Functionality of a bicistronic construction containing HEXA and HEXB genes encoding β-hexosaminidase A for cell-mediated therapy of GM2 gangliosidoses
Alisa A. Shaimardanova, Daria S. Chulpanova, Valeriya V. Solovyeva,Aleksandr M. Aimaletdinov, Albert A. Rizvanov
Abstract Tay-Sachs disease and Sandhoff disease are severe hereditary neurodegenerative disorders caused by a deficiency of β-hexosaminidase A(HexA) enzyme, which results in the accumulation of GM2 gangliosides in the nervous system cells. In this work, we analyzed the efficacy and safety of cell-mediated gene therapy for Sandhoff disease and Sandhoff disease using a bicistronic lentiviral vector encoding cDNA of HexA α- and β-subunit genes separated by the nucleotide sequence of a P2A peptide (HEXA-HEXB). The functionality of the bicistronic construct containing the HEXA-HEXB genetic cassette was analyzed in a culture of HEK293T cells and human umbilical cord blood mononuclear cells (hUCBMCs). Our results showed that the enzymatic activity of HexA in the conditioned medium harvested from genetically modified HEK293T-HEXA-HEXB and hUCBMCs-HEXA-HEXB was increased by 23 and 8 times, respectively, compared with the conditioned medium of native cells. Western blot analysis showed that hUCBMCs-HEXA-HEXB secreted both completely separated HEXA and HEXB proteins, and an uncleaved protein containing HEXA + HEXB linked by the P2A peptide. Intravenous injection of genetically modified hUCBMCs-HEXA-HEXB to laboratory Wistar rats was carried out, and the HexA enzymatic activity in the blood plasma of experimental animals, as well as the number of live cells of immune system organs (spleen, thymus, bone marrow, lymph nodes) were determined. A significant increase in the enzymatic activity of HexA in the blood plasma of laboratory rats on days 6 and 9 (by 2.5 and 3 times, respectively) after the administration of hUCBMCs-HEXA-HEXB was shown. At the same time, the number of live cells in the studied organs remained unchanged. Thus, the functionality of the bicistronic genetic construct encoding cDNA of the HEXA and HEXB genes separated by the nucleotide sequence of the P2A peptide was shown in vitro and in vivo. We hypothesize that due to the natural ability of hUCBMCs to overcome biological barriers, such a strategy can restore the activity of the missing enzyme in the central nervous system of patients with GM2 gangliosidoses. Based on the obtained data,it can be concluded that intravenous administration of hUCBMCs with HexA overexpression is a promising method of the therapy for GM2 gangliosidoses. The animal protocol was approved by the Animal Ethics Committee of the Kazan Federal University (No. 23) on June 30, 2020.
Key Words: bicistronic vector; cell-mediated gene therapy; GM2 gangliosidosis; P2A peptide; Sandhoff disease; Tay-Sachs disease; umbilical cord blood mononuclear cells; β-hexosaminidase
Introduction
GM2 gangliosidoses are a group of autosomal recessive metabolic disorders related to lysosomal storage diseases(LSDs). These diseases are caused by a deficiency of the lysosomal enzyme β-hexosaminidase (Hex) which is responsible for the degradation of GM2 gangliosides.Gangliosides are the main glycolipids of the neuronal membranes, which ensure their normal vital activity. GM2 gangliosidoses include three diseases, namely Tay-Sachs disease (TSD; OMIM 272800), Sandhoff disease (SD; OMIM 268800) and AB variant (OMIM 272750) (Solovyeva et al.,2020). TSD and SD are caused by mutations in the genes encoding the α- and β-subunits of the Hex enzyme, HEXA and HEXB enzymes, respectively. Deficiency of the Hex enzyme leads to the accumulation of GM2 gangliosides in various cells and tissues of the body, mainly in the central nervous system (CNS). The AB variant of GM2 gangliosidosis is caused by the mutations in theGM2Agene, which encodes the GM2 activator protein required for the presentation of the substrate to the Hex enzyme. In the absence of the GM2 activator protein, the Hex enzyme is unable to cleave the substrate,despite the fact that the enzyme activity in patients is normal.All three diseases have similar clinical manifestations and are characterized by the cognitive and motor dysfunction.The accumulation of GM2 gangliosides in nervous system cells leads to neuronal death and the progression of neurodegeneration and neuroinflammation (Solovyeva et al.,2018). The Hex enzyme has several isozymes that are formed from various combinations of α- and β-subunits: HexA (αβ),HexS (αα), and HexB (ββ). HexA is the only isozymes capable of hydrolyzing GM2 gangliosides. HexA cleaves β-glycosidic bond between the N-acetylgalactosamine residue and the galactose residue of the GM2 gangliosides (Mark et al., 2003).
GM2 gangliosidosis has three forms: infantile, juvenile, and adult. Infantile form is the most severe and is characterized by an acute brain damage, as a result of which most patients with the infantile form do not survive past 4 years of age(Jarnes Utz et al., 2017). Characteristic features of the infantile form are developmental delay and regression,visual impairment, the presence of a cherry red spot in the macula and emphasizing normal color of the actual choroid,contrasting with the pallor of swollen ganglion cells in the affected part of the retina (Smith et al., 2012; Jahnova et al., 2019). Juvenile and adult forms are much less common and progress more slowly. The juvenile form is characterized by cerebellar dysfunction and, as a consequence, gait disturbance, problems with balance, and impairment of motor and cognitive functions (Smith et al., 2012). The adult form is extremely rare and difficult to diagnosis, since the adult form has a wide range of clinical symptoms, such as spinal muscular atrophy, motor axonal neuropathy, cerebellar disorders and mental disorders (Jahnova et al., 2019).
