Positron emission tomography imaging for the assessment of mild traumatic brain injury and chronic traumatic encephalopathy: recent advances in radiotracers
Chu-Xin Huang , Yan-Hui Li , Wei Lu Si-Hong Huang Meng-Jun LiLi-Zhi Xiao, Jun Liu
Abstract A chronic phase following repetitive mild traumatic brain injury can present as chronic traumatic encephalopathy in some cases, which requires a neuropathological examination to make a definitive diagnosis. Positron emission tomography (PET) is a molecular imaging modality that has high sensitivity for detecting even very small molecular changes, and can be used to quantitatively measure a range of molecular biological processes in the brain using different radioactive tracers. Functional changes have also been reported in patients with different forms of traumatic brain injury, especially mild traumatic brain injury and subsequent chronic traumatic encephalopathy. Thus, PET provides a novel approach for the further evaluation of mild traumatic brain injury at molecular levels. In this review,we discuss the recent advances in PET imaging with different radiotracers, including radioligands for PET imaging of glucose metabolism, tau, amyloid-beta, γ-aminobutyric acid type A receptors, and neuroinflammation, in the identification of altered neurological function. These novel radiolabeled ligands are likely to have widespread clinical application, and may be helpful for the treatment of mild traumatic brain injury. Moreover,PET functional imaging with different ligands can be used in the future to perform largescale and sequential studies exploring the time-dependent changes that occur in mild traumatic brain injury.
Key Words: amyloid-β; chronic traumatic encephalopathy; flumazenil; fluorodeoxyglucose;mild traumatic brain injury; positron emission tomography; tau protein; traumatic brain injury
Introduction
Traumatic brain injury (TBI) results from various causes,including closed-head injuries where the skull and dura remain intact, penetrating injuries with breaches to the skull and dura, and direct damage to the brain parenchyma (Blennow et al., 2016). In general, a cerebral insult leading to brain compression and vascular disruption may result in subdural and epidural hematomas, and contusion-related changes involve the frontal and temporal lobes in most cases (McGinn and Povlishock, 2016). Diffuse TBI is a common form of TBI that potentially involves scattered or diffuse neuronal and axonal injury (Adams et al., 1989; Chen et al., 2020). TBI may induce disruption of the blood–brain barrier and can activate immune cells and molecules, thus mediating the neuroinflammatory response (Nasser et al., 2016). Based on the overall level of severity, TBI can be associated with symptoms ranging from mild consciousness alterations to a comatose state or death;these are generally framed clinically within the context of the Glasgow Coma Scale (McGinn and Povlishock, 2016).
Mild TBI (mTBI), which is typically caused by blunt nonpenetrating head trauma and is defined according to a Glasgow Coma Scale score of 13–15 at 30 minutes postinjury (Azouvi et al., 2017), accounts for the vast majority of all TBIs. Most patients who experience mTBI have mild symptoms with rapid recovery (within 3 months) (Levin et al., 1987); however, some individuals experience persistent post-concussive symptoms after mTBI in the chronic phase(Umile et al., 2002), which is known as chronic traumatic encephalopathy (CTE). CTE is a neurodegenerative disease that is mainly associated with repeated concussive and subconcussive injuries. The neuropathology of CTE was first recognized in boxers, and has since been mostly reported in athletes and soldiers with exposure to impact injuries(Henry et al., 2017; McKee et al., 2018; Cheng et al., 2020). In general, CTE presents with nonexclusive symptoms, such as depression or memory and cognitive impairments (Mez et al.,2017). A presumptive diagnosis of CTE is usually made based on a patient’s medical history, whereas a definite diagnosis relies on the neuropathological examination of tau protein in the brain (McKee et al., 2018).
Diffuse axonal injury has been identified in all severities of TBI, and may represent an essential pathological substrate of mTBI. Axonal degeneration arising from diffuse axonal injury is generally associated with a complicated progression,including the disruption of axonal transportation, axonal swelling and disconnection, and Wallerian degeneration(Johnson et al., 2013). Neuronal death is caused by oxidative stress followed by impaired homeostasis (Frati et al., 2017). Axonal degeneration may play a role not only in the acute and subacute periods of TBI, but also in longterm neurodegenerative processes, which may be linked to Alzheimer’s disease (AD) and CTE (Johnson et al., 2013).
mTBI can induce various pathophysiological responses.In the acute phase, astrocytes and microglia may secrete proinflammatory cytokines, which are accompanied by reductions in oligodendrocyte cell numbers and glial reactivity. Moreover, an initial reduction in cerebral blood flow can occur within several days following mTBI, which leads to a subsequent increase in nitric oxide expression and vasodilation (Dixon, 2017). In the chronic phase, amyloid proteins may aggregate in the brain, which can trigger other diseases that are associated with these proteins, such as AD and CTE (Dixon, 2017).
