Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions
Peiyuan Huang, Zhihao Zhao, Liting Duan,
Abstract Dynamic protein-protein interactions are essential for proper cell functioning. Homointeraction events—physical interactions between the same type of proteins—represent a pivotal subset of protein-protein interactions that are widely exploited in activating intracellular signaling pathways. Capacities of modulating protein-protein interactions with spatial and temporal resolution are greatly desired to decipher the dynamic nature of signal transduction mechanisms. The emerging optogenetic technology, based on genetically encoded light-sensitive proteins, provides promising opportunities to dissect the highly complex signaling networks with unmatched specificity and spatiotemporal precision. Here we review recent achievements in the development of optogenetic tools enabling light-inducible protein-protein homo-interactions and their applications in optical activation of signaling pathways.
Key Words: cryptochrome 2; homo-interaction; intracellular signaling; light; light-induced protein-protein interaction; light-oxygen-voltage-sensing domain; light-sensitive proteins;optogenetics; phytochrome; signal transduction
Introduction
Protein-protein interactions (PPIs) regulate biochemical processes underlying most of the cellular activities. A large number of proteins self-associate into homodimers or oligomers to obtain structural and functional advantages,including improved stability, control over the accessibility and specificity of active sites, and increased complexity(Marianayagam et al., 2004). Homo-association of proteins contributes to various signaling transduction processes that transduce extracellular signals into cellular responses. Any dysregulation of signaling events may result in a disease state. Technologies to detect or modulate PPIs, such as coimmunoprecipitation or chemical genetic tools, have greatly advanced our understanding of signaling pathways and relevant diseases. However, these methods have limited temporal and spatial controllability to delineate signaling pathways from complex cellular activities.
Optogenetics is an emerging biotechnology that combines optic and genetic technologies to achieve remote control of cell activities. In optogenetic systems, cells are genetically modified to express photosensitive proteins originally from plants and microbes and thus become responsive to light stimulus. Optogenetics based on light-sensitive ion channels has been successfully used to selectively and rapidly regulate the neuronal activity, which has transformed neuroscience research (Deisseroth, 2011). The rapid development of optogenetics relying on light-inducible PPIs presents many promising toolkits that can be used to study cellular signal transduction in a wide range of biological systems (Zhang and Cui, 2015; Kolar and Weber, 2017; Repina et al., 2017).Optogenetic strategies can exploit the light-gated proteinprotein homo-interactions for the self-association of targeted proteins in order to optically activate specific cell signaling.The light stimulation can be non-invasively delivered to targeted locations for desired duration at specific time points with its illumination intensity adjustable to pre-set levels. Compared to the commonly used pharmaceutical treatment and chemical genetic methods, optogenetic strategies demonstrate great capacity in dissecting complex cell signaling pathways with high specificity, fewer off-target effects, and spatiotemporal resolution. Here in this review, we will introduce recent developments of optogenetic toolkits enabling light-inducible protein-protein homo-interactions,and their applications in different optogenetic strategies to activate intracellular signaling pathways.
Data Sources and Searching Strategy
We searched literature from PubMed, Google Scholar, Web of Science, and an annotated database OptoBase (https://www.optobase.org/). The searching strategy was based on combinations of following terms and words: optogenetics,activation, intracellular signaling, homo-interactions, CRY2,LOV domain, phytochrome, dimerization, oligomerization.The results were further selected by titles and abstracts. Only studies using light-induced protein-protein homo-interactions to activate intracellular signaling pathways were included.
Optogenetic Tools for Light-Gated Protein-Protein Homo-Associations
A variety of photosensitive proteins originated from plants and microbes can undergo light-induced homo-interactions and have been exploited in optogenetic strategies designed for mammalian systems. Here we focus on three types of photosensitive proteins: light-oxygen-voltage-sensing (LOV)domains, cryptochrome 2, and phytochromes, all of which have been extensively used in controlling signaling pathways.
LOV domains
The LOV domain is a class of photoreceptors that widely exist in plants, bacteria, and fungi. Once activated by blue light, the C-terminal Jα helix of the LOV domain undocks from the PER-ARNT-SIM core domain within seconds, and the conformational change leads to various signaling outputs(Glantz et al., 2016). Blue light can induce dimerization of the LOV domain of aureochrome1 fromVaucheria frigida(VfAU1-LOV) (Figure 1A) (Grusch et al., 2014). The improved light-inducible dimer (iLID) based on the LOV2 domain ofAvena sativa(AsLOV2) and its adaptor protein SspB is a pair of optical heterodimers (Guntas et al., 2015). Recently, the iLID/SspB pair has been engineered as iLID + tdSspB system to allow homo-interactions with a tandem-dimer construct of SspB (Figure 1A) (Hope et al., 2020).
