Current and future therapeutic strategies for the treatment of retinal neurodegenerative diseases
Victoria Maneu, Pedro Lax, Nicolás Cuenca
The complex and mostly multiple and unknown aetiology of neurodegenerative diseases always give way to an intricate scenario of dying tissue that involves multiple cell mediators and cell types. All neurodegenerative diseases of the central nervous system (CNS) share common mechanisms, regardless their origin:oxidative stress, neuroinflammation and cell death. Accordingly, retinal degenerative diseases, with or without a genetic cause,as retinitis pigmentosa (RP), glaucoma, agerelated macular degeneration (AMD) or diabetic retinopathy (DR) do not differ in their basic mechanisms of cell death neither one to another, nor from those observed in other CNS diseases as Parkinson’s or Alzheimer’s(Cuenca et al., 2014). Indeed, the therapeutic findings should be able to be more or less easily extrapolated between these conditions, as far as they are directed to common dartboards.Gene therapy, in which we have very high hopes to solve genetic disorders, is currently being traslated from preclinical assays to the clinic for some retinal degenerative diseases,with successful achievement up today for the Leber congenital amaurosis, due to mutations in the RPE65 gene (Garafalo et al., 2020). But our promising therapies still face relevant challenges. In this sense, CRISP/Cas editing tools used to amend genetic missenses, need to fix secondary effects, as those related to the immune response (Yu et al., 2017); stem cell approaches have to procure the functionality of transplanted cells in the recipient, to assure the accurate establishment of synaptic connectivity and cell contacts, and gain success in precise image processing (Cuevas et al., 2019; Garita-Hernandez et al., 2019); and optogenetics also needs to find appropriate vectors for the delivery and expression in suitable cell types, avoiding immunological rejection of the vector systems (Shen et al., 2020).While gene- and cell-based therapies evolve through the tortuous pathway of biological success, combined therapies with antioxidant(as lutein or zeaxanthin), antiinflammatory(as corticosteroids or cannabinoids), and antiapoptotic (as tauroursodeoxycholic acid or proinsulin) molecules appear currently as the widest approach to pharmacologically treat a wide spectrum of retinal degenerative diseases.These compounds provide several advantages.They can slow down the progression of the degenerative process, so preserving the visual capacity for a certain time. Moreover, the administration of neuroprotective factors is essential even when the vision has been completely lost, as they can improve non-visual functions, like the control of circadian rhythms and pupil contraction, as the cannabinoidmediated improvement of circadian rhythmicity in P23H rats, which are mediated by the melanopsin-containing photosensitive ganglion cells (Lax et al., 2019). Non-visual retinal functions have also effects on memory and depression. Therefore, the preservation of this subset of cells, although will not improve the visual function, will surely improve the quality of life of the patients and should not be underestimated. But, far beyond, these molecules will surely increase the success of the new therapies, as they can provide an adequate environment of healthy cells, as a substrate for gene transplant or optogenetic approaches,which could hardly be successful in a damaged tissue surrounded by dying cells. Genetic material can be potentially incorporated to the retina and eventually restore the visual functionality in the zone in which it is injected but, without a global actuation on the whole retina by maintaining the health of the adjacent cells, an inflamed surrounding could end in a complete failure of any therapy. Hence,the concomitant use of antiinflammatory,antioxidant and antiapoptotic agents, as well as neurotrophic and growth factors, will provide an adequate environment of healthy cells that will help to achieve a sustained functional restoration of the visual function (Figure 1),as it has been shown for the combination of progesterone and lipoic acid in a mouse model of RP (Ramirez-Lamelas et al., 2018).
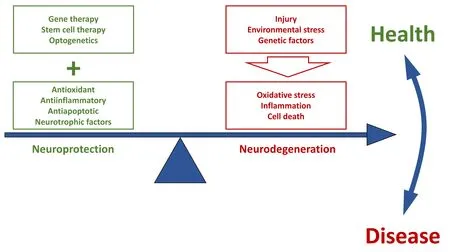
Figure 1|The use of antioxidant, antiinflammatory and antiapoptotic agents, as well as neurotrophic factors, contributes to retinal neuroprotection.