Functional changes have been reported in different forms of TBI, especially in mTBI and its progression to CTE.Studies of the response to TBI in the affective, cognitive,and behavioral domains have yielded mixed outcomes, and rehabilitation and responses following brain injuries vary greatly between individuals (Rapport et al., 2020). However,the recent identification of biomarkers as well as technological improvements have led to great advances in the diagnosis and understanding of the pathological mechanisms of mTBI. In particular, recent progress in imaging techniques,including diffusion tensor imaging (Asken et al., 2018;Wang et al., 2020), susceptibility-weighted imaging (Eldeş et al., 2020), and brain iron imaging (Raz et al., 2011), has improved the diagnosis, management, and understanding of mTBI pathophysiology in comparison with conventional imaging, such as structural magnetic resonance imaging(MRI) or computed tomography (CT), which can reveal only microlesions or no lesions in patients with mTBI. Furthermore,positron emission tomography (PET) functional imaging has contributed to the exploration of mTBI pathology by enabling the detailed assessment of associated metabolic changes.
Compared with MRI and CT, PET molecular imaging provides excellent sensitivity for detecting molecular changes; it can quantitatively measure various molecular biological processes in the brain using different radioactive tracers,thereby providing new insights into normal and abnormal brain functional processes (Byrnes et al., 2014). Radiotracers emit positrons, which are then subject to radioactive decay and collide with electrons to produce two photons. The PET scanner detects these photons (gamma rays) to reconstruct spatial density images of the internal functional activity and metabolic processes of the brain. Specific neurometabolic processes can be detected by specially developed targeted radioactive tracers (Hooker and Carson, 2019). Thus, PET using different tracers provides a non-invasive method of measuring brain metabolism that may yield important information about the pathology of tumors or injuries.
Several recent studies have explored the manifestations of molecular PET imaging in TBI, and have used PET to evaluate different molecular biological changes in the disease course(seeTable 1for a summary). Moreover, the integration of PET imaging with structural and functional MRI has contributed to the progress of basic research and clinical imaging. Hence, PET offers a tool that can better evaluate the clinical significance of different imaging manifestations, and can provide guidance for the clinical diagnosis, treatment, and prognosis of TBI. In this review, we discuss the current advances in PET imaging using different types of radiotracers for assessing mTBI, based on clinical and preclinical research.
Retrieval Strategy
The PubMed and Web of Science databases were searched for all PET studies related to mTBI or CTE published up to October 1, 2020 using the following terms: “traumatic brain injury,” “mild traumatic brain injury,” and “chronic traumatic encephalopathy” in combination with “positron emission tomography.” We also searched the reference lists of retrieved publications to identify other potentially relevant studies.Consequently, 405 publications were retrieved. The results were further screened by title and abstract to those only relevant to PET using different radioligands for TBI—especially mTBI—and subsequent CTE. Data were extracted and summarized according to the different radiotracers and their application and significance.
Positron Emission Tomography Imaging of Metabolism
[18F]Fluorodeoxyglucose (FDG) is the most widely used radiotracer for PET/CT in clinical practice. Given the structural similarities between [18F]FDG and glucose, [18F]FDG is subject to the same mechanisms of metabolism as glucose in the brain; it is metabolized into [18F]FDG-6-phosphate, which becomes trapped inside the cell (Finessi et al., 2020) (Figure 1A). Detection of the presence of [18F]FDG can therefore reflect the metabolic status of glucose in the body (Hess et al., 2014; Finessi et al., 2020). Indeed, abnormal uptake of brain [18F]FDG, as measured by PET, has been used for the non-invasive detection of metabolic dysfunction following TBI(Selwyn et al., 2013; Jaiswal et al., 2019). Studies of moderate or severe TBIs have demonstrated measurable effects on cerebral glucose uptake (Ito et al., 2016; Yamaki et al., 2018),although the detailed changes in brain glucose metabolism in mTBI patients remain to be elucidated. Despite several reports of metabolic assessments of mTBI using [18F]FDG PET,the results among such studies are conflicting, which has led to controversy about the utility of the technique and the underlying mechanisms.
Using [18F]FDG PET, altered brain metabolism in multiple brain regions can be observed in mTBI (Peskind et al., 2011;Mendez et al., 2013; Petrie et al., 2014; Buchsbaum et al.,2015; Komura et al., 2019). In one study, 15 out of 18 patients with mTBI and continuing post-concussive symptoms showed abnormal findings in the temporal lobe by [18F]FDG PET imaging. These abnormal findings were associated with the hippocampus and related structures, providing some insight into the frequent finding of memory disorders after mTBI(Umile et al., 2002). However, another study reported no differences in cerebral glucose metabolism in the frontal and temporal regions in the resting state between five patients with mTBI and healthy controls, as measured by [18F]FDG PET (Chen et al., 2003). According to a review article, several studies have performed [18F]FDG PET in patients with mTBI during task performance and have demonstrated a consistent decrease in glucose uptake in the frontal and temporal regions(Wu et al., 2016), whereas another study showed both increased and decreased metabolic changes associated with mTBI symptoms (Gross et al., 1996).