Cryptochrome 2
Cryptochromes (CRY2) are a type of proteins involved in circadian rhythms of plants and animals. A conserved N-terminal photolyase homology region (PHR) of cryptochrome 2 (CRY2PHR, denoted as CRY2 thereafter) fromArabidopsis thalianacan homo-oligomerize upon blue light stimulation (Kennedy et al., 2010; Shao et al., 2020), and does not require the addition of exogenous cofactors (Figure 1B). Homo-oligomerization of CRY2 can be significantly enhanced [CRY2olig (Taslimi et al., 2014), CRY2high (Duan et al., 2017) and CRY2clust (Park et al., 2017)) or suppressed(CRY2low (Duan et al., 2017)] by directional design and pointmutations. In addition, it can be promoted by CRY2 membrane localization and suppressed by tagging to bulky proteins (Che et al., 2015). Significant CRY2-based clustering can also lead to physical sequestration of target proteins to inhibit relevant biological activities (Bugaj et al., 2013; Lee et al., 2014), which will not be covered in this review.
Phytochromes
Phytochromes have the ability to sense red and far-red light, and to control many aspects of development in plants and microbes. The cyanobacterial phytochrome 1 (Cph1)fromSynechocystisundergoes dimerization under red light,and monomerize under far-red light at the presence of cofactor phycocyanobilin (Figure 1C) (Strauss et al., 2005). TheDeinococcus radioduransbacterial phytochrome (DrBphP)forms a head-to-head parallel dimer and can switch its conformation reversibly under illumination with far-red or near-infrared light within milliseconds without the addition of any exogenous co-factor (Chernov et al., 2017). The C-terminal histidine kinase domains of the DrBphP dimer are separated by ~35 Å gap after red light (660 nm) exposure. They approach each other under near-infrared light (740–780 nm)illumination or in the dark, thus enabling the homo-interaction of proteins fused to the C-termini (Figure 1C) (Redchuk et al.,2017; Leopold et al., 2019).
Activation of Intracellular Signaling via Light-Actuated Homo-Interactions at Different Subcellular Locations
Interactions of signaling proteins may occur at specific subcellular locations to activate the signaling cascades. For example, cell surface receptors, residing on the plasma membrane via their transmembrane domains, can be activated by ligand-binding induced conformational change,dimerization, or oligomerization in order to transmit extracellular stimuli to intracellular signals (Simon, 2000). On the other hand, many serine/threonine protein kinases can be activated via homo-associations in cytosol (Udell et al., 2011).Here we categorize three types of optogenetic strategies(Figure 2) that utilize light-inducible homo-interactions either on the plasma membrane or in the cytoplasm: (1) membranebound homo-interactions, (2) membrane-recruited homointeractions, and (3) cytosolic homo-interactions.Additional Table 1summarizes intracellular signaling pathways that have been successfully modulated by these optogenetic strategies.
Membrane-bound homo-interactions
In this category, activation of intracellular signaling pathways is elicited by the homo-binding of optogenetically engineered proteins of interest that are bound to the cell membrane. In the first approach, full-length transmembrane receptors can be fused to photosensitive proteins (Figure 2A). Secondly,the intracellular domain of transmembrane receptors linked with optical homo-dimerizers can attach to the membrane via additional membrane targeting sequences (Figure 2B). In the third approach, DrBphP with light-controllable conformational change is targeted to the plasma membrane by a membrane targeting sequence and fused with the intracellular domain of transmembrane receptors (Figure 2C).
Membrane-bound homo-interactions by transmembrane domains
This strategy has been used for optical activation of receptor tyrosine kinases (RTKs), which are a large class of cell membrane receptors that play critical roles in regulating cell proliferation, migration, differentiation and survival.Mutations and aberrant activation of RTK signaling can lead to cancer mutagenesis, neurodegeneration and many other diseases (Huang and Reichardt, 2003). Members of the RTK family share conserved molecular structures which are composed of an extracellular domain that binds to ligands,a transmembrane domain, and a catalytic intracellular domain (ICD). Generally, ligands activate RTKs by inducing receptor dimerization or oligomerization which leads to trans-autophosphorylation of the cytoplasmic ICD. Because of their similar modular structures and conserved activation mechanism that requires the homo-interaction of ICD,optically activatable RTKs (Opto-RTKs) have been designed based on light-inducible membrane-bound homo-interactions(Kainrath and Janovjak, 2020; Leopold and Verkhusha, 2020).