Concerning the use of antioxidants, quite a relevant experimental evidence inin vivomodels points that oxidative stress modulate the progression of retinal degenerative diseases (Cuenca et al., 2014). Photoreceptor cells, with a high metabolic rate, have a high accumulation of mitochondria and are great consumers of oxygen. An overproduction of reactive oxygen species (ROS) and/or a reduced ability to neutralize them, increases the oxidative stress leading to the oxidization and inhibition of phosphatases, kinases and other proteins, and the alteration of their downstream signaling pathways, triggering apoptosis and cell death. The administration of antioxidants has shown good results in different degeneration models and has been proven to preserve functional vision both, in animal models and patients. Also, promising results have been obtained in assays with dietary supplementation with antioxidant compounds as vitamin A, zinc, manganese, curcumin,saffron, safranal, ubiquinone coenzyme Q, as well as with small molecules or drugs directed to reduce ROS formation (Fernandez-Sanchez et al., 2015; Newton and Megaw, 2020).The effects of dietary supplementation with lipidic compounds as omega-3 long-chain polyunsaturated fatty acids, as docosahexaenoic acid or eicosapentaenoic acid, which have antioxidant and antiinflammatory properties,are being currently evaluating for their effects to reduce the progression of AMD, DR or RP(ClinicalTrials.gov). Among the quantity of antioxidants, increasing evidence suggests the potential benefits of combined antioxidant therapy, but clinical trials to date are still too heterogeneous in molecules, doses and results,so more studies are needed to establish their safety and efficacy, including the performance of long-term assays.
The neuroinflammation and toxicity that are always present in CNS neurodegenerative disorders are mediated by multiple inflammatory mediators including cytokines,chemokines, trophic factors and small molecules secreted first by activated microglia,and later by macroglia, astrocytes and Müller cells, which perpetuate the inflammatory response, either leading or worsening a neurodegenerative process (Cuenca et al.,2014). Thus, diseases as glaucoma, AMD, or RP,as well as non-inherited retinal conditions, are associated with chronic microglial activation and neuroinflammation. In degenerating retinas,microglial cells change from their “resting”state, in which they secrete antiinflammatory cytokines, antioxidants and growth factors, into an activated form of migrating amoeboid cells,which show a variety of functional phenotypes,with an increased phagocytic activity, that can engulf pathological proteins, and also secrete pro-inflammatory cytokines, ROS, nitric oxide and tumor necrosis factor-α, that promote chronic inflammation, with severe pathological side effects, what can result in irreversible neuronal death (reviewed in (Cuenca et al.,2014)). In some neurological disorders, such as multiple sclerosis, Alzheimer’s and Parkinson’s diseases, the attenuation of microglial activation has a protective effect. Also, the regulation of microglial cells with drugs as minocycline appears as a good approximation to control the inflammatory process in retinal degenerative diseases and is currently under clinical evaluation in RP and DR. Another approximation to block the inflammatory/death processes is the pharmacological inhibition of key kinases of cellular signaling pathways as mammalian target of rapamycin or glycogen synthase kinase-3.These proteins have a complex regulatory role in inflammation and oxidative stress associated to retinal degeneration (Cuenca et al., 2019),and some of them are also currently under clinical evaluation, as the mammalian target of rapamycin selective inhibitor, rapamycin for AMD or DR.
Promising results both inin vitroandin vivoassays for the treatment of degenerative diseases, have been shown for several antiapoptotic compounds, most of them showing further antioxidant and/or antiinflammatory properties. This is the case of urso- and tauroursodeoxycholic acid in models of Alzheimer’s and Parkinson’s diseases, in DR or RP (Boatright et al., 2009), as well as the synthetic progestin norgestrel or proinsulin in RP (Cuenca et al., 2014; Fernandez-Sanchez et al., 2015). Several clinical assays have evaluated the effects of bile acids in CNS degenerative processes, although studies specific on retinal diseases are still lacking. Although apoptosis has been considered to be the predominant death mechanism in all neurodegenerative diseases, other mechanisms as autophagy,necroptosis, pyroptosis or parthanatos also contribute to retinal cells death (Newton and Megaw, 2020). We know that necroptosis and autophagy have relevant roles in Alzheimer´s or Parkinson´s diseases and their potential in retinal therapeutics remains to be seen.
