TC10 as an essential molecule in axon regeneration through membrane supply and microtubule stabilization
Takeshi Nakamura, Shingo Koinuma
Mammalian central nervous system (CNS)neurons lose axon regenerative ability as they mature. This failure to regenerate shows a clear contrast to a remarkable potential of axon growth during embryonic development and after an injury in the peripheral nervous system (PNS) (Hilton and Bradke, 2017). The absence of regeneration in the mature CNS neurons is caused by an inhibitory influence of the environment of the injured axons and the deficit of intrinsic factors that enable regeneration in the PNS (He and Jin, 2016). In the last two decades, gene manipulation strategies and compound screening have identified several neuron-intrinsic players involved in axon regeneration (Ribas and Costa, 2017). The central players are the PTEN/mTOR pathway,which contributes to protein synthesis, and transcription factors (such as SOCS3, KLF family, and SOX11) which control the cell differentiation/de-differentiation status. In addition, cytoskeletal dynamics at growth cones and material transport in axons are essential for axon regrowth; however, it is unclear how the components that regulate these functions are modulated in injured CNS neurons. In this study, we have discussed our recent discovery of an indispensable role of TC10 in CNS and PNS axon regeneration(Koinuma et al., 2020).
TC10, a member of Rho family G-proteins,is implicated in neurite outgrowth through membrane trafficking (Dupraz et al.,2009; Fujita et al., 2013) and microtubule stabilization (Koinuma et al., 2020) (TC10 pathway inFigure 1andAdditional Table 1). In cultured hippocampal neurons,TC10 ablation reduced axon length by 37%without affecting polarization (Koinuma et al., 2020). Exo70 is a component of the exocyst tethering complex and binds to active TC10. Previous studies have indicated that a TC10-Exo70 complex is essential for membrane expansion at the growth cones in cultured hippocampal neurons and NGFtreated PC12 cells (Dupraz et al., 2009; Fujita et al., 2013). Growth cone areas of TC10 knock-out (KO) hippocampal neurons were 41% smaller than those of wild-type (WT)neurons; this decrease is partly due to the defects in exocytosis and membrane supply owing to the absence of the TC10-Exo70 complex. Also, TC10 can limit axon retraction possibly through microtubule stabilization at the growth cone neck (Koinuma et al.,2020). Time-lapse recordings have shown a clear difference in morphological changes between extending WT and TC10 KO axons in cultured hippocampal neurons. Most WT axons extended continuously, whereas KO axons showed frequent retraction during outgrowth. The retraction frequency of KO axons was eight times higher than that of WT axons in embryonic hippocampal neurons at 1.5 DIV. Microtubule bundling at the growth cone neck can limit axon retraction and lead to efficient outgrowth (McNeely et al., 2017).TC10 KO axons had more splayed and less bundled microtubules than WT axons at the growth cone neck. This is in line with the fact that the level of acetylated tubulins, which were used to label stable microtubules, in TC10 KO axons was lower than that in WT axons (unpublished data). PAK, one of TC10 effectors, can phosphorylate and inactivate the microtubule-destabilizing protein stathmin in the neurite-promoting pathway downstream of βPix-d, a microtubulelocalized isoform of βPix RhoGEF (Kwon et al., 2020). βPix has been shown to activate TC10 (Tobón et al., 2018). Thus, we think that βPix-d on microtubules may activate TC10 on vesicles in the proximity of microtubules, and thus the subsequent up-regulation of TC10-PAK signaling leads to stathmin inactivation,microtubule stabilization, and the decrease in retraction events in cultured neurons. This idea is consistent with the fact that TC10 activity on vesicles was higher than that on the plasma membrane (Kawase et al., 2006;Fujita et al., 2013).
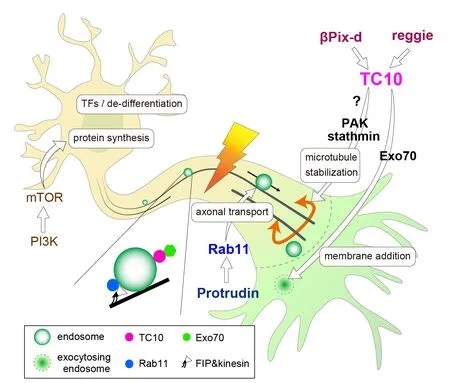
Figure 1|Perspective model of regeneration-competent adult mammalian central nervous system axons.
Prior studies have shown that axon injury increases TC10 levels in the hypoglossal neurons (Tanabe et al., 2000) and the spinal cord, suggesting that TC10 may be involved in axon regeneration. Thus, we examined the regenerative ability of injured hypoglossal axons of TC10 KO mice. In this experiment,a retrograde tracer, Fluoro-Gold (FG), was used to evaluate the axon regenerative ability. When FG is injected into the tongue four weeks after nerve injury, it is taken from axon terminals and retrogradely transported into cell bodies. The axon regeneration rate was calculated from the number of FG-positive cells in the control and injured sides of the hypoglossal nuclei. In WT mice, approximately 60% of motor neurons reprojected into the tongue four weeks after injury; however, in KO mice, only 10% of motor neurons reprojected. The decrease in the number of FG-positive cells by TC10 ablation was not caused by the death of injured motor neurons since approximately 70% of neurons in both WT and KO mice survived for four weeks after nerve injury.This result indicates that TC10 is critically involved in the regeneration of peripheral axons.
