Dendritic spine density changes and homeostatic synaptic scaling: a meta-analysis of animal studies
Thiago C. Moulin , Danielle Rayêe , Helgi B. Schiöth
Abstract Mechanisms of homeostatic plasticity promote compensatory changes of cellular excitability in response to chronic changes in the network activity. This type of plasticity is essential for the maintenance of brain circuits and is involved in the regulation of neural regeneration and the progress of neurodegenerative disorders. One of the most studied homeostatic processes is synaptic scaling, where global synaptic adjustments take place to restore the neuronal firing rate to a physiological range by the modulation of synaptic receptors, neurotransmitters, and morphology. However, despite the comprehensive literature on the electrophysiological properties of homeostatic scaling, less is known about the structural adjustments that occur in the synapses and dendritic tree. In this study, we performed a meta-analysis of articles investigating the effects of chronic network excitation (synaptic downscaling) or inhibition (synaptic upscaling) on the dendritic spine density of neurons. Our results indicate that spine density is consistently reduced after protocols that induce synaptic scaling, independent of the intervention type. Then, we discuss the implication of our findings to the current knowledge on the morphological changes induced by homeostatic plasticity.
Key Words: chronic inhibition; chronic stimulation; dendritic spines; downscaling;excitability; homeostatic plasticity; spine density; synaptic scaling; upscaling
Introduction
In order to maintain neural networks in a stable state, neurons can perceive changes in their excitability and trigger negativefeedback homeostatic mechanisms to restore their activity to a physiological range (Turrigiano and Nelson, 2004). One of the most intensively studied forms of homeostatic plasticity is synaptic scaling, in which the synaptic strengths of a neuron are adjusted in a compensatory manner following a global multiplicative scaling factor (Abbott and Nelson, 2000;Turrigiano, 2012). Moreover, the interaction between synaptic scaling and other forms of neuronal plasticity is necessary to maintain crucial brain mechanisms, such as sensory information processing, learning, memory, and cellular development (Fernandes and Carvalho, 2016; Keck et al.,2017; Mendez et al., 2018).
Neuronal changes from homeostatic plastic have been described for bothin vitroandin vivomodels (Moulin et al., 2020), supporting the notion that this mechanism is a fundamental feature for the proper functioning of the neural system. However, the absence of synaptic scaling is associated with improved neural regeneration and regression of pathological conditions. For instance, during neurogenesis,immature neurons do not adjust their synaptic strengths in a homeostatic manner until fully integrated into the neuronal network (Vlachos et al., 2013a; Strehl et al., 2018). Moreover,there is evidence that synaptic scaling drives Alzheimer’s disease progression (Yamamoto et al., 2015; Rodriguez et al., 2020), as the hypoactivity caused by amyloid-β oligomers induces compensatory synaptic changes that raise intracellular calcium, resulting in neurotoxicity and cell death (Small, 2008).It has also been hypothesized that synaptic scaling plays a role in drug addiction (Moulin and Schiöth, 2020). Thus, due to the implications for the modulation of processes in the healthy and unhealthy brain, the study of the cellular and network modifications caused by synaptic scaling is gaining increasing interest.
It is well known that the global adjustment of synaptic strength is achieved by the concomitant modulation of postsynaptic receptor trafficking and presynaptic neurotransmitter release (De Gois et al., 2005; Wang et al., 2012; Diering and Huganir, 2018). Protocols to induce homeostatic plasticity also result in structural modifications,such as changes in dendritic spine volume or density (Goold and Nicoll, 2010; Keck et al., 2013; Barnes et al., 2017; Moulin et al., 2019). Notably, the consequences of chronic activity blockade at dendritic spines have been comprehensively described (Barnes et al., 2017; Hobbiss et al., 2018); however,to the best of our knowledge, no report has systematically evaluated if these modifications are consistent between synaptic upscaling and downscaling processes.
In this study, we analyzed the experiments assessing the effects of chronic excitability changes to neuronal dendritic spine density, extracted from a systematic review of the synaptic scaling literature (Moulin et al., 2020). Specifically,we focus on the measurement of spine density, as previous reports advocate that the formation of silent synapses and increase in the number of spines are important steps for the interaction between homeostatic and Hebbian types of plasticity (Arendt et al., 2013; Mendez et al., 2018). Our meta-analytic results indicate that dendritic spine density is consistently reduced after protocols to induce synaptic scaling, independent of the administration of inhibitory or excitatory interventions. We then discuss the implication of these findings to the current hypotheses on the morphological changes induced by scaling and the interplay of different types of synaptic plasticity.