A simple but effective activation mode of Opto-RTK is to directly fuse photosensitive proteins to full-length RTKs. Chang et al. (2014) created light-activatable tropomyosin-related kinase (Trk) receptors by introducing CRY2 to the C-termini of full-length Trk receptors, named as optoTrk. The membranelocalized optoTrk were able to trigger canonical downstream signaling cascades (MAPK/ERK, PI3K/Akt, and PLC-γ/Ca2+signaling) via light-induced homo-interaction of CRY2.Functional expression of optoTrkB in rat hippocampal neurons induced filopodia formation upon blue light stimulation. Like wild-type TrkB receptors (Segal, 2003), full-length optoTrkB also underwent internalization and axonal transportation in neurons (Chang et al., 2014).
Membrane-bound homo-interactions by membranetargeting sequence
To exclude the ligand-binding capacities in Opto-RTK constructs, extracellular domain and transmembrane domain of the receptor can be replaced by a short membraneassociated motif in optogenetic constructs. The motif can either be Myr, the myristoylation signal peptide, or Lyn,an alkylation Src-family kinase (Resh, 1999). Light-induced homo-interactions of photosensitive proteins allow the membrane-associated ICD to dimerize, thus leading to the autophosphorylation of ICD and activation of downstream signaling pathways. Each of CRY2, VfAu-LOV, and Cph1 has been successfully used to construct Opto-RTK systems through the membrane-bound homo-interactions.
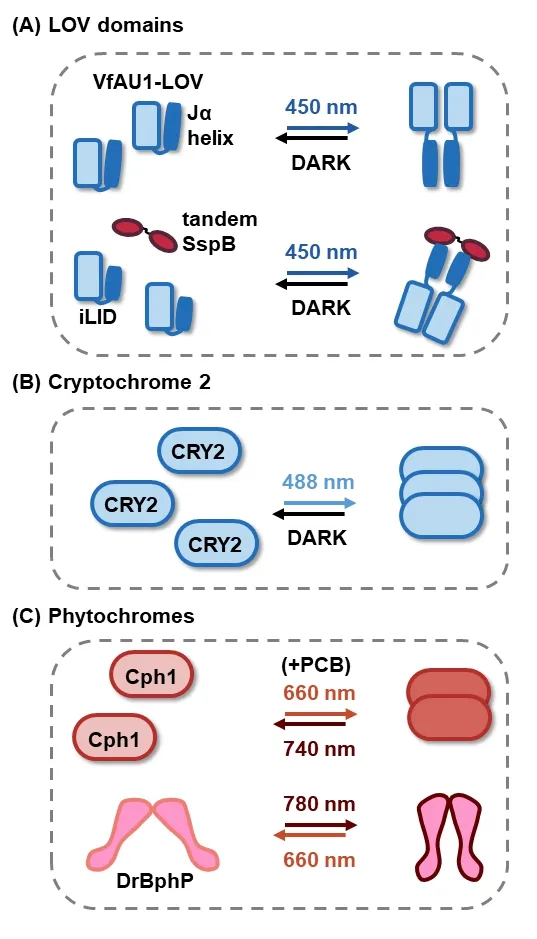
Figure 1| Illustration of light-induced homo-interactions of (A) LOV domains; (B) cryptochrome 2; and (C) phytochromes.
Kim et al. (2014) reported that optical stimulation of fibroblast growth factor receptor 1 (FGFR1) via optoFGFR1, which consists of Myr motif, CRY2, and the cytoplasmic domain of human FGFR1, was able to optically control the polarity and migration of human umbilical vein endothelial cells. For RTKs such as ephrin type-B receptors (EphB) that require formation of high order oligomers to be activated, CRY2olig (Taslimi et al., 2014), a mutated version of CRY2 with higher tendency to cluster, can be used in the design. OptoEphB2, composed of Myr, EphB2 ICD, and CRY2olig tagged with fluorescent protein,was developed to manipulate EphB signaling under blue light via CRY2 oligomerization. Localized OptoEphB2 activation in dendritic areas of hippocampal neurons can induce actin nucleation and polymerization (Locke et al., 2017).