There is no treatment for GM2 gangliosidoses today, but such promising therapeutic strategies as substrate reduction therapy (Bembi et al., 2006; Marshall et al., 2019), bone marrow transplantation (Wada et al., 2000) or hematopoietic stem cell (HSC) transplantation (Stepien et al., 2018), enzyme replacement therapy (Tsuji et al., 2011) and the restoration of the functional enzyme expression using gene therapy(Golebiowski et al., 2017; Gray-Edwards et al., 2018) are being actively investigated at present. HSC transplantation is often used to treat GM2 gangliosidoses and other LSDs, since healthy donor cells synthesize an enough level of functional enzyme and in some cases, usually in less aggressive forms of the disease, this procedure helps to increase the enzyme activity and alleviate the disease symptoms (Stepien et al.,2018). However, HSC transplantation without additional genetic modification does not always have a sufficient therapeutic effect (Matzner and Gieselmann, 2005; Bredius et al., 2007). Cell-mediated gene therapy can increase the efficiency of the enzyme activity restoration and biodistribution in the CNS (Harrison et al., 2013; Sergijenko et al., 2013; Visigalli et al., 2016).
The mechanism of therapeutic action of genetically modified stem cells overexpressing Hex is that after passing through the blood-brain barrier (BBB) the cells enter the CNS and secrete functional enzyme which can enter neighboring cells from the intercellular space by endocytosis and can be delivered to lysosomes through mannose-6-phosphate receptors. Such a mechanism for restoring enzyme activity is called crosscorrection (Solovyeva et al., 2018). Thus, in CNS neurons after cross-correction the activity of the Hex enzyme can reach the level sufficient to prevent the accumulation of GM2-gangliosides. Only 10–15% of HexA activity is required in order to prevent the accumulation of GM2 gangliosides (Coutinho et al., 2012; Doerr et al., 2015).
The therapeutic effect of HSC-mediated gene therapy of various LSDs has been shown in animal models (Harrison et al.,2013; Sergijenko et al., 2013; Visigalli et al., 2016). In addition,a number of clinical cases of transplantation of genetically modified HSCs in LSDs affecting CNS have been described.For example, great success has been achieved in the use of genetically modified HSCs in metachromatic leukodystrophy,characterized by the progressive demyelination due to a deficiency of the arylsulfatase A lysosomal enzyme. In clinical trials it has been shown that this method is safe and can prevent the disease progression at presymptomatic stages(Sessa et al., 2016; Shaimardanova et al., 2020a, b). It has also been shown that genetically modified CD34+stem cells can effectively prevent a demyelination in X-linked adrenoleukodystrophy (Cartier et al., 2009).
Human umbilical cord blood mononuclear cells (hUCBMCs)are a promising tool for LSD therapy, since they, like HSCs,are able to overcome the BBB (Man et al., 2007; Bahbouhi et al., 2009; Takeshita and Ransohoff, 2012; Calderon et al.,2017) and deliver the missing enzyme to the CNS, and also contain a population of CD34+stem cells with neuroprotective properties (Islamov et al., 2015). Moreover, the infiltration with hUCBMCs is increased during neuroinflammation and neurodegeneration, which has been shown in SD model mice(Kyrkanides et al., 2008). The transplantation of hUCBMCs has a therapeutic potential for the treatment of many CNS diseases since these cells exhibit an anti-inflammatory,neuroregenerative, neuroprotective activity. hUCBMCs also show a lower risk of a graft-versus-host disease, which is often observed in HSC transplantation, since hUCBMCs are immature and secrete small amounts of cytokines,immunoglobulins, and HLA class II antigens (Paloczi, 1999;Vyas et al., 2014).
In this work, we aimed to develop a bicistronic genetic construct containing the nucleotide sequences of theHEXAandHEXBgenes, separated by the nucleotide sequence of the P2A self-cleaving peptide of porcine teshovirus 1, and to analyze its ability to restore the enzymatic activity of HexA upon hUCBMC-mediated delivery in Wistar rats.
Materials and Methods
Animals
In this study sixteen 12-week-old male Wistar rats (230–250 g in weight) were used. The animals were randomly divided into two groups: a control group (8 animals injected with 500 µL of phosphate-buffered saline (PBS)) and an experimental group(8 animals injected with genetically modified hUCBMCs). The animals were housed in transparent plastic cages, 12:12 hours light:dark cycle, at a temperature between 21–25°C, with a relative humidity of 40% to 70% withad libitumfood or water access. All experiments were carried out in accordance with ethical standards and current legislation, and the protocol was approved by the Animal Ethics Committee of the Kazan Federal University (No. 23, approved on June 30, 2020).Genetically modified mononuclear cells (hUCBMCs-HEXAHEXB) were injected into the tail vein of rats in an amount of 4 × 106cells in 500 µL of PBS. Animals from the control group were injected with 500 µL of PBS. Prior to injection,whole blood samples were taken from rats in tubes containing sodium citrate anticoagulant. Whole blood plasma was isolated by centrifugation for 20 minutes at 1500 ×gand stored at –80°C. Also, whole blood for plasma isolation was taken 2, 3, 6, 9, 30 days after the administration of hUCBMCs-HEXA-HEXB (the method of obtaining is described in the next subsections). Plasma samples were used to determine the HexA enzymatic activity.
Optimization of the codon composition of cDNA of the HEXA and HEXB genes and their cloning into the pLX303 lentiviral expression vector
The OptimumGeneTMalgorithm (GenScript, Piscataway, NJ,USA) was used to optimize the codon composition of cDNA of theHEXAandHEXBgenes. The nucleotide sequences of mRNA of the humanHEXAandHEXBgenes were taken as a template for codon optimization. De novo synthesis of the genetic cassette containing cDNA of the HEXA and HEXB genes, separated by the nucleotide sequence of the P2A peptide of porcine teshovirus 1 (the nucleotide sequence of the genetic cassette is presented inAdditional file 1), and its cloning into pUC57 plasmid donor vector were carried out by GenScript. The correct assembly of the pUC57-HEXA-HEXB genetic construct was confirmed by restriction analysis (at the EcoRI and SalI restriction sites) and sequencing.