Data and Methods
Systematic review of the synaptic scaling literature
To identify articles that measured the effects of scalinginducing stimulation protocols on the dendritic spine density,we used the openly-available data from a recent systematic review of the field (Moulin et al., 2020). Briefly, in the original review, two distinct PubMed searches were performed to find articles related to homeostatic plasticity. The first was based on popular keywords (“homeostatic plasticity” OR “synaptic scaling”), which returned 664 articles (Search #1). The second search looked for publications that might have been missed by the first search, based on the most common methods of the field (“(mEPSC* OR mIPSC* OR patch clamp*) AND (scaling OR homeostat* OR chronic* inhibit* OR chronic* excitat*) NOT review”), which returned 618 studies (Search #2). Sixty-one duplicated articles were removed.
The first screening stage analyzed titles and abstracts,eliminating articles that were not written in English, not describing original results, or not describing experiments of chronic stimulation or inhibition of neurons. The second step examined the full text of the publications. They were included if they reported the effects of intensity- and time-controlled chronic neuronal activation or inhibition; used interventions with known effects on synaptic transmission and/ or firing of neurons; and explored changes in the neuronal population caused by synaptic homeostatic plasticity – defined by the descriptions present in the publication.
As the original search was performed on May 31, 2018, we updated the dataset by looking for recent articles discussing synaptic scaling and dendritic spines density. On PubMed,we searched for “(homeostatic plasticity OR synaptic scaling)AND spine NOT review AND 2018/04/01:2020/11/25[dp]”,performed on November 25, 2020, which returned 71 results(Search #3). Screening stages were performed following the same criteria as the previous systematic review. The selection and inclusion process considering all searches is illustrated inFigure 1.
Meta-analysis data extraction
Within the articles selected by the systematic review, we looked for those that performed experiments measuring the effect of synaptic scaling on dendritic spine density. Twentyone articles were included in the final sample following this criterion (Figure 1). Outcomes were divided between those studying chronic inhibition and chronic excitation, and we extracted experimental information from cohort and comparison levels. Each cohort corresponds to a different set of animals. If more than one cohort was used in the same experiment (e.g., for testing at different time points),the source of the data for each cohort was clearly defined.
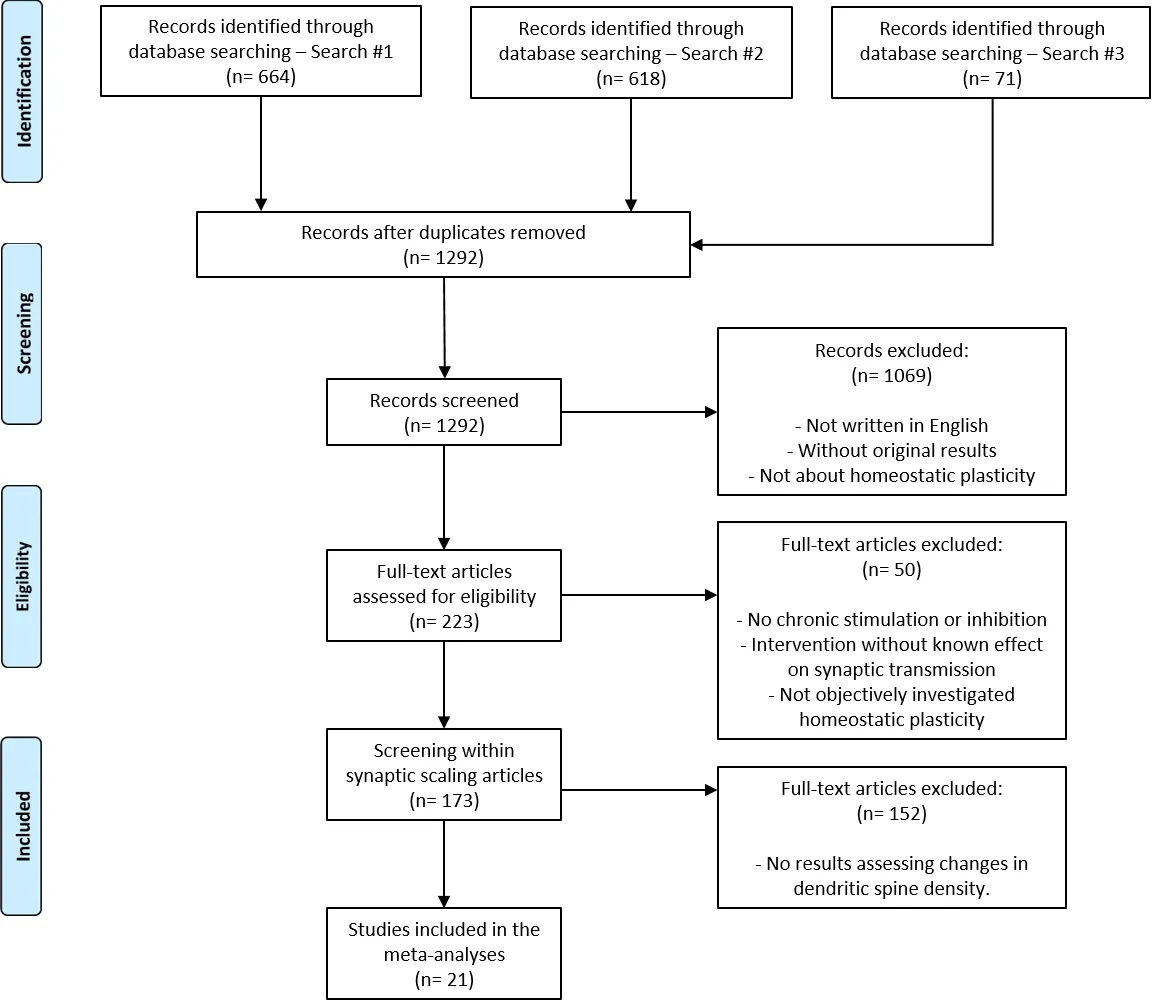
Figure 1|PRISMA flowchart of article search and selection.
