Amplification effects of magnetic field on hydroxylamine-promoted ZVI/H2O2 near-neutral Fenton like system
Wei Xing,Mingjie Hung,Xiohui Wu,Fugng Zhng,Dn Li,To Zhou,∗
a School of Environmental Science and Engineering,Huazhong University of Science and Technology,Wuhan 430074,China
b Key Laboratory of Water and Wastewater Treatment (HUST),MOHURD,Wuhan 430074,China
c Three Gorges Base Development Co.,Ltd,Yichang 443002,China
Keywords:Fenton like Zero valent iron Hydroxylamine Magnetic field
ABSTRACT This study has demonstrated an interesting amplification effect of magnetic field (MF) on the hydroxylamine (HA)-promoted zero valent iron (ZVI)/H2O2 Fenton-like system.Sulfamethoxazole (SMX) could be efficiently degraded at near neutral pH.Conditional parameters affecting the SMX degradation in the ZVI/H2O2/HA/MF system,e.g.,pH and the dosages of ZVI,HA and H2O2,were investigated.Unlike the acid-favorable ZVI/H2O2 and ZVI/H2O2/HA systems,the MF-assisted system exhibited good performances even at pH up to 6.0 and highest degradation rate at pH of 5.0.•OH was still identified as the responsible oxidant.A mechanism involving the MF-enhanced heterogeneous-homogeneous iron cycle was proposed in the near-neutral ZVI/H2O2/HA system.Without MF,HA-induced reductive dissolution of the surface iron oxides occurred and thus leaded to homogeneous Fenton reactions.After the introduction of MF,the gradient magnetic field formed on the ZVI particles would induce the generation of concentration cells of Fe(II) and local corrosion of iron.Large amounts of aqueous and bounded Fe(II) catalyzed H2O2 to efficiently produce •OH,while HA maintained the surface and bulk cycles of Fe(II)/Fe(III).The result of study is expected to provide a green,energy-free method in improving the effectiveness of ZVI-based Fenton-like technologies at weak-acidic circumstances.
With the elevated demand of the medical level,the usage of antibiotic grows rapidly.Sulfamethoxazole (SMX),a typical antibacterial agent usually applied in urethral infection,has been increasingly adopted,and amounts of its residues have been put into surface water,ground water and the sewage system [1,2].However,the conventional wastewater treatment plants are always not effi-cient in the removal of SMX,leading to its substantial emigration to water environment [3].As a result,the concern about the carcinogenic risk of SMX in aqueous ecosystem has been provoked in the public.
Advanced oxidation processes (AOPs) are recognized as the effective technologies for removing the refractory organic pollutants.Among of them,Fenton oxidation is favorable because of its mild condition and high efficiency (Eq.1) [4].Nevertheless,the high consumption of ferrous iron by side reactions (Eq.2) not only elevates the dosage of agent,but also brings large amount of iron sludge [5].Instead of direct and once dosing of Fe2+,the use of zero valent iron (ZVI) as sustained release agent may effectively decrease the side reaction [6].In the acidic circumstance,ZVI is liable to be oxidized by water and protons (Eqs.3 and 4),resulting in sustained generation of Fe2+which supports the continuous Fenton reaction.But the ZVI based Fenton-like technologies are always limited by the reactivity of iron particles [7].Numerous researches reported that the intrinsic and/or newly formed iron oxides inhibited the iron corrosion and thus the degradation of organic pollutants showed a lag phase [5,6].Furthermore,at neutral and alkaline pH,this Fenton-like reaction is always blocked.Obviously,the bottleneck of low reactivity and pH-sensitivity of iron particles should be conquered in ZVI based Fenton-like technology before which become an applicable substitute for conventional Fenton reaction.
