Highly cient photocatalytic NO removal and in situ DRIFTS investigation on SrSn(OH)6
Wendong Zhng,Yun WngYi WngYi LingFn Dong
a Chongqing Key Laboratory of Inorganic Functional Materials,Chongqing Key Laboratory of Green Synthesis and Application,Chongqing Normal University,Chongqing 401331,China
b Yangtze Delta Region Institute (Huzhou) &Institute of Fundamental and Frontier Sciences,University of Electronic Science and Technology of China,Huzhou 313001,China
c State Centre for International Cooperation on Designer Low-carbon and Environmental Materials (CDLCEM),School of Materials Science and Engineering,Zhengzhou University,Zhengzhou 450001,China
Keywords:Highly cient NO removal In situ DRIFTS investigation SrSn(OH)6;Reaction mechanism
ABSTRACT A novel SrSn(OH)6 photocatalyst with large plate and particle size were synthesized via a facile chemical precipitation method.The photocatalytic activity of the SrSn(OH)6 was evaluated by the removal of NO at ppb level under UV light irradiation.Based on the ESR measurements,SrSn(OH)6 photocatalyst was found to have the ability to generate the main active species of O2•−,•OH and 1O2 during the photocatalytic process.Moreover,SrSn(OH)6 photocatalyst not only exhibits high photocatalytic activity for NO removal (79.6%),but also has good stability after “ve cycles.The in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was used to investigate the NOx transfer pathway and the intermediate products distribution during the adsorption and photocatalytic NO oxidation process.The present work not only provides an cient material for air pollutants puri“cation at room temperature but also in-depth understanding of the mechanism involved in the photocatalytic NO removal process.
Air pollution is one of the most serious environmental issues,which results in extremely hazardous effects and risks on public health and ecological security [1,2].However,conventional methods including physical,chemical and biological methods are diˇ-cult to remove the low-concentration of air pollutants (such as nitrogen oxide (NOx),volatile organic compounds (VOCs),etc.) [3,4].Furthermore,most of the above-mentioned methods have disadvantages of requiring complicated techniques,harsh conditions,high costs and time consuming,which greatly limit their practical application.Hence,it is highly desirable to develop a green,stable andcient method to improve air quality.
Photocatalysis is considered as a promising method for air pollutants puri“cation that needs only photocatalyst,sunlight,O2and H2O [5,6].Over the past few decades,many attempts have been made to develop a large amount of photocatalysts for the“eld of environmental remediation,including metal-based photocatalysts (e.g.,TiO2,Bi4O5Br2,SrTiO3,(BiO)2CO3,Ag3PO4,WS2,Au/TiO2and Ag/AgCl) [7-10],polymeric photocatalysts (e.g.,g-C3N4,poly(diphenylbutadiyne),and polyimide) [11-13],and elementalbased photocatalysts (e.g.,Bi,Bi/TiO2,S,and P) [14-16].However,most of the above-mentioned photocatalysts are still far from satisfactory because of their low sunlight harvesting ability,high charge carriers recombination and poor photocatalytic stability.Therefore,there is an urgent need to develop novel photocatalysts with highlycient and stable photoactivity.
Very recently,Liet al.prepared SrSn(OH)6viaa facile homogeneous precipitation method,and the as-obtained SrSn(OH)6samples exhibited excellent UV photocatalytic performance for the degradation of benzene and rhodamine B [17].However,to the best of our knowledge,application and reaction mechanism of SrSn(OH)6for photocatalytic removal of air pollutants has not been reported.Herein,SrSn(OH)6photocatalyst was synthesized by a facile chemical precipitation method in water bath.More importantly,this study focuses on photocatalytic NOxremoval over SrSn(OH)6.Interestingly,SrSn(OH)6exhibited high photocatalytic activity and good stability in the removal of NO under the irradiation of UV light.In addition,on the basis ofin situDRIFTS investigations,the detailed reaction mechanism during the photocatalytic oxidation of NOxwas proposed.
Details of SrSn(OH)6synthesis,characterization method,photocatalytic activity evaluation method,andin situDRIFTS investigation method (S4) are described in Supporting information.
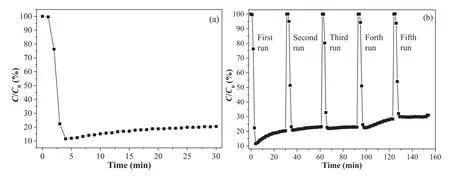
Fig.1.(a) Photocatalytic activity and (b) recycling tests of SrSn(OH)6 for NO removal under ultraviolet light irradiation (λ=280 nm).