Therefore, both independent and non-independent data could be identified and individually extracted at the comparison level. Importantly, to avoid confounders to the assessment of the synaptic scaling effects, we selected experiments that described the effects of only chronic inhibition or excitation interventions to induce scaling (i.e., trials in which genetic or pharmacological manipulations were employed in parallel with the scaling-inducing method were not included).
The comparison-level data referred to the comparison between control and treated groups. We extracted the mean and error for synaptic density in each group, either from figures, using a software to digitize data points from the article’s image files (Gsys 2.4, Hokkaido University, https://www.jcprg.org/gsys/2.4/gsys-e.html) or from the article’s text(which was preferred if available). The extracted variables were: brief protocol characteristics (intervention method,type, and animal model); mean effect of the control and treated group; the standard deviation of the control and treated group; sample size of the control and treated group. In case the sample size informed was a range (e.g., 7–10 animals)or the sum for all groups, the total sample was equally divided into each group.
Meta-analysis parameters
All analyses were performed with the metafor package in R 4.0.2 (Viechtbauer, 2010). We applied the multi-level metaanalysis random-effects model or the standard two-level meta-analysis random-effects model, as stated in each figure.Restricted maximum-likelihood was used as the estimator. All results present effect sizes as Hedge’s g.
Publication bias was estimated through Egger’s regression(Egger et al., 1997), while funnel plots were presented for each meta-analysis in order to demonstrate the presence or absence of asymmetry. Trim-and-fill analyses (Duval and Tweedie, 2000) were performed in order to calculate the number of missing studies and to correct the effect estimate.As there is no way to perform these types of analyses on multi-level data, we adjusted the weights of outcomes corresponding to the same cohort by dividing the sample size by the number of outcomes in the cohort to prevent data redundancy.
Authorship bias was assessed as described in (Moulin and Amaral, 2020). Briefly, we generated a co-authorship network from articles in both the excitation and inhibition metaanalysis. To isolate the research group’s influence on the results, we performed modularity analysis (Blondel et al.,2008) on the network and used the generated clusters to define a higher level on the multi-level analysis model, as described previously.
Results
Meta-analyses of dendritic spine density assessments
In meta-analyses of preclinical studies, each article usually contributes to several different outcomes (Vesterinen et al.,2014); therefore, the non-independency of these results should be considered. Thus, we grouped outcomes originated from the same article, and from the same cohort of animals or cultures within an article. To calculate estimates, we used multi-level fully random-effects models, which separates the article and cohort levels of influence on the overall variance.We also plotted the standard two-level random-effects model estimate for qualitative comparison.