Several recent studies of metabolic activities in TBI have been conducted using rodent models. Jaiswal et al. (2019)explored the time course of metabolic changes in adult male rats following blast head injury. Within 3 hours post-injury,increased glucose metabolism detected by [18F]FDG uptake was observed in the amygdala, somatosensory cortex, andcorpus callosum, whereas decreased uptake was detected in the midbrain structures and dorsal auditory cortex. Seven days after injury, a region of increased uptake was observed in a cluster centered on the cortex–amygdala transition zone,and no regions of decreased uptake were detected (Jaiswal et al., 2019). Brabazon et al. (2017) combined [18F]FDG PET with anatomical and functional MRI to investigate the complex alterations that occur following moderate controlled cortical impact injury in adult rats. These authors demonstrated that [18F]FDG PET was able to detect glial activation, but that the interpretation was confounded by cell damage; in contrast, MRI was able to detect neuronal damage, but the interpretation was confounded by glial activation (Brabazon et al., 2017). This finding demonstrates the importance of considering different pathological perspectives using different imaging modalities; this approach could be generalized to improve the study of mTBI.
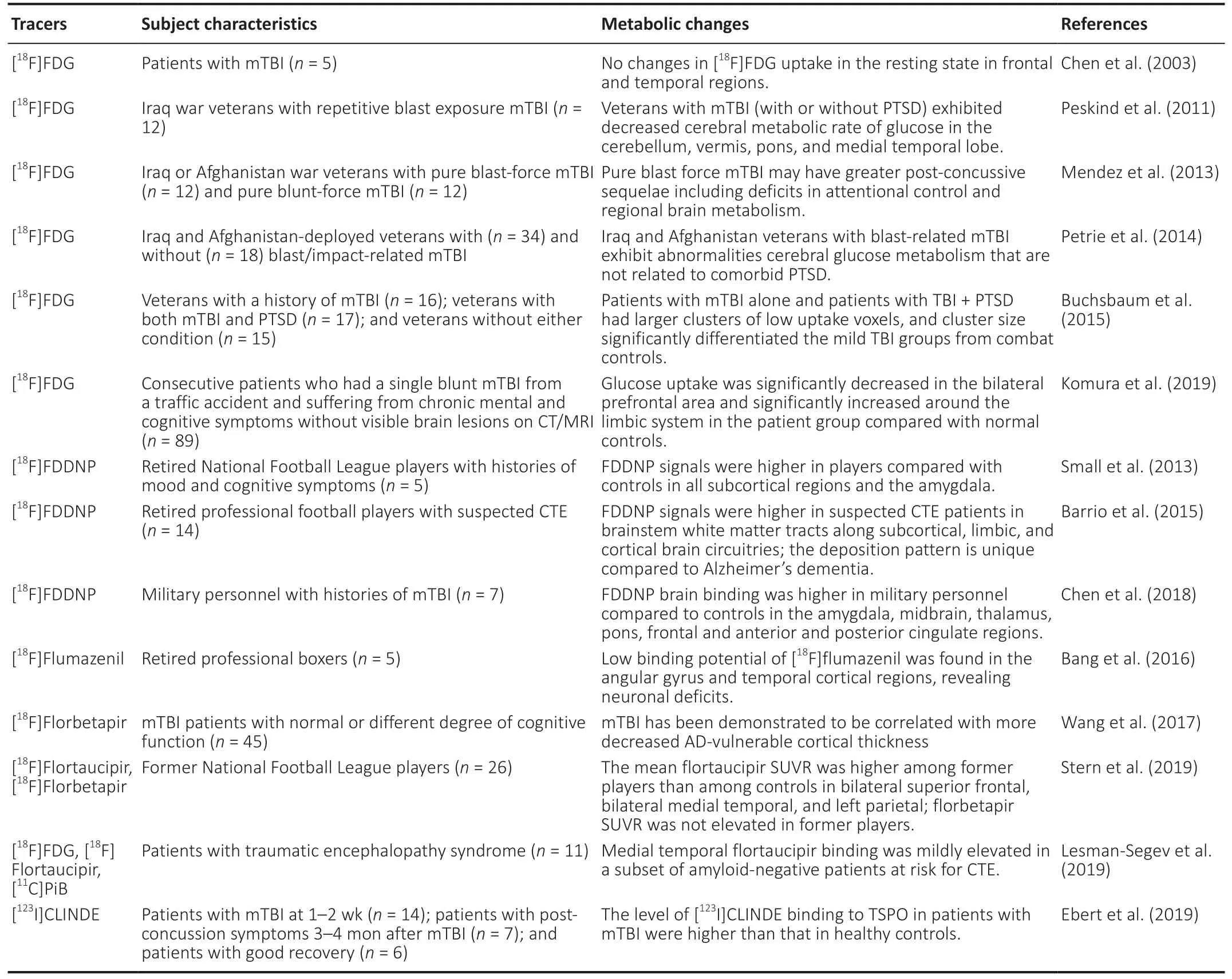
Table 1 |Summary of studies applying positron emission tomography imaging for mTBI using different tracers
It is particularly important to determine whether the inconsistencies in the findings among studies using [18F]FDG PET to investigate mTBI can be explained by the intrinsic heterogeneity among patients with mTBI, or whether they occur because mTBI mainly affects specific functional regions of the brain, leading to changes in uptake in the corresponding regions only. Thus, further investigations are needed to better elucidate the underlying reasons for this heterogeneity, and to clarify the role of [18F]FDG PET in TBI, and especially in mTBI.
