNW@CeVO4 samples.(b) Corresponding plots of ln(C0/C) against irradiation time for the photocatalytic degradation of RhB under full spectrum light over different catalysts.(c) Photodegradation of MB and 4-NP under full spectrum light irradiation over the as-prepared AgNW@CeVO4 catalysts.(d) Schematic diagram of possible photocatalytic mechanism for AgNW@CeVO4 heterogeneous system.
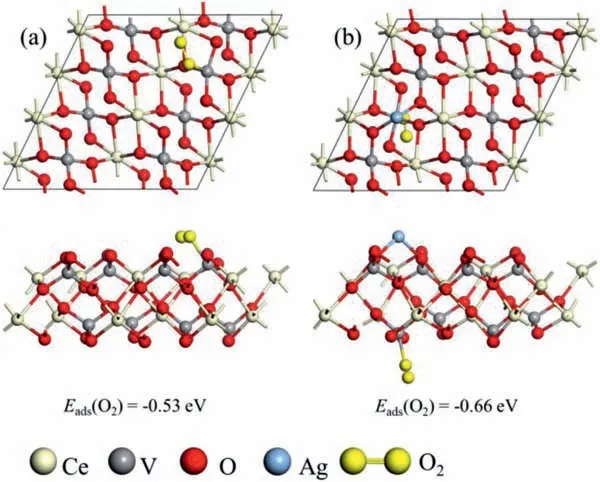
Fig.4.Optimized O2 adsorption structure on (a) CeVO4 and (b) Ag-CeVO4.
To investigate surface catalysis of AgNW@CeVO4photocatalyst,the adsorption of the O2molecules on the surfaces of CeVO4(110)are studied on the basis of density functional theory (DFT).Fig.S8 (Supporting information) shows optimized structure of pristine CeVO4and Ag-CeVO4.Based on the optimized structures of CeVO4and Ag-CeVO4,the effect of introducing Ag on O2adsorption and separation of electron and hole is further explored.Adsorption modes and adsorption energy of O2on the CeVO4and Ag-CeVO4surfaces are presented in Fig.4.The adsorption energy for of O2on the CeVO4and Ag-CeVO4are −0.53 and −0.66 eV,respectively.It is obvious that an adsorption energy gain of −0.13 eV can be obtained when the presence of Ag.The increase of adsorption energy insinuates that the presence of Ag is beneficial to the reduction of oxygen molecules on the surface of AgNW@CeVO4.It can be speculated that the photocatalytic activity of CeVO4is promoted by introducing Ag.
The Muliken charge analysis was achieved based on the charge changes of Ag atom before and after adsorption on CeVO4.The results show that the positive charge of Ag atom (0.485 e) is mainly contributed by the surrounding O atoms and the O atoms around the V atoms located directly under the Ag atom.Through the charge differential density diagram after O2adsorption as shown in Fig.S9 (Supporting information),we can see that the charge depletion region and the charge accumulation region mainly distribute in the O2molecular and V atom region,respectively.However,Muliken charge analysis shows that the positive charge of the V atom did not change significantly,and the negative charge of the surrounding O atoms is reduced.Therefore,we believe that electrons are mainly transferred from O atoms around V to O2molecules through V atoms after the adsorption of O2molecules [47,48].
Subsequently,a possible photocatalytic mechanism of AgNW@CeVO4for the degradation of pollutants is expounded,as illustrated in Fig.3d.Initially,the RhB and MB dye molecules are excited and corresponding electrons migrate to photocatalysts,resulting in forming the reactive excited-state RhB+and MB+under simulated sunlight radiation [49].For 4-NP adsorbed on the surface of AgNW@CeVO4,the nitro group can be transformed into nitrophenolate ions and further reduced to amino group due to the electrons displacement and active hydrogen species derived from borohydride [50,51].Simultaneously,the electrons are excited from valence band (VB) the conduction band (CB) of Ag@CeVO4photocatalysts with the increase of absorbing photon energies,and then transfer to the Ag and V atoms on the interface between CeVO4and AgNWs for contributing to the degradation [52,53].The photoexcited electrons can reduce the dissolved oxygen molecules to produce the superoxide radicals O2•−,and the holes react with H2O molecules to form hydroxyl radicals (•OH) [54–56].The associated superoxide radicals (O2•−),hydroxyl radicals (•OH),and holes (h+) can efficiently degrade contaminants into nontoxic compounds.
In summary,we develop a convenient strategy to fabricate CeVO4based photocatalyst with hollow submicrospheres,and combine AgNWs and CeVO4to rationally construct AgNW@CeVO4photocatalyst with core shell architecture.The introduction of Ag NWs not only endow the photocatalyst with one dimensional continuous structure,but also expand the optical absorption range of CeVO4photocatalyst.Moreover,AgNWs afford rapid charge transport channels and reservoir for the electrons in the AgNW@CeVO4heterostructure,which promote the separation efficiency of photogenerated electrons and holes.The theory analysis further validates that introducing Ag can strengthen O2adsorption on CeVO4surface,which facilitates the separation of electron and hole and the advance of photocatalytic activity of CeVO4.Benefiting from the electron transfer and boosted optical absorption in the presence of the AgNWs,the resultant AgNW@CeVO4reveals prominent photocatalytic activity towards the decomposition of organic pollutants under simulated sunlight irradiation.Thereinto,AgNWs can further improve the photocatalytic efficiency of the AgNW@CeVO4based on the plasmonic effect under solar light irradiation.Significantly,the good structural stability and recyclability of AgNW@CeVO4are observed due to the strong synergistic effect,which ensure longterm usability of photocatalyst and great promise in water purification.From these findings,this is a valuable reference in designing Ce based compounds with improved photochemical abilities.From these findings,this study is a valuable reference into developing efficient Ce-based recyclable photocatalysts with excellent photochemical abilities for application in environmental purification.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study is financially supported by the National Natural Science Foundation of China (Nos.21701166,51472236,21603109),the National Basic Research Program of China (973 Program,No.2014CB643803),the Fund for Creative Research Groups (No.21521092),and Key Program of the Frontier Science of the Chinese Academy of Sciences (No.YZDY-SSW-JSC018),the Henan Joint Fund of the National Natural Science Foundation of China (No.U1404216).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.07.060.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