For what is exposed, and taking into account that multiple cell mediators are involved in the retinal degeneration, at present, our best chance to minimize the degenerative process seems to apply a multiple approach therapeutic strategy, including the concomitant administration of more than one compound which can minimize the oxidant, inflammatory and apoptotic environment and slow the retinal cell death. In this sense, the chemical design of hybrid compounds from motifs addressed to different targets, can provide multiple modes of action and could be a good approach to maintain the cell health and to improve thetissue status. Nowadays, several molecules are under development, and its efficiency is to be seen in the next years. Besides, the success of prospective therapeutics will be greatly influenced by the development of suitable ocular delivery systems that can provide long lasting effects with less administration frequencies. Minimally invasive procedures are needed to carry the therapeutic agents up to the retina. The administration of drugs as eye drops for the treatment of retinal diseases is a rare chance, although some formulations are being tested in clinical trials, as it is the case of nerve growth factor in glaucoma. Other great promising therapeutic agents as smallinterfering RNAs, which are administered initially by intravitreal injection (as for DR or AMD), could also be administered topically as eye drops due to its low size, and new formulations with suitable carriers are currently under development. But for most of the new pharmacological therapies, as antibodies(tested for AMD or DR) or designed ankyrin repeat proteins (for AMD), neither eye drops(with the need to cross many barriers) nor systemic administration (that requires a high amount of drug, which is expensive and has the risk of systemic adverse effects) are a good option, and drug delivery with injections are often discouraged due to the risk of the sightthreatening complications as endophthalmitis.The field of drug encapsulation in nanoparticles,hydrogels, encapsulated cell technology and synthesis of new materials are continuously evolving and are expected to greatly improve the therapeutic possibilities. As an example,the administration of encapsulated implants of neurotrophic factors as Ciliary Neurotrophic Factor for the treatment of glaucoma, RP and AMD are currently ongoing clinical trials(ClinicalTrials.gov).
Thus, the development of new strategies to treat retinal degenerative diseases as optogenetics or cell- and gene-based therapies create good expectations for the future, but the use of neurotrophic factors, antioxidant,antiinflammatory and antiapoptotic agents,preferably in combinations are, not only our present best chance to slow down the disease progression to blindness, indeed, these agents are also needed to preserve relevant physiological functions and, in our opinion,will be needed also to maintain the retinal homeostasis, what will determine to achieve success after gene therapy or cell transplants.
The present work was supported by Ministerio de Ciencia e innovación FEDER-PID2019-106230RB-I00. Instituto Carlos III, RETICS-FEDER RD16/0008/0016. Retina Asturias/Cantabria.FARPE-FUNDALUCE. Generalitat Valenciana IDIFEDER/2017/064 (to NC).
Victoria Maneu, Pedro Lax,Nicolás Cuenca*
Department of Optics, Pharmacology and Anatomy, University of Alicante, Alicante, Spain(Maneu V)
Department of Physiology, Genetics and Microbiology, University of Alicante,Alicante, Spain (Lax P, Cuenca N)
*Correspondence to:Nicolás Cuenca, PhD,cuenca@ua.es.
https://orcid.org/0000-0002-6767-5710(Nicolás Cuenca)
Date of submission:January 15, 2021
Date of decision:February 8, 2021
Date of acceptance:March 25, 2021
Date of web publication:June 7, 2021
https://doi.org/10.4103/1673-5374.314305
How to cite this article:Maneu V, Lax P, Cuenca N(2022) Current and future therapeutic strategies for the treatment of retinal neurodegenerative diseases. Neural Regen Res 17(1):103-104.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Blanca Molins, IDIBAPS,Spain; Makoto Ishikawa, Akita University, Japan.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Genes for RNA-binding proteins involved in neuralspecific functions and diseases are downregulated in Rubinstein-Taybi iNeurons
- Research advances on how metformin improves memory impairment in “chemobrain”
- Dendritic spine density changes and homeostatic synaptic scaling: a meta-analysis of animal studies
- Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions
- Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease
- Growth differentiation factor 5: a neurotrophic factor with neuroprotective potential in Parkinson’s disease