The cloning of the genetic cassette from the pUC57-HEXAHEXB donor vector into the pLX303 lentiviral destination vector (Cat# 25897, Addgene, Watertown, MA, USA) was performed by LR recombination using the GatewayTMLR ClonaseTMII Enzyme Mix (Cat# 11791020, Thermo Fisher Scientific, Waltham, MA, USA) according to the instructions recommended by the manufacturer. Next, the recombination products were used to transform Escherichia coli TOP10 competent cells, after which positive clones containing pLX303-HEXA-HEXB vector were selected using a selective antibiotic (ampicillin, 100 µg/mL). To generate a preparative amount of pLX303-HEXA-HEXB, a plasmid DNA was isolated using GeneJET Plasmid Maxiprep Kit (Cat# K0492, Thermo Fisher Scientific).
Production of LV-HEXA-HEXB lentiviruses
To produce the LV-HEXA-HEXB recombinant lentivirus, a calcium phosphate co-transfection of HEK293T packaging cell line was performed with three plasmids: envelope plasmid pCMV-VSV-G (Cat# 8454, Addgene), packaging plasmid pCMVR8.74 (Cat# 22036, Addgene) and the pLX303-HEXAHEXB vector plasmid. Viral supernatant was collected three times every 12 hours and stored at 4°C. After 48 hours supernatants were centrifuged for 5 minutes at 2000 ×gand filtered through a nylon filter (Biofil, Yueqing, China) with 0.22 µm pore size. Collected recombinant lentiviruses were concentrated using high speed centrifugation at 131,000 ×gfor 2 hours at 4°C. The viral particle pellet was resuspended in 500 µL of Opti-MEM medium (Gibco®, USA) and stored at–80°C. The viral titer was determined using qPCR as previously described (Barczak et al., 2015).
hUCBMCs isolation and culture
Human Embryonic Kidney 293 cells HEK293T (ATCC: CRL-11268, American Type Culture Collection, Manassas, VA,USA) were used in this study. HEK293T cells were cultured in DMEM medium (PanEco, Moscow, Russia) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 2 mM L-glutamine (PanEco), and a mixture of antibiotics (100 U/mL penicillin + 100 µg/mL streptomycin, Biolot, St. Petersburg,Russia) at 37°C in a humidified atmosphere with 5% CO2.
Human umbilical cord blood samples were provided by the Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan for research purposes in accordance with the principles of theDeclaration of Helsinkiand Good Clinical Practices and the protocol was approved by the Local Ethics Committee of Kazan Federal University (No.23) on June 30, 2020. Umbilical cord blood was collected after obtaining informed consent from a pregnant woman who underwent prenatal screening for contraindications for cord blood donation and human immunodeficiency viruses type 1 and 2 infections, syphilis, hepatitis B and C, acute stage of infections caused by herpes simplex virus 1 and 2,cytomegalovirus, toxoplasma. After the baby was born and the umbilical cord was severed, the blood was collected in disposable plastic blood collection container containing citrate phosphate dextrose adenine solution anticoagulant. Within 12–18 hours after blood collection, mononuclear cells were isolated under aseptic conditions in compliance with generally accepted rules of work. Mononuclear cells were isolated by centrifugation of umbilical cord blood in a Ficoll density gradient (density 1.077 g/cm3) (PanEco, Russia) according to a standard technique. The isolated cells were incubated in Iscove’s Modified Dulbecco’s Medium (IMDM) (PanEco,Moscow, Russia) supplemented with 10% fetal bovine serum,2 mM L-glutamine, and a mixture of antibiotics (100 U/mL penicillin + 100 µg/mL streptomycin) at 37°C in a humidified atmosphere with 5% CO2.
Immunophenotyping of hUCBMCs was performed by immunofluorescence staining with specific antibodies to CD3,CD4, CD8, CD34, and CD45 using MultiTEST CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC (Cat# 340499, BD Biosciences, San Jose,CA, USA) and CD45-FITC/CD34-PE reagents (Cat# 341071, BD Biosciences) and further flow cytometry analysis on a Guava easyCyte 8HT flow cytometer (Millipore, Molsheim, France).
Genetic modification of HEK293T cells and hUCBMCs
HEK293T cells and hUCBMCs were transduced (genetically modified) with LV-HEXA-HEXB with a multiplicity of infection of 10. Forin vitrostudies, immediately after isolation, PBMCs were plated in an amount of 2.5 × 106per 35 mm Petri dish for suspension cultures in complete IMDM medium after which LV-HEXA-HEXB and protamine sulfate (10 µg/mL) were added.The cells were incubated with the recombinant lentiviruses for 4 hours, after which the medium was changed with fresh IMDM. Forin vivostudies, hUCBMCs were plated at 4 × 106cells per 35 mm Petri dish for suspension cultures in complete IMDM medium, after which LV-HEXA-HEXB and protamine sulfate (10 µg/mL) were added. Then the cells were incubated with the recombinant lentiviruses for 4 hours after which they were washed three times with PBS (PanEco, Russia) from viral particles, resuspended in 500 µL of PBS and used for injecting Wistar rats.
Collection of conditioned medium
The collection of conditioned medium (CM) from HEK293T and hUCBMCs was performed 48 hours or 7 days after transduction, respectively. The collected CM was centrifuged for 5 minutes at 12,000 g to remove cellular components,stored at –80°C and used to determine the HexA enzymatic activity.
Western blot analysis
Seven days after transduction, hUCBMCs were washed three times with PBS by centrifugation. To isolate total protein,hUCBMC pellets were incubated for 30 minutes in RIPA buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) containing a mixture of protease and phosphatase inhibitors (Thermo Fisher Scientific), and then centrifuged at 13,200 rpm for 30 minutes. The isolated total protein was stored at –80°C.For subsequent Western blot analysis, the total protein concentration was measured using the PierceTMBCA Protein Assay Kit (Cat# 23225, Thermo Fisher Scientific) according to the manufacturer’s protocol.