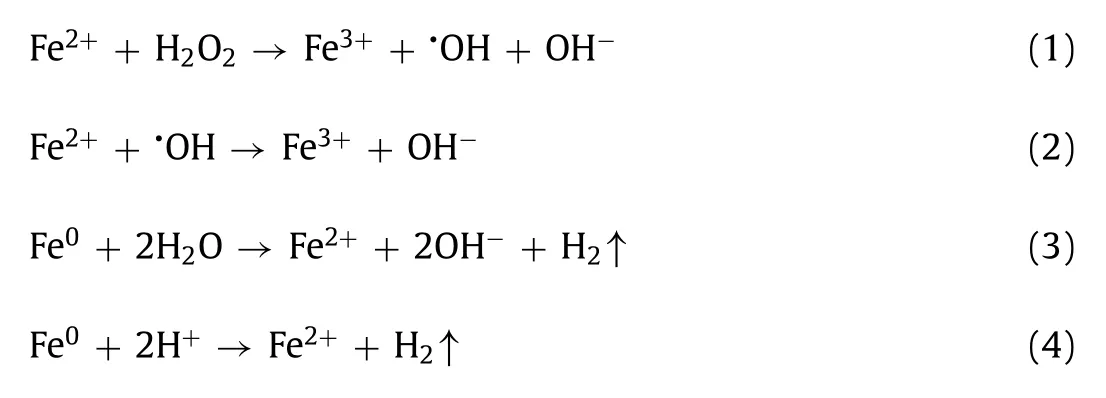
In the last decade,weak magnetic field (WMF) was found effective in enhancing the ZVI technologies [7].It was thought that magnetic field would be distorted around the ferromagnetic iron particles.The high magnetic gradient on iron surface would induce the generation of concentration cells with ferrous/ferric ions,which accelerated the electrochemical corrosion of Fe0[6].Several groups applied WMF to assisting the ZVI based Fenton-like reaction and observed significantly enhanced leaching of iron and degradation of organic pollutants [6,8,9].In our previous research,thekobsof 4-CP degradation by ZVI/H2O2system could be increased by an order of magnitude with WMF at pH lower than 3.However,we also found the iron corrosion and Fenton reaction were significantly suppressed above pH 4,regardless of WMF.It was proposed the intrinsic iron oxides was stable at high pH,and the electron transfer between Fe0and H+/H2O was blocked even at the presence of WMF [6].Therefore,although it was an excellent assisting method at acidic environment,WMF alone could not effectively overcome the low reactivity of iron particle at near neutral pH.
Recently,it was reported that hydroxylamine (HA) could promote the iron corrosion at near neutral pH.Luo et al.observed 100% degradation of rhodamine in 10 min in the Fe@Fe2O3/H2O2/HA system at pH 4.0,comparing with 29% in 60 min in the Fe@Fe2O3/H2O2system [10].The enhanced degradation was attributed to the complexation/reduction-dissolution of iron oxides as well as promoted homogeneous Fe(II)/Fe(III) cycle.Ouyanget al.added HA to ZVI/H2O2system and found the removal ratio of lincomycin was increased from 35% to 65% at pH 5.8[11].These researches evidenced that HA could break the intrinsic and/or newly formed iron oxides on iron particles,thus broaden the effective pH for ZVI based Fenton-like technology.
Based on the above discussion,it was expected that the coexistence of WMF and HA would bring cooperative reinforcement on ZVI based Fenton-like technology at near neutral pH (4.0 - 6.0).The rapid degradation of refractory organic pollutants might be possible with this compounding technology.However,the coupling effect of WMF and HA on ZVI was never reported.Therefore,the main objective of this study was to confirm the feasibility of applying ZVI/H2O2system assisted by both HA and WMF to degrading SMX at near neutral pH.The conditional parameters,e.g.,pH and the dosages of ZVI,HA and H2O2,were also investigated.After the qualification of responsible oxidant,evolution of iron species and characterization of solid samples,a homogeneous/heterogeneous mechanism was proposed.
The ZVI powders (>98%) were purchased from Sinopharm Chemical Reagent Co.,Ltd.and used without pretreatment.The characterization of ZVI,i.e.,SEM picture,XRD and XPS spectra,could be seen in Fig.S1 (Supporting information),which indicated that the ZVI with the diameter range from 20 μm to 200 μm were constituted by Fe° core and iron oxides layer.Other chemicals,such as H2SO4,NaOH,methanol,were also obtained from Sinopharm Chemical Reagent.Hydroxylamine sulfate ((H2NOH)2•H2SO4),SMX,o-phenanthroline and acetonitrile were supplied by Aladdin Chemistry Co.,Ltd.
All experiments were conducted in borosilicate glass reactors(250 mL).Magnetic field was supplied by two pieces of rounded rubber magnets placed under the reactors.The maximum magnetic field intensity of 2.9 mT was measured at the bottom of the reactors by a Gaussmeter (421-MNA-1904-VG,Lake Shore Cryotronics,Inc.).Before degradation experiments,200 mL working solution containing predetermined HA,H2O2and SMX was mixed by a mechanical stirrer (EUROSTA 20 digital,IKA®-Werke GmbH &Co.KG,Germany) and adjusted to certain pH (4.0,5.0,6.0 and 7.0) by NaOH (0.1 mol/L) and H2SO4(0.05 mol/L).The degradation reaction was initiated by the addition of ZVI powders.During the reaction,the working solution was open to air and stirred at 400 rpm.At regular intervals,5 mL of suspension was filtered by 0.22 μm PES membrane (Titan) and mixed with 20 μL methanol.The liquid samples would be sent for analysis of SMX and aqueous iron immediately.
