High temperature H2S selective oxidation on a copper-substituted hexaaluminate catalyst: A facile process for treating low concentration acid gas
Xin Xu,Ganggang Li,Fenglian Zhang,Guoxia Jiang,Zhengping Hao,∗∗
a Research Center for Eco-Environmental Sciences,Chinese Academy of Sciences,Beijing 100085,China
b National Engineering Laboratory for VOCs Pollution Control Material &Technology,Research Center for Environmental Material and Pollution Control Technology,University of Chinese Academy of Sciences,Beijing 101408,China
Keywords:Low concentration acid gas H2S selective oxidation High temperature Hexaaluminate catalyst
ABSTRACT H2S selective catalytic oxidation technology is a prospective way for the treatment of low concentration acid gas with simple process operation and low investment.However,undesirable results such as large formation of SO2 and catalyst deactivation inevitably occur,due to the temperature rise of fixed reaction bed caused by the exothermic reaction.Catalyst with high activity in wide operating temperature window,especially in high temperature range,is urgently needed.In this paper,a series of copper-substituted hexaaluminate catalysts (LaCux,x=0,0.5,1,1.5,2,2.5) were prepared and investigated for the H2S selective oxidation reaction at high temperature conditions (300-550°C).The LaCu1 catalyst exhibited excellent catalytic performance and great stability,which was attributed to the best reductive properties and proper pore structure.Besides,two facile deep processing paths were proposed to eliminate the remaining H2S and SO2 in the tail gas.
H2S-containing acid gas that are generated in large amount from the fossil energy processing industry,constitute a major hazard to human health and the environment [1].An increase in utilization of high-sulfur raw materials and the enforcement of stringent environmental regulations have triggered demand for achieving a higher level of acid gas removal and its efficient treatment[2].
At present,the high concentration of H2S (≥12 vol%) is mainly treated by the Claus process,which consists of a high-temperature(1000–1400°C) thermal section and a low-temperature multistage catalytic section.However,the Claus reaction is restricted by thermodynamic equilibrium,and 3–5 vol% H2S gas will be remained in the Claus tail gas [3].Several technologies exist for the treatment of Claus tail gas,including low-temperature Claus reaction technology,reduction-absorption technology,and H2S selective catalytic oxidation technology.Among them,H2S selective catalytic oxidation technology is widely concerned because it is not limited by the thermodynamics and H2S concentration,and that low operating cost is needed.The reaction equations are as follows (R1,with side reactions R2 and R3).Recently,a lot of processes have been developed based on this reaction,such as SuperClaus,EuroClaus,Clinsulf-Do,Modop and Selectox technologies [4].
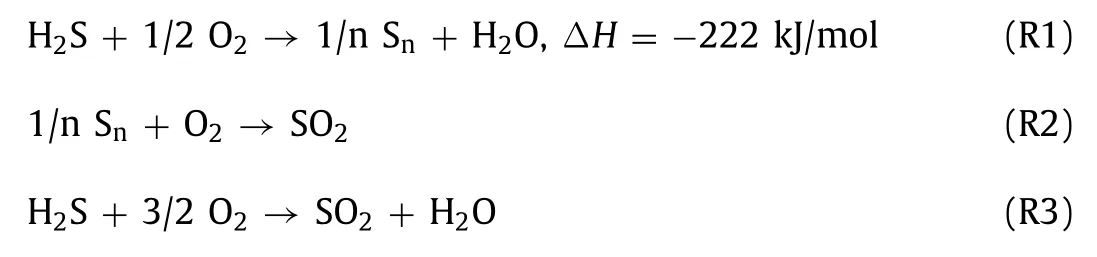
Additionally,in coal chemical industry and some small-scale refineries,such as coking plants,fertilizer plants,the concentration of byproduct H2S is relatively low (<12 vol%).The acid gas is not suitable to be treated by the Claus process because of its low calorific value.Use of auxiliary fuel must be taken in order to maintain a stable flame of high temperature and achieve good combustion efficiency,which complicates the process and increases capital cost.Methods based on absorption and adsorption are alternatives for the treatment of low concentration H2S acid gas.However,the absorbents or adsorbents need periodic regeneration and the desorbed H2S still needs further processing [5].H2S selective oxidation technology can not only be applied to the Claus tail gas,but also for the treatment of low concentration H2S acid gas.More importantly,compared to the traditional absorption and adsorption method,H2S selective oxidation could oxidize H2S to element sulfur directly and continuously,achieving cleaner and more sustainable production [6].