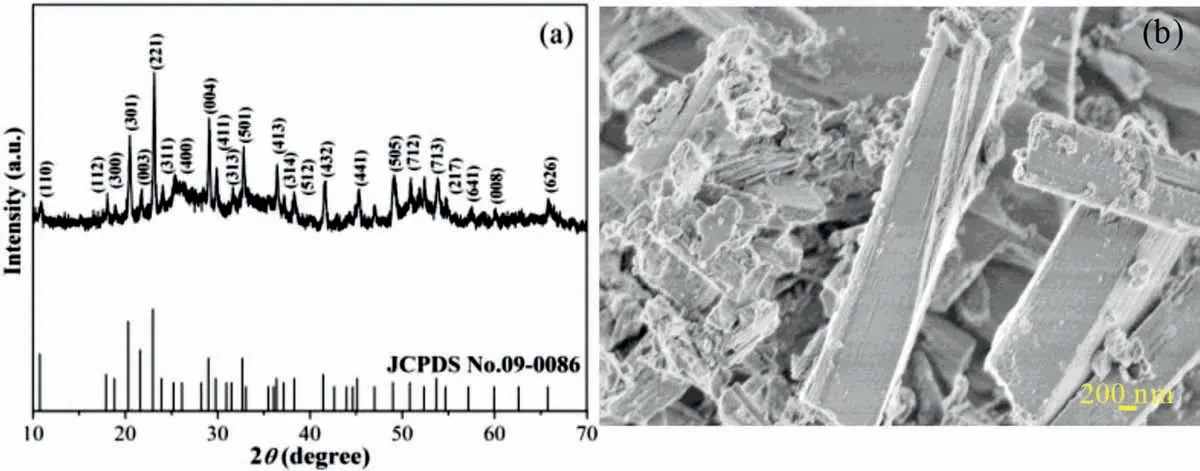
Fig.2.(a) XRD patterns and (b) SEM image of SrSn(OH)6.
As shown in Fig.1a,the photocatalytic removal ratio of NO was up to 79.6% after ultraviolet light irradiation for 30 min with the reactive species,which is morecient than other Bi-based and g-C3N4based photocatalysts [15].Especially,the SrSn(OH)6shows signi“cantly higher photocatalytic activity than that of BiOBr0.5I0.5/BiOI composite (removal rate of 36.2%) [18],oxygen vacancies-mediated TiO2nanocrystals (removal rate of 45.0%) [19],Bi spheres/g-C3N4nanohybrid (removal rate of 59.7%) [20],and defective-Bi2O3(removal rate of 52.0%) [21].However,the concentration of generated NO2rises as high as 195 ppb.Actually,the NO2as intermediate shows more toxicity than NO,which could cause secondary pollution of atmosphere.Therefore,highly-cient inhibition of the toxic NO2generation should be addressed in the future work for practical application of SrSn(OH)6.In addition,SrSn(OH)6only displays slight loss of photocatalytic activity even after “ve recycling tests (Fig.1b).The gradual generation of intermediates and “nal products may occupy the active sites of SrSn(OH)6,which results in the slight loss of photocatalytic activity.These results indicate that SrSn(OH)6has excellent activity and good stability during the photocatalytic removal of NO.
XRD patterns in Fig.2a shows that all the diffraction peaks can be indexed to the hexagonal structure of SrSn(OH)6(JCPDS card No.22-1442) [17].As can be seen from Fig.2b,the as-obtained SrSn(OH)6demonstrates an stick-like structure formed by stacking of large plates and particles size.
As shown in Fig.S5a (Supporting information),SrSn(OH)6featured strong light absorption in the ultraviolet light region.Moreover,the band gap energy (Eg) of SrSn(OH)6which can be determined through the plots of (h)1/2versusphoto energy (Fig.S5b Supporting information) is 3.53 eV.In addition,the conduction band (CB) and valence band (VB) position of SrSn(OH)6can be calculated from the relationshipECB=X−E0−0.5EgandEg=EVB−ECB.Thus,the calculatedECBandEVBof SrSn(OH)6were 0.19 and 3.72 eV,respectively.
As shown in Figs.3a-c,the ESR signals of O2•−,•OH and1O2were increased with prolonged irradiation time,suggesting that O2•−,•OH and1O2are the main active species during the photocatalytic reaction.Fig.3d shows that photogenerated electrons can be rapidly consumed to participate in he generation of abundant active species under the ultraviolet light.
Based on the mentioned above,the valence band position of SrSn(OH)6is 3.72 eV,indicating that the hole oxidation potential of SrSn(OH)6is much higher than those of OH−/•OH (1.99 eV) and H2O/•OH (2.37 eV).Therefore,the•OH radicals could be directly generated from the oxidation of OH−(Eq.1) or H2O (Eq.2).And besides,the conduction band position of SrSn(OH)6is only 0.19 eV,hence,the e−reduction potential of SrSn(OH)6is obviously more positive than that of O2/O2•−(0.33 eV).Thus,the e−reduction potential of SrSn(OH)6could not directly reduce O2to O2•−radicals.The main active species can be formed through a series of reactions as follows: Eqs.1-6.Especially,the1O2radicals can be produced by the further oxidation of O2•−radicals with photogenerated holes (Eq.5) [22].