The meta-analysis for the effect of chronic excitation on spine density is shown inFigure 2. We could observe a strong negative effect both in the standard two-level model and in the multi-level model, although the latter computes a smaller estimate and wider confidence interval (Ptwo-level <0.001;Pmulti-level = 0.002;n= 12 outcomes). The variance component (σ2) was 1.31 for the article level and null for the cohort level. AQ-test for heterogeneity in the multi-level model yieldedQ=24.89 andP= 0.009. We did not observe any effect of the different experimental models (i.e.,in vivoorin vitro) when running the multi-level analysis using it as moderator (P= 0.71); however, only two experiments used anin vivointervention, limiting our conclusions in this regard.
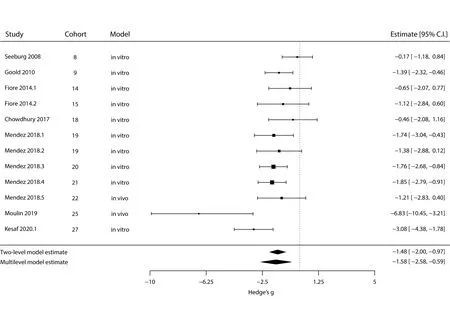
Figure 2|Meta-analysis of dendritic spine density after chronic excitation.
Next, we performed the same type of analysis for the effects of chronic inhibition (Figure 3). Surprisingly, as for chronic excitation, both the standard two-level model and the multi-Figure 2|Meta-analysis of dendritic spine density after chronic excitation.The outer left column identifies the original article; the two middle columns define the experimental cohort and type of model, respectively; while the right column contains each experiment’s effect size (in Hedges’ g) and 95%confidence interval. Square sizes are proportional to the relative weight attributed to each study (for the multi-level model), and bars represent confidence intervals. Diamonds represent estimate results for the standard two-level random-effects model (top) and for the multi-level fully randomeffects model (bottom). Negative effects mean a decrease in spine density,while positive effects would indicate an increase in this measure. CI:Confidence interval. All articles cited in this meta-analysis can be found at the reference list (Seeburg and Sheng, 2008; Goold and Nicoll, 2010; Fiore et al.,2014; Chowdhury et al., 2018; Mendez et al., 2018; Moulin et al., 2019; Kesaf et al., 2020).level model show a reduction in dendritic spine density, with the multi-level model again indicating a smaller effect and broader confidence interval (Ptwo-level 0.002;Pmulti-level= 0.028;n= 27 outcomes). The variance components (σ2) for the article- and cohort-levels were 0.55 and 0.29, respectively,and aQ-test for heterogeneity in the multi-level model yieldedQ= 95.65,P< 0.001. There was again no effect of the experimental model as a moderator (P= 0.058), despite the larger number ofin vivointerventions in this case.

Figure 3|Meta-analysis of dendritic spine density after chronic inhibition.
Analysis of publication and authorship biases
To evaluate whether publication bias could be detected in the meta-analyses, we performed trim-and-fill and Egger’s regression analyses (Egger et al., 1997; Duval and Tweedie,2000).Figure 4shows the funnel plots for the meta-analysis of chronic excitation (Figure 4A) and chronic inhibition (Figure 4B). Trim-and-fill analysis estimated that there was no missing study in either plot, while Egger’s regression did not reach significance in any of the cases (P= 0.072 forFigure 4A;P=0.235 forFigure 4B), indicating no evidence of publication bias.
Finally, we assessed authorship bias according to the methods described in (Moulin and Amaral, 2020). We constructed a co-authorship graph of all authors of studies included in either of the two meta-analyses and segregated clusters by modularity analysis (Additional Figure 1). For the metaanalysis of excitatory interventions, each article belongs to an independent cluster (Additional Table 1); therefore,no authorship bias is detectable. On the other hand, there were clustered articles in the meta-analysis of excitatory interventions (Additional Table 2). To investigate if this had any influence on the meta-analysis, we included the author cluster as a level in the random-effects multi-level model. The variance component (σ2) for the cluster level was null, while the article- and cohort-levelsσ2were maintained at 0.55 and 0.29. We thus conclude that no authorship influence could be detected on effect sizes in the studied meta-analyses. All findings are summarized inTable 1.