Catalysts play an important role in selective oxidation of H2S,which the H2S conversion and sulfur selectivity both depend on the performance of the catalysts.However,the selective oxidation of H2S is a strong exothermic reaction (ΔH=−222 kJ/mol),and reacting every 1 vol% H2S will lead to a 50–60°C temperature rise of the fixed reaction bed [7].Meanwhile,the high formation activation energy of SO2(120 kJ/mol) determines that high temperature is conducive to the generation of SO2[8].To maintain a long catalytic life and high sulfur selectivity,recent researches generally concern the reaction temperature at 160–300°C.The catalysts mainly concentrate on the iron-,vanadium- and cadmiumbased catalysts,which might be overactive to cause the overoxidation of reactant H2S or product S into SO2.Moreover,metal oxides are easy to be vulcanized by H2S under high temperature conditions [9–11].The narrow activity window of existing catalysts limits the further application of H2S selective oxidation technology.The high temperature reaction puts forward requirements for the anti-sintering and anti-poisoning properties of catalytic materials.Moreover,side reactions and by-product SO2will be conducive to generate with the increase of bed temperature.Therefore,it is meaningful and urgent to develop a high temperature resistant catalyst for H2S selective oxidation reaction,which could maintain the high activity and high sulfur selectivity simultaneously.
Hexaaluminate is a class of aluminate compounds with hexagonal layered crystal structure.The general formula can be expressed as ABxAl12-xO19.The A site and B site (Al3+ions) in the crystal lattice could be substituted by metal ions with similar radius [12].Furthermore,the special layered structure with alternate Al2O3spinel phases separated by mirror planes is mainly responsible for their excellent anti-sintering ability and thermal stability [13].Consequently,hexaaluminate with sufficient redox and acid-base surface properties can be achieved and are adaptable for many catalytic reactions.As a preferred oxygen diffusion channel,the mirror layer has a great application prospect in the field of oxygen involved reactions [14].In recent years,hexaaluminate materials have attracted a lot of attention for their application in high temperature reactions,for example,catalytic combustion of methane and selective catalytic oxidation of ammonia [15–18].The selective oxidation of H2S at high temperature conditions requires the catalyst that on the one hand has active sites to selectively oxidize H2S into elemental sulfur,and on the other hand has prominent stability to prevent it from being vulcanized at high temperature.Based on this,the hexaaluminate materials maybe a promising and potential catalytic material for H2S selective oxidation at high temperature.