Tau Positron Emission Tomography
The association between repetitive mTBI and CTE has long been recognized from observations of boxers, in whom this condition was first described as “punch drunk” (Falcon et al., 2019). As one of the potential sequelae of mTBI, CTE is reportedly associated with cognitive deficits and abnormal cerebral deposition of insoluble proteins (Johnson et al.,2013). In particular, CTE is characterized by the abnormal intracellular accumulation of amyloid-beta (Aβ) and tau proteins (Johnson et al., 2013). Several studies have also proposed that a previous history of TBI may be a possible trigger for cognitive decline and the onset of AD, which is pathologically induced by the production and aggregation of Aβ and tau (Fleminger et al., 2003; Julien et al., 2017).Tau may therefore act as a pathological mediator for TBI and subsequent CTE or AD.
Tau is ubiquitous in the healthy adult brain and is not harmful in a normal microtubule-bound state (Sundman et al., 2015).In adult neurons, tau is mainly distributed in the axons.Structurally, native tau exists in an unfolded state with six isoforms, and its normal function depends on the balance of the different isoforms and their phosphorylation status and structural integrity (Shahani and Brandt, 2002). The fundamental roles of tau are in the stabilization of neuronal microtubules, intracellular transport, axonal morphology,and cell physiology (Williams, 2006). A disturbance of any or all of these functions can result in tau dysfunction, meaning that it is no longer bound to microtubules and consequently accumulates into paired helical filaments and neurofibrillary tangles in cell bodies and dendrites (Goedert, 2004; Wang and Mandelkow, 2016) (Figure 1B). This insoluble state of tau is involved in the pathology of many neurodegenerative diseases collectively known as tauopathies, such as AD, Parkinson’s disease, Huntington’s disease, and mTBI-related CTE.
Accordingly, several novel tau-based PET tracers with a high binding affinity and selectivity for tau deposits in the brain have been used in the evaluation of patients with AD, which has helped to improve the understanding of its pathophysiology. However, the clinical application of tau PET imaging in patients with TBI or CTE remains to be clearly demonstrated.
[18F]Flortaucipir PET
[18F]Flortaucipir, also known as [18F]AV1451 or [18F]T807, binds to neurofibrillary tangles and measures the degree of binding to tau aggregates, thus serving as a useful biomarker for tauopathies (Stern et al., 2019). However, because only a few studies have assessed mTBI or CTE using [18F]flortaucipir PET(Lesman-Segev et al., 2019; Stern et al., 2019), its reliability in the assessment of CTE remains controversial.
Stern et al. (2019) used flortaucipir PET to assess the deposition of tau in the brains of 26 former football players who had experienced repetitive head impacts. In this study,the mean flortaucipir standard uptake value ratio (the ratio of radioactivity in a cerebral region to that in the cerebellum,which was used as a reference) of the football players was higher than that of controls in the bilateral superior frontal,bilateral medial temporal, and left parietal brain regions(Stern et al., 2019). In addition, Dickstein et al. (2016)reported the case of a 39-year-old retired football player who had experienced multiple concussions and had progressive neuropsychiatric symptoms, leading to a high risk of CTE.Flortaucipir PET revealed increased uptake of the radiotracer in the bilateral cingulate, occipital, and orbitofrontal cortices, as well as in several temporal areas. These findings demonstrated multifocal areas of tau protein accumulation at the cortical gray matter-white matter junction, and indicated a probable diagnosis of CTE. Another study observed an overlap between flortaucipir uptake in regions showing neurodegeneration on MRI, as well as hypometabolism based on frontotemporal-predominant [18F]FDG PET, in the brain of a former football player who developed pathologically confirmed CTE 4 years after the MRI and PET scans (Mantyh et al., 2020).
However, flortaucipir appears to have a high binding affinity for paired helical tau filaments in AD only, and not in other tauopathies, including CTE. The use of flortaucipir PET to assess tau retention in a postmortem case series of CTE revealed only faint or no changes in uptake, despite the presence of multiple regions of tau aggregation in all of the brains of the patients with CTE (Marquié et al., 2019). Given that available studies assessing tau aggregates in CTE are confined to case reports or small-sample studies, careful interpretations and further investigations are needed to confirm whether flortaucipir is an optimal agent for evaluating CTE.