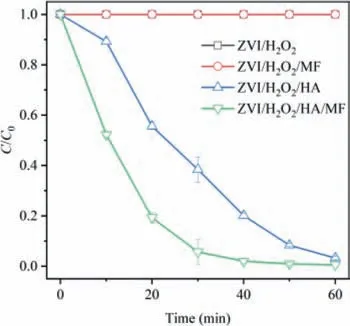
Fig.1.Degradation of SMX in the comparative systems.Initial conditions were: 0.1 g/L ZVI,1.0 mmol/L H2O2,0.4 mmol/L HA,10 mg/L SMX,pH 6.0 and 25 °C.
SMX was quantified by a high-performance liquid chromatography (HPLC,LC-15C,Shimadzu) equipped with a UV-vis detector and a C18 column (Waters,5 μm,4.6 × 300 mm).The mobile phase was a mixture of 0.1% (v/v) acetic acid (40%) and acetonitrile (60%),with a flow rate of 0.7 mL/min.The detection wavelength was set at 270 nm while the temperature was controlled at 35°C.Total dissolved iron and aqueous ferrous iron were spectrometrically measured with an UV-vis spectrophotometer (UV-2600,Shimadzu),according to theo-phenanthroline colorimetric method[12].The data shown in the figures are the mean values of at least duplicate experiments,and the error bars denote the standard deviations.
Fig.1 presents the comparative degradation of SMX in ZVI/H2O2,ZVI/H2O2/MF,ZVI/H2O2/HA and ZVI/H2O2/HA/MF systems at pH 6.Without HA,SMX could not be removed,regardless of MF.The addition of HA would trigger the degradation reaction and 96% removal of SMX could be achieved at 60 min.The degradation reaction could be further accelerated by combination with MF,i.e.,the 95% removal of SMX just consumed 30 min.Besides,a positive relationship between magnetic field intensity and SMX degradation efficiency could be observed in Fig.S2 (Supporting information).Obviously,cooperative reinforcement with HA and MF existed in the ZVI/H2O2system.
The role of HA was explored at first.Aqueous iron was not detected in the ZVI/H2O2(/MF) systems whereas the accumulation of dissolved iron was observed in the HA-assisted systems (Fig.S3 in Supporting information),indicating that HA was able to accelerate the corrosion of ZVI.Furthermore,as shown in Fig.S4 (Supporting information),the degradation of SMX correlated well with the consumption of HA.It indicated that HA absolutely participated in the catalytic oxidation of SMX.The evolution of iron species also showed significant dependences on HA.Fe(II) accumulated at the presence of HA,while its concentration would gradually decrease when HA was completely consumed.By contrast,the concentration of Fe(III) was negligible until the disappearance of HA,after which Fe(III) rapidly accumulated in the bulk solution.Obviously,HA efficiently maintain cycle of Fe(II)/Fe(III),thus promoting the rapid Fenton reaction.In addition,total nitrogen (TN) was measured to probe into the fate of HA (Fig.S5 in Supporting information).It was found that 91% removal of TN was achieved at the termination of reaction.Most of the nitrogen in HA should be transformed to N2,while the residue might exist as NOx[13].

Fig.2.The effect of (a) pH and dosages of (b) HA,(c)ZVI,(d) H2O2 on the degradation of SMX in the ZVI/H2O2/HA/MF system.
Several conditional parameters were investigated and the result was shown in Fig.2.Although the degradation was negligible at pH 7.0,SMX could be totally removed in 60 min at pH lower than 6.0 (include 6.0) (Fig.2a).The pH upper than 7.0 might seriously block the iron corrosion,thus the liberation of Fe(II)was not detected during the reaction.The best pH was 5.0 instead of 4.0 for the ZVI/H2O2/HA/MF system.This result was different from the homogeneous Fenton and HA-assisted Fenton reactions,the most suitable pH of which were recognized as 3.5 and 3.0,respectively [14].Besides,it was reported that higher pH was more favorable for the reaction of•OH with HA,indicating the quenching effect of HA could not explain the more efficient degradation of SMX at pH 5.0.Therefore,heterogeneous reaction was thought also responsible for the degradation of SMX.It was evidenced by Fig.S6 (Supporting information),which showed that the liberation of dissolved iron was accelerated by low pH.Liuet al.reported that dissolved Fe(II) could provide more bounded Fe(II) on Fe@Fe2O3at near neutral pH (5.0-8.0),and the bounded Fe(II) was more reactive than aqueous Fe(II) [15].Therefore,it was possible that the catalytic decomposition of H2O2by bounded Fe(II) on ZVI surface was more favorable at higher pH,which leaded the better degradation of SMX at pH 5.0 than pH 4.0.