Thus,a series of copper substituted LaCuxAl12-xO19(LaCux,×=0,0.5,1,1.5,2,2.5) hexaaluminate catalysts were synthesized with a pH-controlled coprecipitation method and their catalytic performance for H2S selective oxidation reaction were investigated at high temperature conditions (300-550 °C).The physicochemical properties of LaCuxAl12-xO19catalysts were characterized by various techniques and the details about catalysts preparation,characterization,and evolution tests were described in the Supporting information experimental section.For convenience,all catalysts were named as LaCux,such as LaCu0represented LaAl12O19and LaCu1represented LaCu1Al11O19.
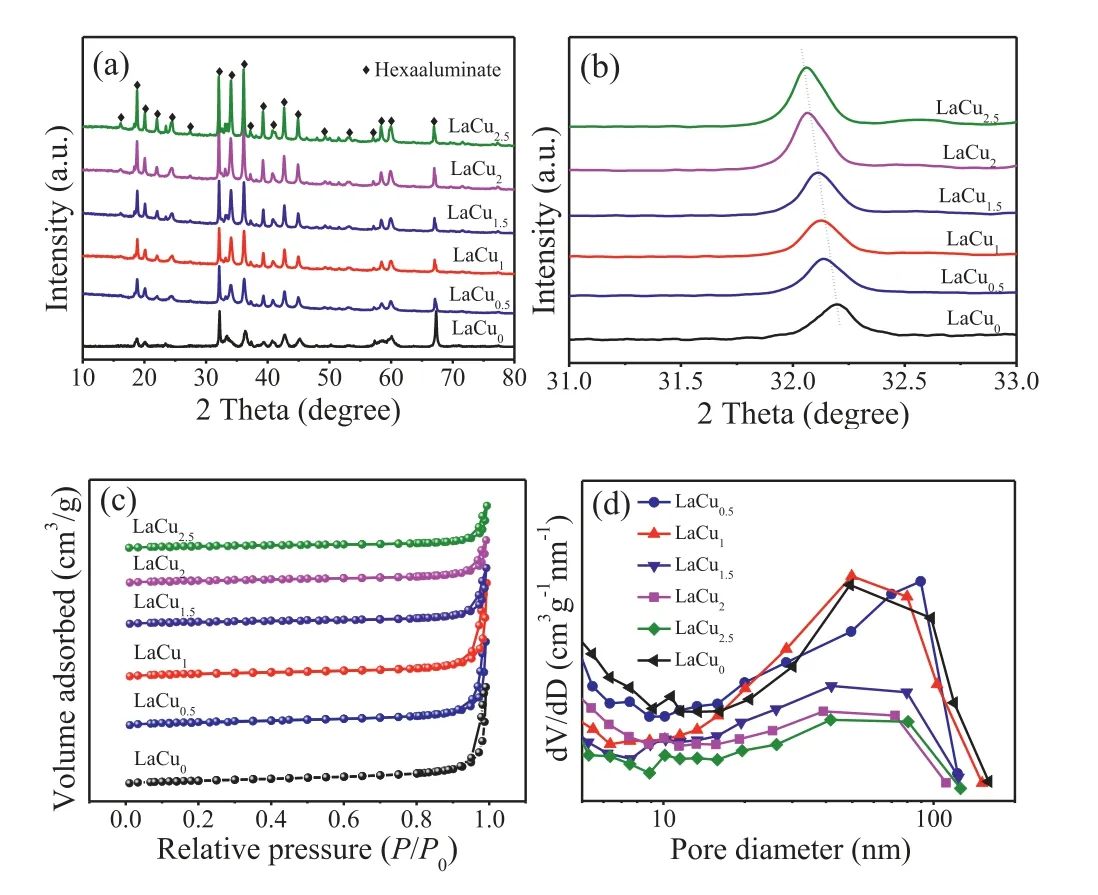
Fig.1.XRD patterns (a,b),N2 adsorption-desorption isotherms (c) and pore size distribution (d) profiles of LaCux (x=0,0.5,1,1.5,2,2.5) catalysts.
Firstly,the structure and textural properties of LaCux(x=0,0.5,1,1.5,2,2.5) catalysts were studied by X-ray diffraction (XRD) and N2adsorption-desorption measurements and the results are shown in Fig.1 and Table S1 (Supporting information).As seen in Fig.1a,all catalysts exhibited standard magneto-plumbite type hexaaluminate diffraction peaks (MP,JCPDS No.33–0699).The diffraction peaks were very sharp and there was no other impurity structure detected,indicating the hexaaluminate crystal phase formed completely after calcination at 1200°C.Compared with the XRD pattern of LaCu0catalyst,the peaks of copper-substituted catalysts were sharper.It is also mentioned in the literature that the substitution of transition metal contributes to the formation of hexaaluminate crystal phase [19].Furthermore,as shown in Fig.1b,with the increase of Cu doping amount,the peaks shifted progressively towards lower angle.This behavior is mainly due to the distortion of crystal lattice after Cu2+with larger radius replacing the smaller Al3+ion,which further confirms that Cu2+was almost doped into the crystal structure of hexaaluminate and partly replaced the position of Al3+.
Fig.1c shows that all catalysts exhibited a characteristics of type IV isotherms with obvious hysteresis loops,implying a mesoporous structure with good pore connectivity of the catalysts [20].Among these catalysts,the LaCu0,LaCu0.5and LaCu1catalysts displayed obvious hysteresis loops,which might contribute to a better pore structure connectivity.Besides,the pore size of the catalysts from Fig.1d mainly distributed between 20–100 nm.The pore size distribution was relatively broad,indicating a variety of different size mesoporous structures.Furthermore,the specific surface area,pore volume and average pore diameters of the catalysts are also given in Table S1 (Supporting information).As shown,the specific surface area of the catalysts almost decreased with the increase of Cu doping amount.Particularly,the LaCu1catalyst had a large pore volume (0.16 cm3/g) and average pore diameter (27.8 nm).Since the mesoporous structure is conducive to the diffusion of reactant molecule and could facilitate it accessible to the active phase,the larger pore volume and pore size would be benefit for the catalytic performance [21].
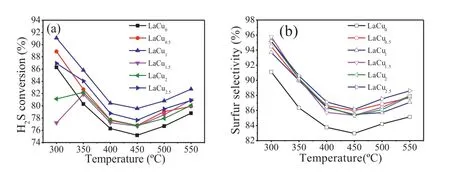
Fig.2.H2S conversion (a) and sulfur selectivity (b) on LaCux (x=0,0.5,1,1.5,2,2.5) catalysts.(Reaction conditions: T=300-550 °C,GHSV=5000 h−1,[H2S]=5000 ppm,[O2]=2500 ppm).