2-(1-{6-[(2-[18F]Fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile PET
2-(1-{6-[(2-[18F]Fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile ([18F]FDDNP) is another imaging probe that has been used to detect neuronal aggregates of both tau and Aβ proteins. One study included seven military personnel and 15 retired football players with histories of mTBI and cognitive and/or mood disturbances,and demonstrated that there was elevated FDDNP binding in similar regions in the pons and in frontal, anterior, and posterior cingulate regions compared with cognitively intact controls. These findings suggest the potential value of FDDNP PET for the early detection of CTE in individuals with different risks (Chen et al., 2018). Increased uptake of [18F]FDDNP has also been detected in former football players after repetitive mTBI in several studies (Small et al., 2013; Barrio et al., 2015;Raji et al., 2016; Omalu et al., 2018). Because [18F]FDDNP can bind to both tau and Aβ proteins at the same time, it lacks diagnostic specificity as a tracer; however, it may have increased applicability when combined with other relevant examinations and radiotracers.
[11C]Pyridinyl-butadienyl-benzothiazole 3 PET
[11C]Pyridinyl-butadienyl-benzothiazole 3 (PBB3) is another PET probe that was developed for tauopathies, and has recently been applied in the evaluation of the chronic stages following mild repetitive or severe TBI. In a case-control study, Takahata et al. (2019) reported that patients with TBI showed higher [11C]PBB3 binding capacities in the neocortical gray and white matter segments compared with healthy controls. Moreover, patients with CTE showed increased [11C]PBB3 binding capacity in the white matter compared with individuals without CTE. Overall, this study demonstrated an association between increased [11C]PBB3 binding capacity and TBI-related neuropsychiatric syndrome (Takahata et al., 2019).
Amyloid-Beta Positron Emission Tomography
Axonal injury after TBI can cause the axonal accumulation of amyloid precursor protein, which can then be aberrantly cleaved to form Aβ. The accumulation of Aβ peptides following TBI supports the pathophysiological link between TBI and the subsequent development of neurodegenerative diseases, including AD (Johnson et al., 2010). An accumulation of Aβ plaques can be identified in humans and rodents in the acute or subacute phase, and may even persist for years after TBI (Johnson et al., 2010, 2013). Therefore, Aβ may serve as a potential biomarker for assessing the severity and prognosis of TBI.
Aβ peptides consist of 40–42 amino acids and are produced by the amyloidogenic cleavage of Aβ precursor protein(β-APP), which is localized on the cell membrane or within the intracellular vesicles of many cells, including neurons(Figure 1C). Aβ42and Aβ40are the two major noxious forms of Aβ in the brain; however, the soluble oligomeric Aβ40is more abundant than the Aβ42fibrillar form, which is a major constituent of amyloid plaques. Soluble Aβ40is internalized by pinocytosis and degraded by lysosomes, whereas the fibrillar form of Aβ is internalized by microglia via phagocytosis. It has been proposed that Aβ aggregation may be caused not only by its excessive production, but also by its inadequate clearance (Reiss et al., 2018). The rate of Aβ production and release is normally very high in the brain, and the aggregation of Aβ around neuronal and glial cells can activate calcium ion channels, leading to the disruption of synaptic plasticity,inhibition of hippocampal long-term potentiation, and production of reactive oxygen species (Seeman and Seeman,2011; Reiss et al., 2018).
Aβ and β-APP have traditionally been explored in terms of the pathology of AD. However, their presence and significance in TBI have attracted greater interest in recent years. The diffuse axonal injury that arises from TBI may result in the disruption of axonal transportation, axonal swelling, and subsequent Wallerian degeneration. Furthermore, both β-APP and the enzymes necessary for its cleavage and the subsequent formation of Aβ peptides rapidly accumulate in damaged axons (Johnson et al., 2013). This suggests that the focal axonal injury caused by mTBI may also be responsible for the rapid accumulation and deposition of Aβ plaques following the traumatic injury. Indeed, Aβ deposition was detected in 52% of a cohort of 114 athletes and veterans with neuropathologically diagnosed CTE, and was much more frequent in subjects with CTE compared with healthy controls(Stein et al., 2015). Additionally, Yang et al. (2014) conducted a forensic analysis of rats with primary brainstem injury and demonstrated axonal injury and related changes in Aβ and β-APP in these animals.
Several radioactively labeled molecules, including [18F]FDDNP,[18F]florbetapir, and [11C]Pittsburgh compound B (PiB), can selectively combine with Aβ in the brain, and have therefore been applied as pathological biomarkers of Aβ aggregates. The autoradiographic signal of the tracers provides a quantitative estimate of Aβ deposits after brain injuries.