Grusch et al. (2014) developed a generalized series of Opto-RTKs based on homo-associating photosensory proteins that respond to different wavelengths. Blue light can be used to initiate RTK signaling when homo-interacting VfAu-LOV domains are integrated in the design. For instance, OptohEGFR, which consists of Myr motif, VfAu-LOV, and ICD of human epidermal growth factor receptor (hEGFR), can successfully trigger canonical RTK signaling pathways (Grusch et al., 2014). Choi et al. (2020) reported that optical activation of FGFR signaling based on VfAu-LOV domain can sufficiently induce human pluripotent stem cells differentiation free of supplementing ligands under blue light illumination. OptohRET, a light-activatable version of human rearranged during transfection (hRET) receptor, was also developed by using VfAu-LOV (Grusch et al., 2014). Khamo et al. (2019) used a membrane-associated optogenetic TrkA system based on VfAu-LOV, along with its tyrosine sites-mutated versions and other pathway-specific inhibitors, to characterize TrkA signaling activation in details of exploiting different tyrosine sites on TrkA-ICD.
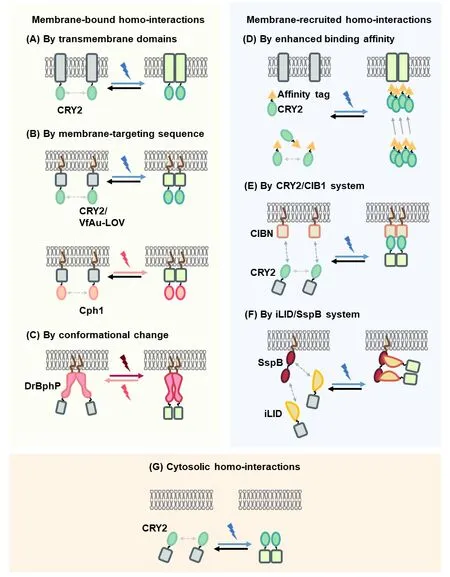
Figure 2| Optogenetic strategies to activate the POI by light-inducible homo-interactions.
Red light offers deeper penetration to tissue and less phototoxicity compared to blue light. With the red-lightresponsive homodimerizer Cph1, Reichhart et al. (2016)developed red-light activatable FGFR1 and TrkB receptors.Proof-of-concept experiments showed that the downstream MAPK/ERK pathway in transfected insulinoma cells was activated by red light stimulation through 10-mm thick mouse tissue. Tichy et al. (2019) presented an expandable library of blue or red light-inducible homodimerizers for rapidly constructing target kinase proteins. Altogether, these methods demonstrate the promise of optogenetic tools to precisely regulate RTK signaling pathways using light stimulation of different wavelengths.
Besides RTKs signaling, many other intracellular signaling pathways can be optically regulated by the approach of membrane-bound homo-interactions via a membrane targeting sequence. For example, light-induced homointeraction of CRY2 can be used to trigger T cell receptor(TCR) signaling. Since evidence suggest the involvement of the oligomerization of T cell receptor ζ-chain in the activation of canonical TCR signaling, Ma et al. (2020) developed an optical control of TCR signaling by tagging CRY2 to the C-terminus of intracellular domain of ζ-chain. The light-inducible clustering increased the phosphorylation levels of ζ-chain and other downstream signaling components. Humphreys et al. (2020)recently reported a novel optogenetic bone morphogenetic protein signaling system (optoBMP) by employing homointeractions of VfAu-LOV domains to initiate the dimerization of membrane-associated intracellular domains of type I and II BMP receptors under blue light. Kim et al. (2020a)recently reported an optogenetically activatable Fas module(optoFas) consisting of a membrane-anchoring Lyn tag, the cytoplasmic domain of Fas, CRY2, and GFP to precisely dissect sophisticatedin vivosignaling networks. Repetitive lightactivation of Fas signaling could affect mice behaviors related to hippocampus-dependent memory (Kim et al., 2020a).