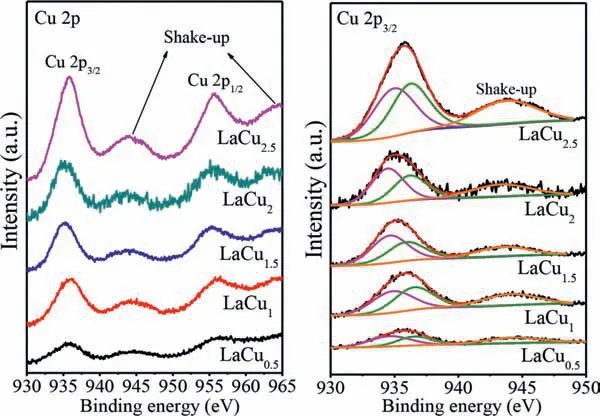
Fig.3.XPS spectra of LaCux (x=0.5,1,1.5,2,2.5) catalysts.
The catalytic performance of H2S selective oxidation reaction on LaCux(x=0,0.5,1,1.5,2,2.5) catalysts were investigated and the results are shown in Fig.2.As seen in Fig.2a,the H2S conversion of different Cu doping amount catalysts quite varied at 300°C.The order is as follows: LaCu1>LaCu0.5>LaCu2.5>LaCu0>LaCu2>LaCu1.5.When the temperature was above 350 °C,there were little differences in activity and all catalysts tended to a similar change trend.At first,the H2S conversion decreased with the temperature rising to 450°C and then it was a slight increase up to 550°C.Among these catalysts,the LaCu1catalyst displayed the highest H2S conversion,about 91.5% at 300°C,and more than 80%even at 450°C.In terms of sulfur selectivity (Fig.2b),the variation of different Cu doping amount catalysts is basically similar.The sulfur selectivity decreased firstly with the temperature rising from 300°C to 450°C,and then increased slightly up to 550°C.Overall,almost all the catalysts can achieve more than 86% sulfur selectivity at the investigated temperature ranges except for LaCu0catalyst.Combined with the activity results of different transition metal substituted LaB(B=Fe,Co,Ni,Cu,Mn) catalysts as presented in Fig.S1 (Supporting information),it can be inferred that the transition metal substituted of hexaaluminate could improve the catalysts performance for H2S selective oxidation.Particularly,the stability behavior of LaCu1catalyst for H2S selective oxidation at 550°C was also investigated.As shown in Fig.S2 (Supporting information),the catalyst could operate steadily for 48 h at a high temperature of 550°C almost without any loss of activity,displaying a high thermal reaction stability.
The chemical properties of catalyst are significant for the catalytic performance.In view of this,various methods were adopted to explore the chemical status of the active species.X-ray photoelectron spectroscopy (XPS) was employed to characterize the chemical status of the transition metals.The Cu 2p XPS spectra of the catalysts are presented in Fig.3.According to the literature,the peaks at 935.8 eV and 955.1 eV are the signal peaks of Cu 2p3/2and Cu 2p1/2,respectively,showing a 19.9 eV spin-orbit splitting and the signal peaks near 945 eV and 965 eV are satellite peaks of Cu 2p.It is well known that shake-up peaks of Cu-XPS are present in the spectra of d9 Cu2+-containing samples but are absent in d10 Cu+spectra [22].Thus,the Cu species mainly existed in the form of Cu2+in the hexaaluminate crystal structure.Moreover,the peaks of Cu 2p3/2could be deconvoluted into two main contributions located at around 933.6 eV and 935.2 eV,ascribed to Cu2+in the tetrahedral and octahedral coordination,respectively[23].The UV-vis diffuse reflectance spectra were performed to obtain detailed information on the oxidation state and coordination of Cu species.As shown in Fig.S3 (Supporting information),two characteristic bands were observed.According to previously published reports,the absorption band at 210–320 nm results from the O2−→Cu2+charge transfer,while the broad band at 600-800 nm were associated with Cu2+in an octahedral configuration,more or less tetragonally [24].Therefore,the results of XPS and UV-vis DRS implied that Cu2+ions were existed in tetragonal and tetragonally distorted octahedral sties of the hexaaluminate catalysts.