Further, the total protein samples were resuspended in the sample loading buffer (10% glycerol, 50 mM Tris pH 6.8, 2 mM EDTA, 2% sodium dodecyl sulfate, 144 mM 2-mercaptoethanol, 0.01% bromophenol blue) and proteins were denatured for 5 minutes at 95°C. Electrophoresis of the samples was carried out in a 4–12% SDS-PAGE gradient gel on a Mini-PROTEAN® 3 Cell device (Bio-Rad, Hercules, CA,USA). Then the proteins were transferred onto the Immun-BlotTMPVDF Membrane (Bio-Rad) on a Trans-Blot® SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad). Membranes were blocked by incubation in Blocking buffer (Tris-buffered saline(TBS), 0.05% Tween 20, 5% skim milk powder) for 1 hour.Then the membranes were incubated with primary rabbit polyclonal antibodies to HEXA (Cat# PAA195Hu22, Cloud-Clone Corp., USA) diluted at a ratio 1:300 in blocking buffer and HEXB (Cat# PAA637Hu22, Cloud-Clone Corp., USA)diluted at a ratio 1:250 in blocking buffer for 12 hours at 4°C.After the end of incubation with primary antibodies, the membranes were washed 3 times for 10 minutes in 30 mL of Tris-buffered saline (TBS)-T buffer (TBS, 0.05% Tween 20) and then incubated with secondary polyclonal goat antibodies to rabbit immunoglobulin G conjugated with horseradish peroxidase (1:1000 dilution, Cat# A6154, Sigma-Aldrich, USA)for 2 hours at room temperature. After the end of incubation,the membranes were washed 3 times for 10 minutes in 30 ml of TBS-T buffer. Next, the membranes were stained with mouse antibodies to β-actin conjugated with horseradish peroxidase (1:5000 dilution, Cat# A00730, THETMbeta Actin Antibody [HRP], GenScript), and incubated for 1 hour, after which the membranes were washed 3 times for 10 minutes in 30 ml of TBS-T buffer. Imaging of the immune precipitate was performed using ECL Western Blotting Substrate (Cat# W1001,Promega, USA) on a ChemiDocXRS+ instrument (BioRad).
Determination of HexA enzymatic activity
The enzymatic activity of HexA was determined in the CM harvested from native and genetically modified HEK293T and hUCBMCs, as well as in the plasma of rats before and after injection of hUCBMCs-HEXA-HEXB. The concentration of total protein in CM and plasma was determined using PierceTMBCA Protein Assay Kit (Thermo Fisher Scientific).The samples were normalized according to the total protein concentration of the sample with the lowest concentration.Next, 60 µL of CM or 30 µL of plasma and 25 µL or 12.5 µL of a fluorescent substrate 3.2 mM 4-methylumbelliferyl-N-acetylbD-glucosamine-6-sulphate, MUGS (TRC Canada, Canada),respectively, were added to a well of a 96-well plate. The mixture was incubated for 1 hour at 37°C. The reaction was stopped by adding 200 µl of glycine carbonate buffer (0.17 M glycine, 0.17 M sodium carbonate, pH 10.0). Fluorescence was measured on an Infinite M200Pro instrument (Tecan Trading AG, Switzerland; excitation at 365 nm and emission at 450 nm). When measuring the enzymatic activity in blood plasma,the data were also normalized relative to the background fluorescence of the plasma samples.
Determination of the number of live cells in immune system organs
On the 30thday after injection of genetically modified hUCBMCs-HEXA-HEXB or PBS the rats were exposed to CO2to euthanatize. Thymus, spleen, popliteal lymph nodes and tubular bones were removed. Lymphoid organs were homogenized and resuspended in cool Hank’s solution (pH 7.2–7.4) (PanEco, Russia) in a volume of 5 mL for thymus,spleen, in a volume of 1 mL for popliteal lymph nodes and bone marrow. The resulting suspension was filtered through two layers of nylon and washed twice by centrifugation at 200×gfor 5 minutes. Then, 10 µL of the suspension was added to 0.4 mL of a 3% solution of acetic acid with trypan blue, and the number of stained and unstained cells in the organs was counted in a cell counting chamber and the ratio of live and dead cells was determined.
Cytokine multiplex analysis
Bio-Plex ProTMRat Cytokine 23-Plex Assay kit (Cat# 12005641,Bio-Rad) was used to analyze rat serum samples according to the manufacturer’s recommendations. The kit allows detecting the following proteins: granulocyte colonystimulating factor (G-CSF), granulocyte-macrophage colonystimulating factor (GM-CSF), growth-regulated oncogene/keratinocyte chemoattractant (GRO/KC), interferon gamma(IFN-γ), interleukin 1 alpha (IL-1α), interleukin 1 beta (IL-1β),interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5),interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 10 (IL-10),interleukin 12 (IL-12p70), interleukin 13 (IL-13), interleukin 17(IL-17A), interleukin 18 (IL-18), macrophage colony-stimulating factor (M-CSF), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1 alpha (MIP-1α),macrophage inflammatory protein 3 alpha (MIP-3α), RANTES,tumor necrosis factor alpha (TNF-α), vascular endothelial growth factor (VEGF). To determine the concentration of cytokines, 50 µL of serum samples were used. The data obtained were analyzed using MasterPlex CT software and MasterPlex QT software (MiraiBio Hitachi Software, San Francisco, CA, USA).
Statistical analysis
Statistical analysis was achieved using GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA), oneway analysis of variance (ANOVA) followed by Tukey’s Honest Significant Difference (HSD) test. A value ofP< 0.05 was considered statistically significant.