Fig.3.ESR spectra of radical adduct trapped by (a) DMPO-O2•−,(b) DMPO-•OH,(c) 4-oxo-TEMP-1O2 and (d) TEMPO-e− over SrSn(OH)6 under ultraviolet irradiation(λ=280∼360 nm).

Fig.4.In situ DRIFTS spectra of the adsorption (a) and photocatalytic removal (b) of NO on the surface of SrSn(OH)6.

Fig.4a showsin situDRIFTS spectra of the adsorption of NO on the surface of SrSn(OH)6.The peaks at 1148 and 1190 cm−1can be assigned to the original NO.The peaks at 1320,1345,1368,1394 and 1415 cm−1can be assigned to NO2,which is the preliminary oxidation product during the adsorption process (Eq.6).The peaks at 1464 cm−1can be assigned to NO2−,other peaks at 1480,1515,1540 and 1559 cm−1can be attributed to NO3−,which is the subsequent reaction product of NO2and H2O (Eq.7).These results indicate that the NO2,NO2−and NO3−yield increased rapidly during the adsorption stage in the NO and O2environment [23,24].
After the light was turned on Fig.4b),a new peak appeared rapidly at 1351 cm−1during the irradiation process,which is regarded as the oxidation of NO2and NO into NO3−by•OH,O2•−and1O2−radicals (Eqs.8-11.In addition,the hole oxidation potential of SrSn(OH)6is higher than those ofEϕHNO3/NO (0.94 eVvs.NHE),EϕHNO2/NO (0.99 eVvs.NHE),andEϕNO2/NO (1.03 eVvs.NHE);hence,the photo-generated holes of SrSn(OH)6could directly oxidize NO to NO2,NO2−and NO3−(Eq.12).As the illumination time was prolonged,the intensities of the peaks at 1515,1540 and 1555 cm−1gradually decreased.However,the new peak (1411 cm−1) of NO2gradually became stronger,which reflects the high yield of NO2owing to the reaction of NO3−and NO (Eq.13) [25-26].

Based on the ESR and thein situDRIFTS results,the photocatalytic NO oxidation mechanisms were provided in Fig.5.The roles of O2•−,•OH and1O2in NO oxidation were illustrated.
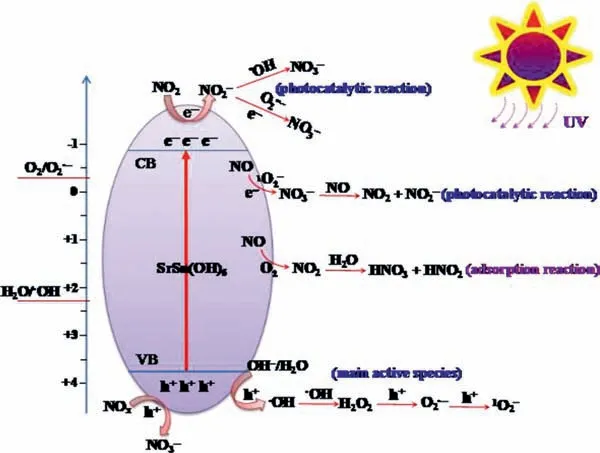
Fig.5.The proposed photocatalytic NO oxidation mechanisms.
In summary,the SrSn(OH)6photocatalyst with stick-like structure was synthesized by a simple chemical precipitation method in water bath.The as-obtained SrSn(OH)6exhibited excellent photocatalytic activity and good stability.Interestingly,it was found that SrSn(OH)6photocatalyst demonstrated the ability to generate the main active species of O2•−,•OH and1O2and could oxidize NO into nitrate.To clarify the adsorption and reaction mechanism,the intermediates and “nal products that distributed on the surface of SrSn(OH)6were determined and analyzed byin situDRIFTS.And thus the photocatalytic NO oxidation mechanism on SrSn(OH)6was proposed.The present work could provide new insights into the SrSn(OH)6with high activity for photocatalytic NOxremoval.
Declaration of competing interest
The authors declare that they have no known competing “nancial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research is “nancially supported by the National Natural Science Foundation of China (No.51708078),the Science and Technology Research Program of Chongqing Municipal Education Commission (No.KJZD-K201900502),and the Natural Science Foundation of Chongqing (No.2018jcyjA1040),the Innovative Research Team of Chongqing (No.CQYC201903221).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.07.065.
 Chinese Chemical Letters2022年3期
Chinese Chemical Letters2022年3期
- Chinese Chemical Letters的其它文章
- Direct catalytic nitrogen oxide removal using thermal,electrical or solar energy
- Construction and applications of DNA-based nanomaterials in cancer therapy
- Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution
- Nanostructured materials with localized surface plasmon resonance for photocatalysis
- Recent progress of Pd/zeolite as passive NOx adsorber: Adsorption chemistry,structure-performance relationships,challenges and prospects
- Microfluidic methods for cell separation and subsequent analysis