[18F]Florbetapir PET
[18F]Florbetapir is a specific ligand that binds to Aβ but not to neurofibrillary tangles in human tissue; the binding intensity is quantitatively associated with the density of Aβ. This tracer has therefore been applied in many studies exploring the pathology of mTBI, AD, and other types of cognitive deficits. Wang et al. (2017) performed [18F]florbetapir PET in 45 patients with mTBI, who varied in cognitive function from normal to impaired, and in 45 control subjects. Because cortical thickness can reflect multiple pathological changes caused by cellular injury, these authors compared tau in the cerebrospinal fluid as well as the cortical thicknesses of AD-vulnerable regions between mTBI and control subjects,partially depending on the Aβ pathology. They found an association between mTBI and decreased cortical thickness in the AD-vulnerable regions (Wang et al., 2017). Other studies have also examined whether TBI and post-traumatic stress disorder are risk factors for Aβ deposition in the brain using[18F]florbetapir, and have demonstrated an increased standard uptake value ratio for the Aβ radiotracer in patients with TBI and post-traumatic stress disorder compared with healthy controls. These findings indicate an association between Aβ and the link between TBI and post-traumatic stress disorder(Mohamed et al., 2018). In addition, serial [18F]florbetapir PET conducted at different time points following injury in survivors of severe TBI was used to dynamically measure changes in Aβ levels in specific brain regions, which were proposed to be predictive of cognitive decline (Gatson et al., 2016).Overall, these studies support the use of [18F]florbetapir as a PET radiotracer to identify the presence of Aβ pathology in patients with mTBI.
[11C]PiB PET
[11C]PiB PET also shows potential value for measuring Aβ plaques in patients following TBI. Among 448 participants with normal cognitive function and 141 participants with mild cognitive deterioration (including a portion of individuals with self-reported TBI in each group), PiB PET revealed that individuals in the mild cognitive deterioration group who had TBI (standard uptake value ratio > 1.5) had a nearly five-fold higher odds of elevated Aβ aggregates compared with those without TBI. However, there was no difference in the uptake of [11C]PiB between cognitively normal individuals with and without TBI. This result supports the association between amyloid accumulation and TBI in individuals with mild cognitive deterioration (Mielke et al., 2014). Another study reported that 15 patients with TBI presented significantly higher [11C]PiB distribution volume ratios in the cortical gray matter and striatum compared with 11 controls (Hong et al., 2014). In addition, Takahashi et al. (2019) reported that the PiB PET binding potential of a patient with cognitive impairment after repetitive TBI was significantly increased in the bilateral prefrontal cortex compared with elderly healthy controls. This finding indicates that an early degenerative process is related to mTBI, even when structural damage is not visible on MRI. In contrast, Kawai et al. (2013) failed to detect any Aβ plaques according to the [11C]PiB levels of 12 patients with post-traumatic neuropsychological impairment at the chronic stage following TBI.
These conflicting results highlight the need for longitudinal[11C]PiB PET studies to clarify the time course of Aβ deposition in the traumatized brain, and to elucidate the association between TBI and injury severity, elapsed time from injury, and neuropsychological impairment.
Positron Emission Tomography Imaging of Gamma-Aminobutyric Acid Type A
MRI does not detect any abnormalities following TBI in some cases, including those with severe brain dysfunction.In contrast, flumazenil PET can reflect neural integrity and viability. [11C]Flumazenil was the first radioligand to be developed for PET imaging of the gamma-aminobutyric acid type A (GABAA) system (Maziere et al., 1984).
GABAAand its receptors, which are prevalent in the neocortex and cerebellum, mediate the principal inhibitory neurotransmitter in the central nervous system. The strength of inhibition can be regulated by targeting specific domains of GABAAreceptor subtypes, including the benzodiazepine binding site. Flumazenil is a selective, neutral antagonist of the GABAAreceptor, and binds reversibly and with high affinity at the benzodiazepine binding site (Odano et al., 2009;Bang et al., 2016) (Figure 1D). Thus, the binding potential of flumazenil may be a sensitive marker of neuronal viability,receptor density, and affinity (Odano et al., 2009; Bang et al.,2016). To date, PET imaging using the flumazenil radiotracer has provided valuable information regarding various neurological conditions and psychiatric disorders.
PET has been used to assess changes in central GABAergic functions in many disorders. The use of PET with flumazenil as the radiotracer has revealed altered benzodiazepine receptor binding potential, reflecting variations in the neuronal integrity of different brain regions. Shiga et al. (2006) used[11C]flumazenil PET to study 10 patients with TBI and 10 healthy controls, and reported normal MRI findings and areas with abnormally low cerebral metabolic rate of oxygen in all patients. However, there was low flumazenil uptake in the binding potential images of 60% of the patients, which may reflect low cerebral metabolism caused by neuronal deficits in the TBI group. Another study identified regional neuronal damage based on reductions in the [11C]flumazenil binding potential in patients with neuropsychological impairment following diffuse axonal injury. These findings indicate the clinical value of flumazenil PET for the functional diagnosis of neuropsychological impairments after TBI (Kawai et al.,2010). Moreover, a female patient with memory deficits and sleep-related symptoms following TBI presented no MRI abnormalities, but cerebral damage was evident by the decreased uptake that was observed via [18F]FDG PET and [11C]flumazenil PET. Furthermore, [18F]FDG PET showed considerable improvements in glucose metabolism during the recovery stage compared with the acute stage after injury;however, neural loss remained, as detected by [11C]flumazenil PET (Nishida et al., 2011).