Results
Development of the bicistronic vector pLX303-HEXA-HEXB
In this work to create a bicistronic vector, codon composition of nucleotide sequences of theHEXAandHEXBgene cDNA were optimized. The wild-type nucleotide sequences of theHEXAandHEXBgenes contain tandem rare codons that can stop translation or reduce its efficiency. When optimizing the codon composition of the wild-typeHEXAgene, the Codon Adaptation Index (CAI) was improved from 0.77 to 0.93. When optimizing the codon composition of the wild-typeHEXBgene, the CAI was improved from 0.71 to 0.93. To increase the mRNA stability, GC composition was optimized and extended regions with a high content of GC pairs were removed. In addition, during the optimization process potential cis-acting sites were removed. As a result of codon optimization, the length of amino acid sequences of theHEXAandHEXBgenes did not change and they consisted of 481 and 541 amino acid residues, respectively. After that, a genetic cassette consisting of theHEXAandHEXBgenes separated by the P2A peptide nucleotide sequence was synthesized and then cloned into the pUC57 donor vector. The correct assembly of the pUC57-HEXA-HEXB genetic construct was confirmed by restriction analysis and sequencing (data not shown). Cloning of the HEXA-HEXB genetic cassette from the pUC57-HEXAHEXB donor plasmid into the pLX303 destination lentiviral vector was carried out by LR recombination using Gateway technology. Further, the resulting plasmid pLX303-HEXA-HEXB(Figure 1A) was produced in a preparative amount and used for the production of lentiviral particles.
Analysis of LV-HEXA-HEXB functionality in vitro
The functionality of the HEXA-HEXB genetic cassette was verified on HEK293T cell culture. For this, genetic modification(transduction) of HEK293T cells was carried out with the LVHEXA-HEXB recombinant lentivirus. CM was harvested 48 hours after viral transduction and HexA enzymatic activity was analyzed. It was shown that in the CM of genetically modified HEK293T-HEXA-HEXB cells, the enzymatic activity of HexA was by 23 times higher than in the CM of native HEK293T cells (Figure 1B) which indicates the secretion of active HexA enzyme by genetically modified cells.
Further, mononuclear cells were isolated from human umbilical cord blood and immunophenotyped. As a result of immunofluorescence analysis of the expression of CD3, CD4,CD8, CD34 and CD45 markers on the surface of isolated cells was determined (Figure 2). In the isolated hUCBMCs expression was as follows: CD3+35.6%, CD4+45.63%, CD8+25.3%,CD3+CD8+24.1%, CD4+CD8+5.67%, CD34+0.06%, CD45+94%and CD34+CD45+2.08% cells. Then hUCBMCs were genetically modified with the LV-HEXA-HEXB recombinant lentivirus.
Seven days after viral transduction, in the CM of genetically modified hUCBMCs-HEXA-HEXB HexA enzymatic activity was by 8 times higher than in CM of native hUCBMCs (Figure 1C),which indicates successful genetic modification and active HexA secretion by genetically modified cells. Western blot analysis was performed to assess the expression of recombinant αand β-subunits of the HexA enzyme in genetically modified hUCBMCs-HEXA-HEXB. The molecular weights of the mature α- and β-subunits of the HexA protein are 54 kDa and 29 kDa (Utsumi et al., 2002), however, these proteins are characterized by heterogeneity in molecular weight due to glycosylation (Sonderfeld-Fresko and Proia, 1989). Seven days after genetic modification, Western blot analysis showed the presence of HEXA and HEXB proteins in hUCBMCs-HEXA-HEXB,the molecular weight of which was about 70 kDa and 30 kDa,respectively. The presence of an uncleaved protein (HEXA +HEXB) was also noted, the molecular weight of which was approximately 200 kDa (Figure 1D).
Analysis of LV-HEXA-HEXB functionality in vivo
It has been observed that after the administration of hUCBMCs-HEXA-HEXB, the activity of the HexA enzyme was significantly increased in rat plasma. The highest activity of HexA was observed 6–9 days after injection of hUCBMCs-HEXA-HEXB. On the 6thday and on the 9thday after injection of the cells, the enzymatic activity was by 2.5 times higher and by 3.4 times higher, respectively, than the enzyme activity in the plasma of control animals, which were injected with PBS. On the 30th day, a decrease in the enzyme activity was observed (Figure 3).
On the 30thday after injection of genetically modified hUCBMCs-HEXA-HEXB, the number of live cells in the immune system organs (spleen, thymus, bone marrow, lymph nodes)of rats was investigated. There was no statistically significant difference in the number of live and dead cells in hUCBMCHEXA-HEXB-treated rats compared with PBS-treated control animals (Figure 4). As well as no significant difference in the secretion level of inflammatory cytokines and chemokines in the serum of control and experimental rats was observed(Table 1).
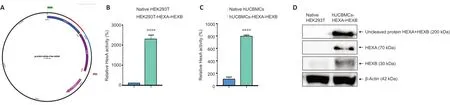
Figure 1|Functional analysis of the bicistronic genetic construct containing the cDNA of the HEXA and HEXB genes separated by the nucleotide sequence of the P2A peptide of porcine teshovirus 1 (HEXA-HEXB).