Both environmental factors and neuronintrinsic mechanisms are crucial for axon regeneration (He and Jin, 2016). Thus, an injury-inducible Cre driver line,Atf3:BAC Tg (Kiryu-Seo et al., 2016), was used to distinguish the effects of the extrinsic environment from neuron-intrinsic functions.The specific induction of Cre recombinase in injured neurons was confirmed by direct observation of a recombination marker(green fluorescent protein expression).Injury-specific TC10-ablated motor neurons lost their regenerative ability at a similar level to motor neurons in conventional KO mice.These results indicate that TC10 promotes axon regeneration in motor neurons mainly in a neuron-intrinsic manner.
In addition, we examined the effect of TC10 ablation on the regenerative ability of damaged CNS axons. Kurimoto et al. (2010)showed that intraocular inflammation and elevation of intracellular cAMP together enabled marked regeneration of axons in retinal ganglion cells (RGCs). Two weeks after crush with forceps at a point ~1 mm behind the optic disk, optic nerves were stained with an anti-GAP43 antibody to quantify the number of regenerated RGC axons at increasing distances from the crush sites.TC10 KO mice showed a significant decrease in the number of GAP43 positive axons compared with the control up to 1.5 mm distal to the crush site. These results indicate that TC10 is implicated in treatment-induced nerve regeneration of RGCs.
Although TC10 ablation reducedin vitroaxon outgrowth and CNS and PNS axon regeneration, TC10 was largely dispensable for brain development including the formation of major axon tracts (Koinuma et al., 2020). This discrepancy could be explained by complementation by molecules that have overlapping functions with TC10.Another possibility is that the slower axon growth in TC10 KO neurons could eventually catch up with normal growth in WT neurons during development. A satisfying explanation for this discrepancy awaits results of further experiments.
Our finding of the indispensable role of TC10 in axon regeneration suggests the importance of a network that regulates cytoskeletal dynamics at growth cones and material transport in regenerating axons in the proximity of microtubules and neighboring endosomes (Figure 1).In this network, we postulate two central pathways. One presumed consists of reggie/flottilin, βPix-d, and TC10. The molecular linkage between lipid raft protein reggie and TC10 has been reported (Bodrikov et al., 2011). Overexpression of reggie promoted retinal axon regeneration in rat optic nerves (Koch et al., 2013). We presume that some mechanistic connection exists between reggie and βPix-d upstream of TC10. Another pathway consists of Protrudin and Rab11. Protrudin has been shown to bind to GDP-bound Rab11 and promote neurite outgrowth. Recent studies have shown that both Protrudin and Rab11 are implicated in axon regeneration (Koseki et al., 2017; Petrova et al., 2020). Rab11 binds to kinesins through FIP adaptor molecules and is centrally involved in axonal transport.A close colocalization of TC10 and Rab11 at recycling endosomes was recently found(enlarged view inFigure 1; Fujita et al.,2013). Thus, we suggest that TC10/Rab11-double positive endosomes are the nexus of the regulation of cytoskeletal dynamics and material transport in axon regeneration.
We have postulated a tentative mechanistic model of regeneration-competent adult mammalian CNS neurons as shown inFigure 1. Combinatorial manipulations of multiple pathways are expected to induce long-distance axon regeneration (Ribas and Costa, 2017). Furthermore, the functional recovery of damaged axons requires guidance, synaptogenesis, and remyelination in addition to axon regrowth. Recent studies have shown that enhancing the neural activity of RGCs by visual stimulation combined with genetic activation of mTOR pathway promotes long-distance axon regeneration in target regions in the brain after optic nerve crush (Lim et al., 2016). This indicates that enhancement of neural activity is a powerful tool for the correct navigation of regrowing axons to their targets. The findings of aforementioned studies including our present work could contribute to more efficient and safer strategies to promote CNS repair.
We thank Ms. Misa Miyaji from Tokyo
University of Science for her skilled illustrative work.
This work was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science 20K06880 (to TN).
Takeshi Nakamura*, Shingo Koinuma
Division of Cell Signaling, Research Institute for Biomedical Sciences, Tokyo University of Science,Noda, Japan
*Correspondence to:Takeshi Nakamura, DSc,
tnakamr@rs.tus.ac.jp.https://orcid.org/0000-0003-2974-6837(Takeshi Nakamura)
Date of submission:December 18, 2020
Date of decision:February 4, 2021
Date of acceptance:March 25, 2021
Date of web publication:June 7, 2021
https://doi.org/10.4103/1673-5374.314297 How to cite this article:Nakamura T, Koinuma S(2022) TC10 as an essential molecule in axon regeneration through membrane supply and microtubule stabilization. Neural Regen Res 17(1):87-88.
Copyright license agreement:The Copyright License Agreement has been signed by bothauthors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Eric Birgbauer, Winthrop University, USA.
Additional files:
Additional Table 1: Studies on TC10 discussed in this perspective.
- 中国神经再生研究(英文版)的其它文章
- Genes for RNA-binding proteins involved in neuralspecific functions and diseases are downregulated in Rubinstein-Taybi iNeurons
- Research advances on how metformin improves memory impairment in “chemobrain”
- Dendritic spine density changes and homeostatic synaptic scaling: a meta-analysis of animal studies
- Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions
- Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease
- Growth differentiation factor 5: a neurotrophic factor with neuroprotective potential in Parkinson’s disease