As discussed above,HA was indispensable for the occurrence of SMX degradation in the ZVI/H2O2/MF system at near neutral pH.Moreover,the degradation rate of SMX was positively related to the dosage of HA when the latter was lower 0.4 mmol/L (Fig.2b).However,the prohibitive concentration of HA would negatively affect the degradation reaction.The possible reason was that the competitive consumption of reactive oxygen species by excess HA[14].
ZVI dosages were also investigated as shown in Fig.2c.The moderate dosage of ZVI (0.1 and 0.2 g/L) would be favorable for the ZVI/H2O2/HA/MF system,while the excessive (0.4 g/L) and inadequate (0.05 g/L) would inhibit the degradation of SMX.Slow liberation of Fe(II) might limit the degradation reaction at low dosage of ZVI.However,existence of superfluous fresh iron surface would also consume oxidants,e.g.,H2O2,•OH,which thus led to suppression of Fenton reaction [5].
Fig.2d shows the effect of H2O2dosage on SMX degradation in the ZVI/H2O2/HA/MF system.At concentration lower than 1 mmol/L,H2O2benefited the degradation of SMX for supplying the oxidant.However,the degradation would be blocked by H2O2when its concentration was upper than 4 mmol/L.Similar phenomenon was observed in our previous research in which we proposed that excessive amounts of H2O2would inactivate the ZVI surface and inhibit the release of ferrous ion [6].The speculation was also evidenced by the detection of dissolved iron (Fig.S7 in Supporting information) in this study.
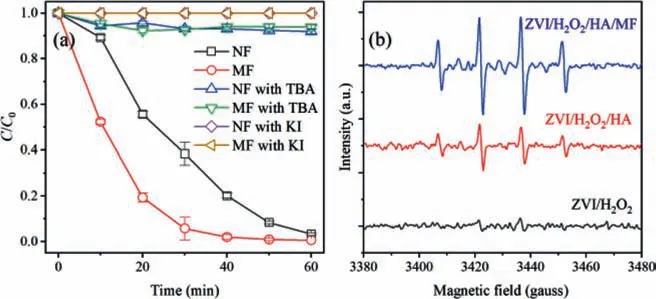
Fig.3.(a) The quenching effect of TBA and (b) the EPR spectra.
The enhancement by MF at different conditions is illustrated within the inset of Figs.2a–d.Obviously,at most of investigated conditions,MF could accelerate the degradation of SMX.It indicated the introduction of MF was beneficial to the ZVI/H2O2/HA system.
To clarify the mechanism for SMX degradation in the ZVI/H2O2/HA/MF system,the oxidant was qualified with quenching experiments and EPR test.Tertiary butyl alcohol (TBA) and potassium iodide (KI) could significantly inhibit the degradation reaction,regardless of MF (Fig.3a).It evidenced that the responsible oxidant should be•OH,of which the signal was also observed in the EPR spectra (Fig.3b).The intensity difference of the quartet peak with hyperfine constants ofαN=αH=14.9 G indicated that MF enhanced the generation of•OH [16].Therefore,the degradation of SMX should be attributed to the oxidation by•OH,which was generated from the decomposition of H2O2.Compared with the system without MF,the formation of•OH was accelerated in ZVI/H2O2/HA/MF system,leading to rapid degradation of SMX.
Since the Fenton reaction was usually thought responsible for•OH formation in the ZVI technologies,the evolution of aqueous iron was measured.Like the previous researches [6,8,9],the liberation of aqueous iron was promoted in the ZVI/H2O2/HA/MF system(Fig.S3).Obviously,the corrosion of iron particles was accelerated by the introduction of MF.The mechanism was usually proposed relating to the magnetic gradient around iron particles.