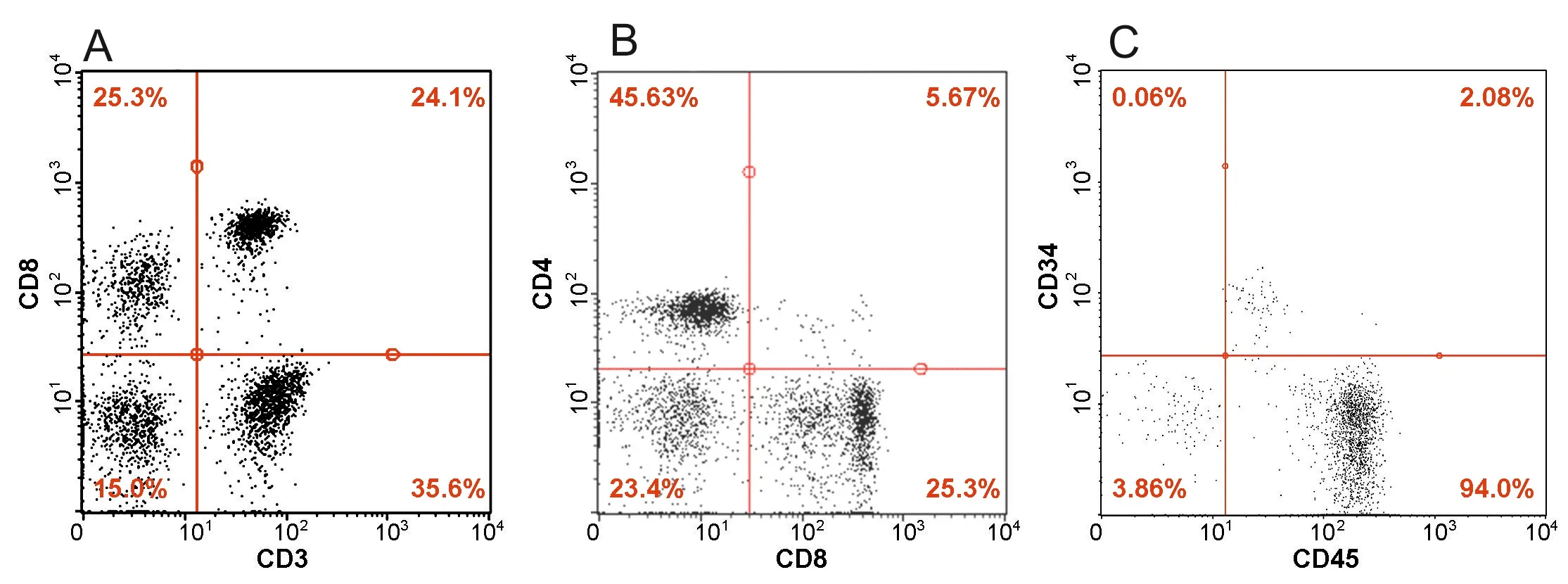
Figure 2| Immunofluorescence analysis of expression of CD3, CD4,CD8, CD34 and CD45 markers by mononuclear cells isolated from human umbilical cord blood.

Figure 3|Enzymatic activity of HexA in rat blood plasma after the administration of genetically modified hUCBMCs-HEXA-HEXB.

Figure 4|The number of live cells in organs of the immune system of rats on the 30th day after injection of hUCBMCs-HEXA-HEXB.
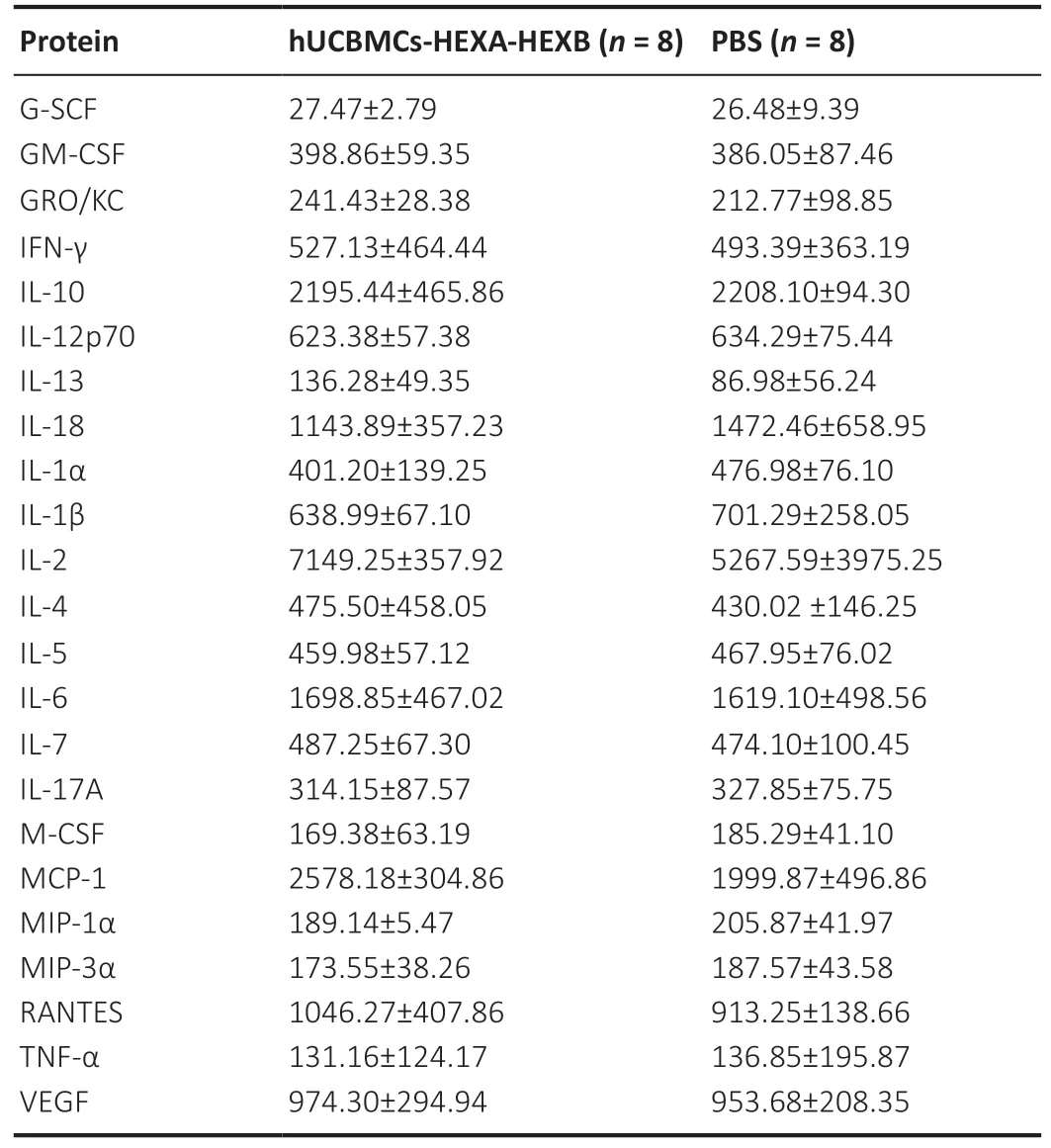
Table 1 |Protein concentration of cytokines (pg/mL) in the rat serum
Discussion
The efficiency of existing methods to treat GM2 gangliosidoses, such as substrate reduction therapy and enzyme replacement therapy, is limited due to the fact that,when administered intravenously, therapeutic substances cannot overcome the BBB (Solovyeva et al., 2018). Thus,intrathecal or intracerebral administration of therapeutic substances (Matsuoka et al., 2011) has an advantage, for example, intracerebral administration of the recombinant HexA enzyme to SD model mice led to a dose-dependent restoration of HexA enzymatic activity in neurons (Tsuji et al.,2011). However, intracerebral administration is restrictedly used in clinical practice since its high invasiveness and poor distribution of the recombinant HexA protein in the CNS(Solovyeva et al., 2018). The transplantation of donor HSCs is successfully used in TSD therapy, however, the expression of the missing enzyme at the physiological level is often insufficient to prevent further development of the disease(Harrison et al., 2013; Sergijenko et al., 2013; Visigalli et al., 2016). Another promising treatment approach is the restoration of functional enzyme expression using gene therapy. Several serotypes of adeno-associated viruses (AAV)(e.g., 9 and 10) have been reported to be able to cross the BBB. This property makes AAV-based vectors promising for the therapy of neurodegenerative diseases, including GM2 gangliosidoses (Merkel et al., 2017). However, in clinical study of LSD patients it has been shown that even with intracerebral administration of AAV9, which encodes the missing enzyme,the viral vector is poorly distributed in the CNS and does not lead to a therapeutic effect (Sevin et al., 2018). Poor biodistribution and high invasiveness are the limiting factors for direct administration of viral vectors in clinical practice,since the delivery of functional enzymes to all damaged cells is critical for successful treatment (Solovyeva et al., 2018).Cell-mediated gene therapy can solve many existing problems,for example, due to the ability of stem cells to migrate toward various tissues and organs (including overcoming the BBB),this method can provide good biodistribution and the least invasiveness. In general, cell-mediated gene therapy involves the use of genetically modified cells that overexpress the therapeutic gene. In the case of LSDs, the transplantation of HSCs genetically modified to overexpress missing enzymes has shown its efficacy (Sano et al., 2005; Gentner et al., 2010;Visigalli et al., 2010, 2016; Harrison et al., 2013; Sergijenko et al., 2013; Parker et al., 2020).