Membrane-bound homo-interactions by conformational change
Light-induced conformational change is another way of introducing homo-interactions. Leopold et al. (2019) reported a novel design of Opto-RTK that can be regulated by far-red(FR) and near-infrared (NIR) light by fusing DrBphP to the cytoplasmic domains of TrkA or TrkB. DrBphP, as a head-tohead parallel dimer, changes its structure under NIR light so that the cytoplasmic RTK domains fused at the head of the dimer come close together and become activated. Absorption of FR light drives the two heads of DrBphP apart so that the membrane-bound RTK domains become separated,and RTK signaling is inactivated. This engineering approach was further adapted to other RTKs such as EGFR and FGFR1 which can be rapidly activated by NIR light (Leopold et al.,2020). Since DrBphP-based Opto-RTKs activate on the near infrared spectrum, the system can be multiplexed with other optogenetic tools or fluorescent probes using well separated activation spectrum (Leopold et al., 2019).
Membrane-recruited homo-interactions
Light-induced recruitment of signaling proteins from the cytoplasm to the cell membrane is also proven efficient to activate intracellular signaling pathways with lower background activities compared with strategies using membrane-bound homo-interactions. The membranerecruited homo-interactions can be achieved via three ways,enhanced binding affinities (Figure 2D), CRY2/CIB1 pair (Figure 2E), and iLid + tdSspB system (Figure 2F).
Membrane recruitment by enhanced binding affinity
In this strategy, light-gated oligomerization of CRY2 can induce the assembly of kinase-coupling domains, thereby effectively enhancing their binding affinities for inactivated endogenous transmembrane proteins. Bugaj et al. (2015) reported a method termed Clustering Indirectly using Cryptochrome 2(CLICR) to activate transmembrane receptors where CRY2 is fused to a specific membrane-receptor targeting domain,for example, the N-terminal src-homology 2 (SH2) domain from PLC-γ that non-covalently binds to RTKs. Light-induced oligomerization of SH2-CRY2 significantly enhances its binding affinity to RTKs localized on the plasma membrane. The authors showed that CLICR allows light-induced activation of endogenous RTKs such as Platelet-derived growth factor receptor beta. This modular method can also be adapted to the phosphotyrosine-binding-like F3 domain from talin, which binds to β3-integrin receptors upon oligomerization (Bugaj et al., 2015). Kyung et al. (2015) reported CRY2-mediated optogenetic control of intracellular calcium signaling through activation of Ca2+release-activated channels. CRY2 is fused to the truncated cytosolic form of stromal interaction molecule(STIM1) to construct optoSTIM1. Light-induced CRY2-STIM1 oligomers can translocate to the plasma membrane, bind to endogenous Ca2+release-activated channels to trigger Ca2+influx. OptoSTIM1 activation was able to induce calcium increase in zebrafish embryos and human embryonic stem cells (Kyung et al., 2015). Bohineust et al. (2020) achieved optogenetic manipulation of calcium signals in single T cells via OptoSTIM1 system.
Membrane recruitment by CRY2/CIB1 system
Upon blue light stimulation, CRY2 can simultaneously form oligomers and bind to its associating partner, theArabidopsisCIB1 (cryptochrome-interacting basic-helix-loophelix) protein (Che et al., 2015; Shao et al., 2020). A twocomponent system utilizing both CRY2 homo-interaction and CRY2/CIB1 hetero-dimerization activates RTK signaling by membrane recruitment of cytosolic ICD. In this strategy, the truncated CIB1 (CIBN, aa 1–170) is localized on the cell plasma membrane via a C-terminal membrane-targeting peptide CAAX. Blue light recruits CRY2-tagged ICD to the plasma membrane via CRY2/CIBN interaction and meanwhile induces the autophosphorylation of ICD via CRY2 homo-interaction.This membrane recruitment strategy was proven to have the best efficiency in activating TrkA and TrkB signaling compared to membrane-bound or cytosolic versions (Duan et al., 2018;Huang et al., 2020a). This is possibly due to the phenomenon that CRY2 homo-interaction can be augmented after being recruited to the membrane at a higher local concentration on the membrane (Che et al., 2015). In addition, the cytosolic CRY2-tagged ICD may be less prone to form pre-existed inactive dimers before blue light illumination. Zhang et al.(2020) used the CRY2/CIB1 membrane recruiting system to induce light-activation of Fas receptor signaling that leads to cell apoptosis. Krishnamurthy et al. (2020) designed a membrane-localized double-CIBN module to recruit CRY2-fused FGFR ICD from the cytoplasm to the plasma membrane upon blue light exposure. This membrane-recruited version of OptoFGFR addressed hyperactivity issues of membranebound OptoFGFR in the dark and was effective in inducing embryogenesis inXenopus laeviswith minimal toxicity(Krishnamurthy et al., 2020).