As an alternative to [11C]flumazenil, [18F]flumazenil can also be used as a PET radiotracer to examine neuronal damage.Odano et al. (2009) performed both [11C]flumazenil and [18F]flumazenil PET in eight male subjects, and found no apparent differences between the kinetic behaviors of the two ligands.The use of structural MRI and [18F]flumazenil PET in patients with repetitive TBI, which is a result of mild and accumulative brain damage and most commonly occurs in patients with multiple concussions, revealed no evidence of morphometric abnormalities; however, a low binding potential of flumazenil(reflecting neuronal loss) was observed in the angular gyrus and temporal cortical regions (Bang et al., 2016).
Positron Emission Tomography Imaging of Neuroinflammation
Neuroinflammation in the central nervous system is involved in many disorders. In the pathology of TBI, various biomechanical processes of neuroinflammation may occur,resulting from disruptions in brain structures and functions.To establish a protective mechanism to defend against the deleterious consequences of an insult, microglia can be activated to restore homeostasis in response to TBI. However,activated microglia may also release proinflammatory molecules, leading to further damage and neurodegeneration,in some situations (Donat et al., 2017). Neuropsychiatric consequences may occur in patients with TBI, and even in those with mTBI; the use of PET with emerging tracers has made the measurement of microglia activation and neuroinflammation possible (Coughlin et al., 2015; Donat et al., 2017).
Translocator protein PET
Translocator protein (TSPO) is a five-transmembrane protein that spans the outer mitochondrial membrane. It normally exists in a quiescent state with low expression levels in the healthy human brain. Under injury conditions, activated microglia significantly upregulate the expression of TSPO in the brain (Best et al., 2019) (Figure 1E).
[11C]PK11195, a first-generation TSPO ligand, has been used to study microglia activation and neuroinflammation in animal experiments (Folkersma et al., 2011; Best et al., 2019). TSPO PET using [11C]PK11195 in a rat model of TBI at 1 day after a controlled cortical impact revealed no differences in whole brain uptake compared with sham-treated controls, but there was a significant increase in uptake in the TBI model after 10 days (Folkersma et al., 2011). Several new-generation TSPO radiotracers, such as [11C]DPA-713 (Coughlin et al.,2015), [18F]DPA-714 (Israel et al., 2016), and [123I]6-chloro-2-(4′-iodophenyl)-3-(N,N-diethyl)imidazo[1,2-a]pyridine-3-acetamide (CLINDE) have also been synthesized and applied in the assessment of TBI. In a study of nine former National Football League players and nine age-matched healthy controls, the former players showed significantly higher [11C]DPA-713 binding to TSPO in several cerebral regions, including the supramarginal gyrus and right amygdala. These results suggest that brain injury and repair may be associated with a history of TBI (Coughlin et al., 2015). The measurement of neuroinflammation using [123I]CLINDE in 14 patients with mTBI without structural damage at 1–2 weeks and 3–4 months after injury and 22 healthy controls has recently been studied. Both at 1–2 weeks after injury in all 14 patients, and at 3–4 months in seven patients with post-concussion symptoms and in six patients with good recovery, the levels of [123I]CLINDE binding to TSPO were higher than those in healthy controls (Ebert et al., 2019).
Although the findings from these studies lend support to the use of TSPO PET as a biomarker to monitor responses in patients with mTBI, the clinical application of current TSPO PET radiotracers is restricted and requires further exploration.