In our study, we investigated the functionality and safety of hUCBMCs-mediated delivery of the HexA using a bicistronic genetic construct, simultaneously encoding the α- and β-subunits of this enzyme. It is known that hUCBMCs are the most attractive candidates for transplantation, since they are immature and less likely to cause graft versus host disease. Many studies have described a detailed immunophenotype of hCUBMCs, since it is important to analyze the immunophenotype for understanding the mechanisms responsible for regulation and migration of the cells, and the experimental results may also depend on the immunophenotype of the used cells (Pranke et al., 2001).We showed that isolated hUCBMCs contained 35.6% CD3+,45.63% CD4+, 25.3% CD8+, 24.1% CD3+CD8+, 5.67% CD4+CD8+,0.06% CD34+, 94% CD45+and 2.08% CD34+CD45+cells. Thus,it has been shown that the used hUCBMCs contained the population of CD34+stem cells, which can exhibit various trophic and neuroprotective effects (Diaz-Flores et al., 2014).
To date, a small number of investigations has been devoted to the study of bicistronic constructs for gene therapy of GM2 gangliosidoses. However, this approach seems reasonable,since HexA is a heterodimer consisting of α- and β-subunits,and in order to achieve a therapeutic effect, it is important to achieve equivalent expression of two genes,HEXAandHEXB.Predominant expression of one of the described above genes results in the formation of the high number of HexS (αα) or HexB (ββ) homodimer isozymes which are unable to cleave GM2 gangliosides and useless for the treatment of TSD and SD.
Self-cleaving 2A peptides are promising candidates for the production of multicistronic vectors due to their small size and ability to self-cleave. 2A peptides consist of 16–20 amino acids and are derived from viral RNA. They provide a better correlation between the expression level of two linked genes before and after the 2A peptide sequence compared to other strategies used to achieve simultaneous expression of several genes in one vector, including constructs with multiple promoters, splicing signals, and nucleotide sequence of internal ribosome entry site (Shaimardanova et al., 2019).Several studies have evaluated the effectiveness of bicistronic vectors for the therapy of GM2 gangliosidoses. For example,the P2A peptide construct has been successfully used in experiments with SD model mice. The authors obtained AAV9 containing bicistronic HEXB-HEXA cassette. Intravenous administration of AAV9-HEXB-HEXA led to an increase in the enzymatic HexA activity and a decrease in the GM2 ganglioside accumulation in the brain of mice, as well as to an increase in the survival rate of model animals by 56% (Woodley et al., 2019). It has also been shown that a bicistronic lentiviral vector containing theHEXAandHEXBgenes separated through the internal ribosome entry site sequence reduces the accumulation of GM2 gangliosides in fibroblasts obtained from a SD patient (Ornaghi et al., 2020).