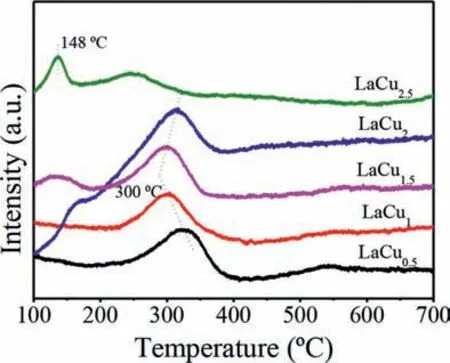
Fig.4.H2-TPR profiles of LaCux (x=0.5,1,1.5,2,2.5) catalysts.
As known,the reducibility of catalyst plays a vital role in the catalytic performance especially in the oxidation reactions.Therefore,the reductive properties of LaCux(x=0.5,1,1.5,2,2.5) catalysts were studied by the H2temperature-programmed reduction(H2-TPR) experiments.Fig.4 shows the H2-TPR profiles of hexaaluminate catalysts with different Cu doping amount.The major reduction peaks were all below 600°C.It is well known that different positions of the reduction peaks indicate the different reducibility,while the positions of the reduction peaks might be ascribed to the valence state changes and the difference in the crystallographic sites of metal ions [24].Researchers generally believe that the crystallographic positions (octahedral sites) located near the mirror layer are easier to be reduced [25].Based on this,the major reduction peaks appearing at 200–350°C were ascribed to the reduction of Cu2+in the substituted positions of Al3+.Moreover,the different reduction temperature might be due to the different crystallographic positions of Cu2+,tetragonal or octahedral sties as mentioned in XPS analysis.In addition,with the Cu doping amount greater than 1.5,the catalysts showed a small reduction peak at 100–200°C,especially for the LaCu2.5catalyst.The peaks could be ascribed to the reduction of Cu2+in the copper oxides,which indicates that the amount of Cu doping in the crystal structure of hexaaluminate is limited,and excessive Cu doping will lead to the form of the amorphous copper oxides.
According to the above physicochemical analysis,the LaCu1catalyst displayed the best reductive properties,which was due to the reason that the appropriate Cu doping amount provides a proper proportion of Cu2+in tetragonal or octahedral sties.Moreover,combined with the physical N2adsorption-desorption measurements,the LaCu1catalyst has a large specific surface area and pore structure,which is conducive to the diffusion of reactant molecule.Therefore,it indicated that excellent reducibility resulted from a proper substitution of Cu and more accessible of reactant molecule owing to the textual properties are responsible for highest reactivity of LaCu1catalyst.While for the catalysts with the Cu doping greater than 1.5,there were small decline in catalytic activity,which might because of the inferior reducibility,less pore structures and the form of amorphous copper oxides with excess Cu substituted amount.
Although the copper-substituted hexaaluminate catalysts exhibited excellent catalytic activity,it still cannot achieve 100% H2S conversion and sulfur selectivity unfortunately.In order to get a better sulfur yield,two facile deep processing paths are proposed for the treatment of the tail gas which includes the remaining H2S and SO2generated in the reaction.The processes are presented in Scheme S1 (Supporting information).As shown,one deep processing is that firstly convert the generated SO2to H2S through a hydrogenation method,then eliminate the H2S completely by a traditional H2S selective oxidation technology (generally at low temperature conditions).Another path is to eliminate H2S and SO2directly by adopting the catalytic section of Claus process,because the ratio of H2S and SO2concentration in the tail gas is close to 2:1,which is suitable for the Claus reaction (2H2S+SO2→3/n Sn+2H2O).
In summary,the copper-substituted hexaaluminate catalysts exhibited excellent catalytic performance and great thermal reaction stability for H2S selective oxidation.Among these catalysts,the LaCu1catalyst showed the best H2S conversion (91.1%,300°C) and sulfur selectivity (93.7%,300°C),and can maintained them at 82.5%and 87% respectively even at 550°C.Hexaaluminate materials have excellent thermal stability because of its special hexagonal layered structure which make LaCu1catalyst stable during the high temperature reaction,almost operating steadily for 48 h at 550°C without any loss of activity.Moreover,the appropriate Cu doping amount provides a proper proportion of Cu2+in tetragonal or octahedral sties and a larger specific surface area and pore structure,which improve the reductive properties of the LaCu1catalyst.Besides,two facile deep processing paths are proposed for the treatment of the low concertation H2S acid gas completely.The technology could reduce the process operation and equipment investment,which has a good application prospect in the field of industrial acid gas treatment.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.21976176,22006148),the Key R&D Program of Shandong province (No.2019JZZY010506)and the Fundamental Research Funds for the Central Universities.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.07.053.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