Membrane recruitment by iLID/SspB system
Hope et al. (2020) also developed a membrane-recruitment strategy by using iLID + tdSspB system that consists of the iLID and a tandem construction of its binding partner SspBnano(tdSspB). The membrane-targeting Lyn tag was affixed to tdSspB, allowing iLID-tagged ICDs of TrkA or TrkB receptors to translocate onto the plasma membrane and homo-interact under blue light. Compared with the above mentioned two approaches based on CRY2, the iLID + tdSspB system can avoid the formation of cytosolic oligomers and precisely control the formation of dimers instead of high-order oligomers.
Cytoplasmic homo-interactions
Light-induced homo-interaction of CRY2 can be a robust method to modulate protein activities in the cytosol (Figure 2G). Duan et al. (2018) designed Opto-iTrkA and Opto-iTrkB(Huang et al., 2020a) by appending CRY2 to the N-termini of ICD of TrkA or TrkB receptors (iTrkA/iTrkB). It is found out that homo-association of iTrkA or iTrkB in the cytosol via Opto-iTrkA or Opto-iTrkB respectively was efficient in activating downstream signaling pathways and the neurite growth of PC12 cells (Duan et al., 2018; Huang et al., 2020a).Interestingly, it was demonstrated in another report that by comparing different light-activation modes of OptoFGFR chimeras, cytoplasmic OptoFGFR1 was not able to cause signaling upregulation and neuronal differentiation in PC12 cells (Csanaky et al., 2019).
Many serine/threonine protein kinases in the cellular signaling network also form dimers in the cytosol to be autophosphorylated and activated. Radziwill et al. has reported optogenetic control of RAF kinase (Wend et al.,2014) and the connector enhancer of kinase suppressor of ras 1 (Fischer et al., 2016), both of which can activate downstream ERK signaling. Hörner et al. (2018) further presented the optogenetic control of focal adhesion kinase signaling by fusing CRY2 to the C-terminus of focal adhesion kinase protein. Bugaj et al. (2013) reported the optical activation of the Wnt/β-catenin pathway using light-induced CRY2 oligomerization. In canonical Wnt signaling activation,Wnt ligands bind to membrane-based Frizzled and LRP6 coreceptors, forming higher-order clusters of LRP that allow β-catenin to translocate into cell nucleus for regulating target-gene expression (Bilic et al., 2007). By tagging CRY2 to the LRP6 C-terminal domain (LRP6c), blue light irradiation could induce the formation of LRP6c clusters and upregulate β-catenin-promoted transcriptional activity in neural stem cells (Bugaj et al., 2013).
Applications
Optogenetically modified protein kinases have been successfully incorporated into drug-screening systems for testing pharmacological inhibitors. Leveraging LOV-based Opto-RTKs, Inglés-Prieto et al. (2015) developed a lightassisted screening method of small molecules against RTKMAPK/ERK signaling. Chatelle et al. (2016) applied optoRAF to select inhibitors of RAF family protein kinases. By monitoring ERK activity via serum response element-dependent expression of fluorescent proteins, the input and output of the screening platforms are both optical, thus making them all-optical platforms which hold great promise in identifying drug targets with high throughput, few operational steps, and low cost (Agus and Janovjak, 2017).
Optogenetic methods based on light-inducible homointeractions are powerful tools to investigate how stem cells determine their fates. Repina et al. (2020) engineered illuminating devices for optogenetic control of Wnt signaling in human embryonic stem cells. Varied light intensities were used to induce different extents of CRY2-LRP6c (Bugaj et al., 2013) clustering, making human embryonic stem cells expressing light dose-dependent reporter genes. Bunnag et al. (2020) constructed Toll-mCh-CRY2 that can optogenetically induce Toll-like receptor signaling pathways in intestinal stem cells in the midgut ofDrosophila. Light-induced clustering of Toll-mCh-CRY2 was shown to drive intestinal stem cells overproliferation. In another optically controlled human pluripotent stem cells culturing system reported by Choi et al.(2020), optical activation of FGFR signaling based on homointeracting VfAu-LOV domains sufficiently induced human pluripotent stem cells differentiation free of supplementing ligands under blue light illumination. Huang et al. (2020b)applied the OptoTrkA system into hair-follicle-derived stem cells. Optical activation of TrkA signaling promoted hairfollicle-derived stem cells proliferation and migration, and hair-follicle-derived stem cells also exhibited increased tendency in differentiating into neuronal and glial cells.