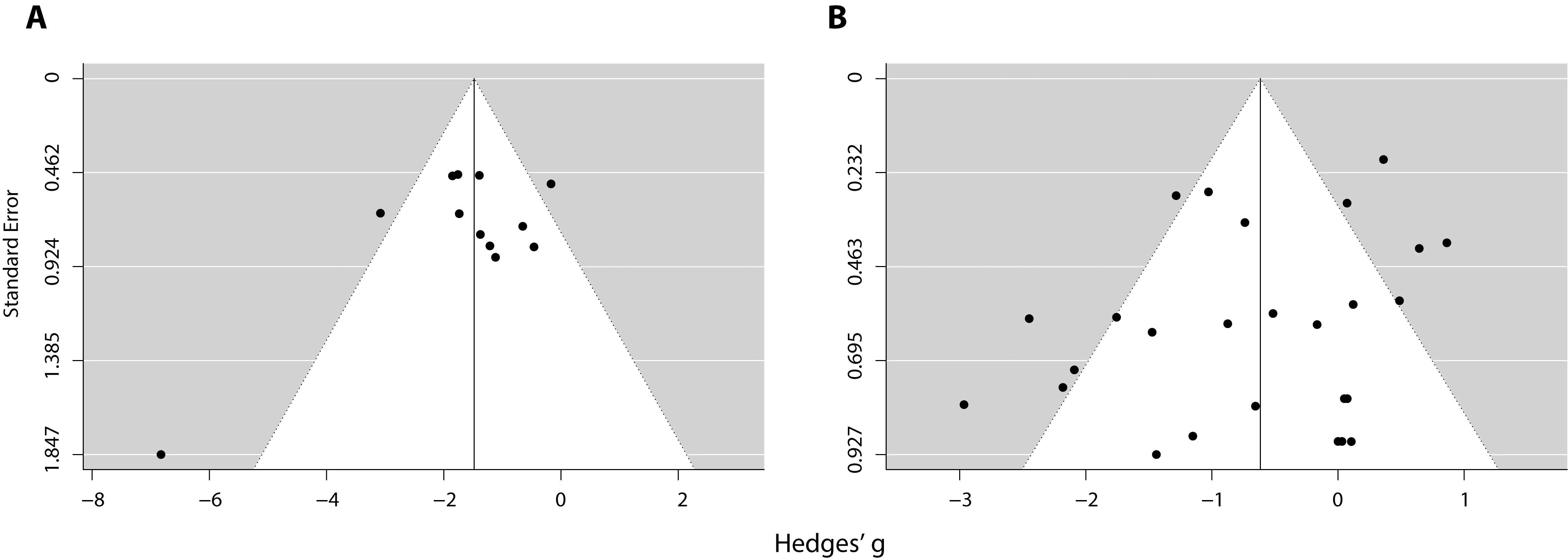
Figure 4|Funnel plots of chronic excitation (A) and chronic inhibition (B)interventions on extinction experiments.

Table 1 |Standard random-effects model and multi-level random-effects model analyses
Discussion
Structured methods of literature synthesis and meta-analysis from preclinical data have been substantially improving in recent years (Vesterinen et al., 2014), particularly concerning animal models of human disorders (Sena et al., 2014). These are useful tools to appraise the available evidence and evaluating whether a given conclusion shows signs of bias (Macleod et al., 2015; Carneiro et al., 2018). A recent systematic review showed that the analysis of morphological parameters, such as dendritic spines, is highly neglected in the field (Moulin et al.,2020); thus, a summarization of the existing literature could bring new insights into the outcomes of chronic alterations in global activity. In this study, we employed a systematic approach to evaluating the existing data on the morphological effects of protocols to induce homeostatic plasticity. Specifically,we performed a meta-analysis of experiments measuring the effects of chronic stimulation or inhibition on the spine density in neuronal dendrites.
Interestingly, although our screening had no limiting criteria on the experimental models, all selected articles describe analyses of hippocampal or cortical neurons from rodents. Moreover,most of the reports restricted their assessment to look at synapses of excitatory neurons (e.g., by labelling only CaMKIIαexpressing cells) or did not describe the neuronal type under analysis. This observation is in line with previous reports showing that few synaptic scaling papers investigate exclusively inhibitory synapses (Moulin et al., 2020). This somewhat narrow range of models used for dendritic spine assessment figures as a limitation of the field and, consequently, restricts the generalization of our meta-analytic results. With that in mind, our discussion focuses on the evidence provided by synaptic scaling protocols within this scope.