We have obtained a bicistronic genetic construct encoding theHEXAandHEXBgenes, and have shown its functional activity and safety in Wistar rats. Thus, the pLX303-HEXAHEXB expression plasmid consisting of codon-optimized cDNA of theHEXAandHEXBgenes separated by the nucleotide sequence of the P2A peptide was produced. On the basis of pLX303-HEXA-HEXB, a LV-HEXA-HEXB recombinant lentivirus was obtained and used for genetic modification of HEK293T cells and hUCBMCs. It has been shown that in the CM of genetically modified HEK293T-HEXA-HEXB and hUCBMCs-HEXA-HEXB cells, the HexA enzymatic activity was by 23 times and by 8 times higher, respectively, compared to CM collected from native cells. Western blot analysis showed that genetically modified hUCBMCs-HEXA-HEXB overexpressed a protein larger than 200 kDa in weight, while the weight of the α- and β-subunits of the Hex enzyme was expected to be about 70 kDa and 30 kDa, respectively, which were also present in the cell lysates in a significant amount. The reason for the presence of the protein of a higher weight is probably the fact that the uncleaved HEXA + HEXB product is synthesized in hUCBMCs-HEXA-HEXB (i.e., α- and β-subunits linked through the P2A peptide are presented). Indeed, one of the drawbacks of bicistronic constructs based on 2A peptides is the poor cleavage of gene products before and after the 2A peptide (Ho et al., 2013; Ahier and Jarriault, 2014). However,the P2A peptide used in our work, according to the literature,is one of the most preferred 2A peptides for creating bi- and multicistronic constructs, since the expression products of such vectors are the most equivalent, and the efficiency of P2A cleavage is higher compared to other 2A peptides (Kim et al., 2011; Wang et al., 2015; Liu et al., 2017). Several methods have been described that can improve cleavage of the 2A peptide, for example, the insertion of additional cleavage sites (Yang et al., 2008), a spacer consisting of glycine and serine residues (Wang et al., 2015) or the use of a 2A peptide tandem (Liu et al., 2017). However, in our study, we used the P2A peptide sequence without modifications, and the efficiency of additional modifications of the P2A peptide is yet to be evaluated. Despite the fact that the products of protein synthesis were not cleaved, a statistically significant increase in the enzymatic activity of HexA was observed in the CM of genetically modified hUCBMCs-HEXA-HEXB.
Next, we evaluated the ability of hUCBMCs-HEXA-HEXB to synthesize a functionally active HexA enzyme after their intravenous administration to rats. We have shown that the highest activity of HexA was observed 6–9 days after injection of hUCBMCs-HEXA-HEXB and was by 2.5–3.4 times higher than the enzyme activity in the plasma of control animals injected with PBS. On the 30thday, a decrease in the enzyme activity was observed. We assume that this may be due to the partial elimination of human hUCBMCs from the rat body. At the same time, the number of live cells in the organs of the immune system (spleen, thymus, bone marrow, lymph nodes)of the studied animals remained unchanged. In addition, we assume that the administration of hUCBMCs-HEXA-HEXB does not induce an immune response in the animals, since the levels of inflammatory cytokines and chemokines in the serum of rats from the control and experimental groups were not statistically different. These data provide evidence of the safety of cell-mediated gene therapy using hUCBMCs-HEXAHEXB.
Thus, we have shown that intravenous administration of hUCBMCs-HEXA-HEXB to Wistar rats provides an increase in the enzymatic activity of HexA in the blood plasma of the studied animals and does not affect the number of live cells in the immune system organs. We assume that due to the ability of hUCBMCs to overcome biological barriers, including the BBB, in combination with their genetic modification, it is possible to achieve the distribution of HexA in the CNS and provide a therapeutic effect by cross-correcting the HexA level in neurons affected with TSD and SD.
However, further investigation is required to confirm this hypothesis due to several limitations of the work. Namely,since wild-type animals were used in the study, in order to assess the therapeutic effect of the developed cell-mediated gene strategy, it is necessary to investigate its efficiency in GM2 gangliosidosis model animals. In addition, the question arises of the advisability of using the nucleotide sequence of the P2A peptide and the bicistronic constructs, since these sequences do not allow obtaining completely cleaved proteins. Moreover, the use of lentiviral vectors can lead to unwanted malignant transformation of transduced cells,therefore it is possible to consider other viral vectors for the delivery ofHEXAandHEXBgenes, such as adeno-associated vectors.
In conclusion, the results obtained indicate that hUCBMCHEXA-HEXB transplantation can be an effective method for the treatment of GM2 gangliosidoses. However, further studies are required to confirm the therapeutic potential of this method.
Acknowledgments:This study was partially accomplished in the Center of the National Technology Initiative at the M.M. Shemyakin–Yu.A.Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences.
Author contributions:AAS and DSC performed almost all the experiments, analyzed and interpretated data and wrote the manuscript;AMA carried out animal experiments; VVS and AAR conceived and designed the study; VVS edited the manuscript. All authors revised the manuscript and approved the final version.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was funded by the subsidy allocated to Kazan Federal University for the state assignment #0671-2020-0058 in the sphere of scientific activities.
Institutional review board statement:Human umbilical cord blood samples were provided by the Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan for research purposes in accordance with ethical standards and current legislation. hUCBMCs acquisition and the animal experiment protocol was approved by the Animal Ethics Committee of the Kazan Federal University (No. 23) on June 30, 2020.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional file 1: The nucleotide sequence of the genetic cassette HEXAP2A-HEXB.
- 中国神经再生研究(英文版)的其它文章
- Genes for RNA-binding proteins involved in neuralspecific functions and diseases are downregulated in Rubinstein-Taybi iNeurons
- Research advances on how metformin improves memory impairment in “chemobrain”
- Dendritic spine density changes and homeostatic synaptic scaling: a meta-analysis of animal studies
- Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions
- Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease
- Growth differentiation factor 5: a neurotrophic factor with neuroprotective potential in Parkinson’s disease