With non-invasive and spatiotemporal control of cell signaling, optogenetic activation of signaling events based on light-gated homo-interactions are powerful tools to investigate neurological events. For example, by using CRY2-based membrane-bound photoactivatable optoTrkB,Woo et al. (2019) have found that local activation of TrkB signaling generates actin waves and can recruit key proteins to stimulated axonal areas during neuron polarization in cultured neurons. They further proved TrkB signaling can be activated by non-invasive blue light LEDs on the hairremoved mouse head (Hong and Heo, 2020). Letellier et al.(2020) recently showed that activation of optoFGFR1 was able to phosphorylate endogenous neuroligin 1 protein that can selectively increase dendritic spine density in mouse hippocampal neurons. Also, the photoactivation of EphB2 signaling can activate the cAMP/Ca2+responsive element binding protein in pyramidal neurons and can enhance longterm fear conditioning memory in genetically modified mice(Alapin et al., 2018). In another example, the CRY2-based lightinducible intracellular Ca2+releasing regulator, OptoSTIM1, has been engineered for manipulating Ca2+signaling in the brain of awake mice through non-invasive light delivery (Kim et al.,2020b).
Summary
Intracellular signaling pathways regulate sophisticated cellular functions through distinct spatiotemporal profiles of activation. Optogenetic manipulations of signaling events provide high spatial and temporal resolutions by the ability of applying light at targeted subcellular areas and desiredtime points, which makes them promising tools to dissect the complex signaling network.
As listed inAdditional Table 1, the majority of optogenetics tools based on light-gated homo-interactions are responsive to blue light, which limits the penetration depth and usually requires the invasive insertion of optic fiber for light delivery in deep tissues. The development of novel optogenetic modules enabling light-inducible homo-association can provide more possibilities to circumvent this limitation. For example, the near-infrared light-activatable optogenetic protein from bacterial phytochrome, DrBphP, was just recently developed that can permit application of optogenetic systems at deeperin vivolocations (Chernov et al., 2017).Meanwhile, currently available tools are being engineered and optimized for different properties and performances.For example, different versions of CRY2 bearing differential homo-associating capacities and the photocycle kinetics (Kim et al., 2020b) are available to meet various needs of optical control. Among them, the use of CRY2high (Duan et al., 2017),CRY2oligo (Taslimi et al., 2014), and CRY2clust (Park et al.,2017) may help increase the penetration depth of optogenetic applications due to their much-enhanced light-mediated homo-binding capacities.
The conditions of light stimulation in cited optogenetic applications are also summarized inAdditional Table 1.Depending on the association and dissociation kinetics of different homo-interacting toolkits, intermittent light illuminations are often utilized to ensure sufficient optical stimulation and minimize phototoxicity as well as photobleaching of fluorophores. Whether to use brief or prolonged light stimuli is also depending on the nature of the targeted intracellular signaling and expected outcomes, as transient or continuous activation of signaling pathways may lead to varied cellular outputs.
In summary, we expect that more tools for light-inducible homo-interactions will be developed, and more optogenetic applications enabled by those tools will be established to control diverse signaling pathways, which will greatly facilitate the research on intracellular signaling pathways.
Acknowledgments:We thank all members of Dr. Liting Duan’s research team for proof-reading the manuscript.
Author contributions:Literature search: PH and ZZ; manuscript writing and revision: PH and LD; figure drafting: ZZ and PH; review supervision:LD. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by a Shun Hing Institute of Advanced Engineering Grant (No. 4720247), and a General Research Fund/Early Career Scheme (No. 24201919) from the Research Grants Council of Hong Kong Special Administrative Region (to LD).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ljiljana Nikolic, University of Belgrade, Serbia.
Additional files:
Additional Table 1: Intracellular signaling pathways that have been optically controlled via different strategies of light-gated homointeractions
- 中国神经再生研究(英文版)的其它文章
- Genes for RNA-binding proteins involved in neuralspecific functions and diseases are downregulated in Rubinstein-Taybi iNeurons
- Research advances on how metformin improves memory impairment in “chemobrain”
- Dendritic spine density changes and homeostatic synaptic scaling: a meta-analysis of animal studies
- Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease
- Growth differentiation factor 5: a neurotrophic factor with neuroprotective potential in Parkinson’s disease
- The promise of neuroprotection by dietary restriction in glaucoma