In the above discussion about pH effect,we thought the Fentonlike reaction catalyzed by bounded Fe(II) might also contribute to the generation of•OH and the following degradation of SMX.To further confirm the heterogeneous process,o-phenanthroline was also adopted to complex the Fe(II)aqand bounded Fe(II).Although never reported were the different rates foro-phenanthroline complexing with Fe(II)aqand bounded Fe(II),the reactivity of bounded Fe(II) was recognized higher than Fe(II)aq[17].It was expected the inhibition role ofo-phenanthroline was more significant in Fenton reaction catalyzed by Fe(II)aq.Interestingly,the addition of ophenanthroline brought different results between the ZVI/H2O2/HA and ZVI/H2O2/HA/MF systems (Fig.S8 in Supporting information).Thereupon,it was thought that bounded Fe(II) made more contribution in the ZVI/H2O2/HA/MF systems compared with the control system.XPS was applied to semi-quantifying the bounded Fe(II)on iron particles.The spectra of the solid samples collected during the reaction are shown in Figs.4a and b.The quantity of bounded Fe(II) was obviously larger at the surface of iron reacted in the system with MF than the control system.The ratios of ferrous iron to total iron (FeII/Fetotal) and ferric iron to total iron (FeIII/Fetotal) was respectively calculated with the peak area in Fe 2p core level spectra [18].As illustrated by Fig.4c,Fe(II) accounted for about 66%of the total iron species on the surface of particles in the system with MF,while just 55% in the control system.Therefore,it was possible that the increased bounded Fe(II) formed at the presence of MF also contributed to the enhanced OH generation and SMX degradation.
Fig.4.XPS spectra of the iron particles collected from the (a) ZVI/H2O2/HA/MF and(b) ZVI/H2O2/HA systems,and (c) the calculated area percentage of Fe(II),Fe(III) on the iron surfaces.

Fig.5.SEM images of (a) the pristine iron particles and the solid samples collected from the (b) ZVI/H2O2/HA and (c) ZVI/H2O2/HA/MF systems.
Fig.5 shows the SEM images of the iron particles.Smooth surface could be observed with the pristine iron particles,while the rugged surface would appear on the particles after the reaction in solution.It was hard to qualified the oxides formed on iron surface during the reaction,since the XRD spectra showed that Fe0was still the major constituent of ZVI particles after reaction (Fig.S9 in Supporting information).However,noticeable discrepancy could be observed between the surface morphologies of iron particles in the two comparative systems.MF would induce the ZVI surface more porous after the reaction.This observation was similar to our previous research,in which the iron surface morphology of pits and tubercles induced by MF was attributed to the local corrosion under the magnetic gradient [6].Obviously,the magnetic gradient also affected surface evolution of the iron particles in the ZVI/H2O2/HA/MF system.
Based on the above discussion,the mechanism including heterogeneous and homogeneous processes was proposed for the ZVI/H2O2/HA/MF system (Scheme 1).As a typical complexant and reductant,HA could chelate and reduce the oxides on iron surface,leading to the dissolution of oxides [10].In near neutral circumstances,HA would maintain the homogenous cycle of Fe(II)/Fe(III)as well as the Fenton reaction.
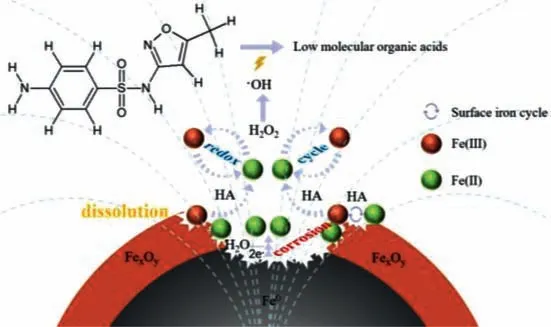
Scheme 1.Proposed mechanism in the ZVI/H2O2/HA/MF system.
In the presence of MF,the concentration cell of Fe(II) formed under the magnetic gradient would lead to local corrosion of iron[19].As a result,the MF-induced specific iron corrosion would lead to the accelerated liberation of Fe(II) and appearance of the porous iron surface.MF would further force the production of abundant bounded Fe(II) on the surface,except for the simultaneous release of aqueous Fe(II).Both the aqueous and bounded Fe(II) would catalyze the Fenton reaction,leading to the generation of•OH and degradation of SMX.In the near neutral reaction systems,especially,the MF-induced generation of the surface bounded Fe(II)would be more important in catalyzing H2O2effectively and heterogeneously,since direct complexion corrosion of the exposed(chelated) active Fe0sites would be more hard.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21677055 and 22006045),the China Postdoctory Science Foundation (No.2020M672361) and the Project of Three Gorges Corporation (No.JD-ZC-FW-20-001).Huazhong University of Science &Technology Analytic and Testing Centre is thanked for the advanced analytic operations.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.07.072.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