First, we observed that chronic stimulation of the neuronal circuitry led to spine density loss, which is consistent with influential reports of the field (Goold and Nicoll, 2010; Fiore et al., 2014). It was suggested that the reduction of spine numbers could facilitate the disruption of the connectivity in the neuronal groups specified during learning processes,thus impairing memory recall, facilitating forgetting, and assisting memory extinction (Mendez et al., 2018). Moreover,synaptic downscaling has been indicated as the mechanistic link to the extensively documented decrease in spine density in human epilepsy (Swann et al., 2000; Goold and Nicoll,2010). Homeostatic downregulation may also play a role in the effects of electroconvulsive therapy, shown to reduce spine density in rodent models of depression (Maynard et al.,2018). Our results support these speculations by showing that spine density loss is a robust outcome of chronic stimulation experiments, bothin vitroandin vivomodels.
Furthermore, our meta-analysis of studies investigating prolonged inhibition of neuronal activity also indicated a reduction in the density of dendritic spines. These results are surprising, given that previous evidence suggests that neurons can respond to chronic inhibition activity by promoting the emergence of new synapses, while globally increasing the strength of existing synapses (Arendt et al., 2013). Moreover,it is argued that synaptic upscaling may facilitate long-term potentiation (LTP) through mechanisms such as silent synapse formation, which would be expected to increase spine density(Hulme et al., 2013).
The assumption that synaptic upscaling prompts the formation of new spines is supported by evidence describing that neuronal dendrites from hippocampal slices have a greater spine density when synapses are inactivated for a few hours (Kirov and Harris, 1999) – a protocol originally intended to block Hebbian plasticity. However, a recent reportin vivoshows that neuronal activity deprivation causes a reduction in the number of spines, which is then compensated by increasing spine volume, not density, through synaptic scaling mechanisms (Barnes et al., 2017). We suggest that these seemly conflicting results can be explained by the modulation of NMDAR-dependent spine pruning that takes place after neuronal inactivation (Vlachos et al., 2013b). Methods such asex vivobrain slices, common for reports investigation Hebbian plasticity, would first induce pruning after denervation, which in turn would be prevented by NMDA receptor inactivation in animals undergoing activity-blockage protocols, resulting in a relative higher spine density. Conversely, forin vitroneuronal cultures orin vivoimaging approaches, used for synaptic scaling experiments, chronic inhibition would induce the observed decrease in spine density, while excitatory synaptic strength and spine volume increases in a homeostatic manner.
Accordingly, it is hypothesized that during upscaling, the synapses of a given neuron are strengthened by the global insertion of AMPA receptors. In turn, the number of AMPA receptors in the synapse is correlated with spine size(Matsuzaki et al., 2001; Zito et al., 2009). Thus, our results indicating that spine density can be reduced after chronic inhibition offers additional support for insertion of AMPA receptors in existing synapses as the basis for synaptic scaling,rather than silent synapse formation.
Lastly, we could not detect any evidence of publication or authorship biases in our meta-analyses. It suggests that these results were unlikely to be affected by unpublished experiments or skewed by author-dependent features, such as location or methodological approach. Nevertheless, more studies are necessary to better characterize the relation between spine density and synaptic strength after synaptic scaling protocols, especially for chronic inhibition protocols.
Author contributions:TCM conceived and designed the meta-analyses,prepared the figures, and wrote the manuscript. TCM and DR extracted data and performed the statistical analysis. HBS contributed with supervision,manuscript editing, and funding acquisition. All authors revised the final version of the manuscript.
Conflicts of interest:The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support:This work was supported by scholarships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil(to TCM and DR); supported by the Kungl Vetenskapssamh Scholarship (Royal Society of Arts and Scientists), provided by Uppsala University, Sweden (to TCM); and supported by the Swedish Research Council and the Swedish Brain Research Foundation (to HBS).
Reporting statement:The article was present according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA)statement.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1: Co-authorship network of the spine density metaanalyses.
Additional Table 1: Authorship clustering for meta-analysis of chronic excitation.
Additional Table 2: Authorship clustering for meta-analysis of chronic inhibition.
- 中国神经再生研究(英文版)的其它文章
- Genes for RNA-binding proteins involved in neuralspecific functions and diseases are downregulated in Rubinstein-Taybi iNeurons
- Research advances on how metformin improves memory impairment in “chemobrain”
- Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions
- Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease
- Growth differentiation factor 5: a neurotrophic factor with neuroprotective potential in Parkinson’s disease
- The promise of neuroprotection by dietary restriction in glaucoma