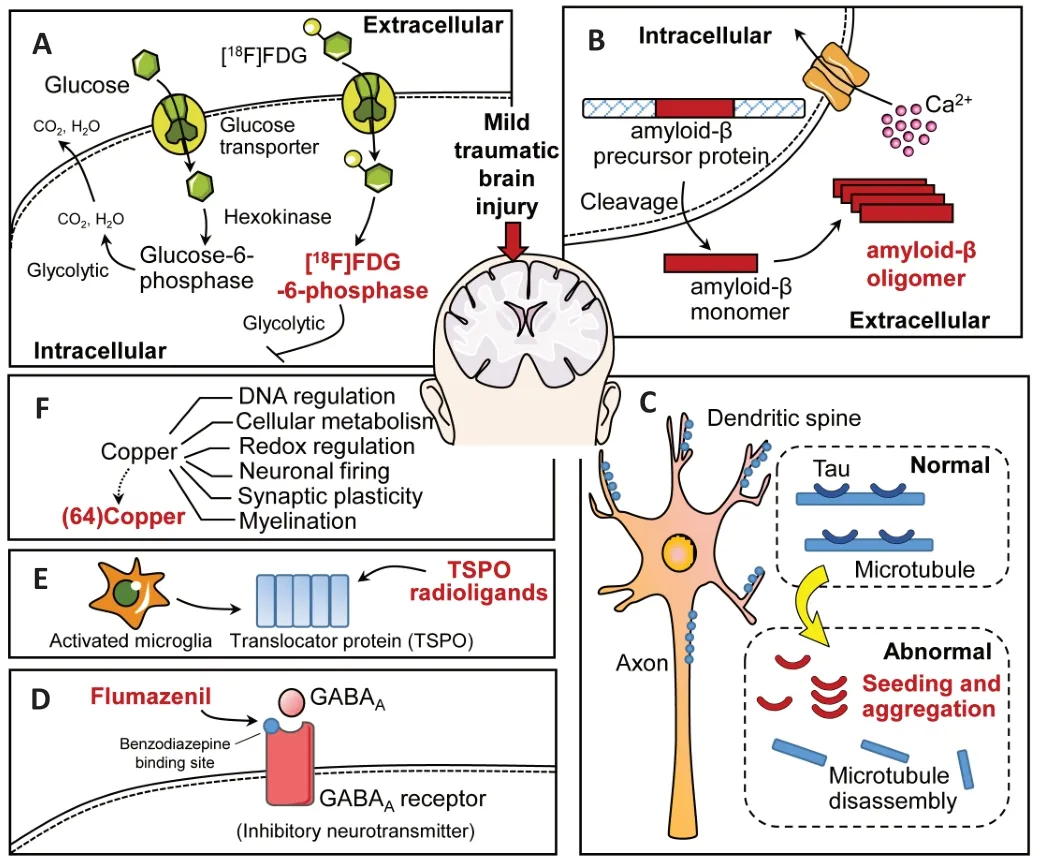
Figure 1| Mechanisms of the different radiotracers that have been used in positron emission tomography studies of traumatic brain injury or chronic traumatic encephalopathy.
[64Cu]CuCl2 PET
Copper is essential for the activity and functioning of enzymes,thereby impacting both physiological and pathological processes in the brain, including DNA regulation, cellular metabolism, redox regulation, neuronal firing, synaptic plasticity, and myelination (Rihel, 2018) (Figure 1F). Copper derived from peripheral copper is physically transported through the blood–brain barrier to enter the brain. Numerous complexes for metabolic processes in the human brain contain Cu+and Cu2+compounds, which largely dominate copper biochemistry. Changes in the environment and pH can lead to a change in the reduction potential of the Cu2+/Cu+redox pair,to ultimately affect the normal development and function of the brain (Scheiber et al., 2014).
Because inflammation can increase the copper content to maintain and repair neurons and surrounding structures(Klevay, 2013), copper-64 chloride ([64Cu]CuCl2) has been used as a novel radiotracer to assess copper metabolism in several diseases, such as skeletal muscle injury (Xie et al.,2017), prostate cancer (Cantiello et al., 2020), and TBI. The application of [64Cu]CuCl2has been demonstrated in C57BL/6 mice with experimental TBI induced by a controlled cortical impact, and increased64Cu uptake was detected in traumatic cortical brain tissue compared with control non-traumatic cortical brain tissue (Peng et al., 2015). These changes were suggested to reflect microglial activation in response to oxidative stress resulting from TBI (Peng et al., 2015).However, the copper response to TBI may differ between humans and rodents; thus, clinical studies in patients are needed for further validation of these findings. Moreover,further studies are needed to determine whether [64Cu]CuCl2PET is also suitable for the detection of mTBI pathology.
Conclusions and Future Perspectives
Recent advances in novel imaging agents have allowed for the identification of altered neurological functions and the underlying pathology of TBI, and particularly of mTBI. Based on its advantages of high sensitivity for metabolic imaging, PET can be a valuable tool for the evaluation of functional changes in different brain regions following mTBI. In addition to glycolysis, tau and Aβ radioligands are the most widely studied PET techniques for TBI, whereas studies using PET imaging to investigate the GABAAsystem and neuroinflammation in TBI remain relatively rare. Different types of radiotracers may help to detect altered functions from a range of different perspectives (Table 1). However, the clinical application of PET in mTBI remains limited to studies with a small sample size; this may partly be attributed to the high cost and risks of exposure to radiation of this technique compared with other imaging techniques, such as MRI, in clinical practice.
With the recent emergence of encouraging data, novel radiolabeled ligands for PET are likely to have more widespread application. However, more evidence for their clinical translation and the further evaluation of novel radiotracers of PET in mTBI are needed. It may be useful to perform early and sequential studies exploring timedependent changes by combining different tracers. In the future, large and well-defined prospective trials combined with multimodal metrics will help to evaluate which functional brain regions may experience more severe damage in mTBI,as well as to determine the characteristics of blood flow,function, and metabolic changes in damaged areas. This information will reveal the true utility of PET, with the ultimate goal of improving the optimal management and outcomes of patients with mTBI.
Author contributions:Manuscript supervision and design: JL; data collection: CXH, YHL, SHH, MJL; manuscript writing: CXH; manuscript review: JL, WL, LZX. All authors approved the final version for publication.
Conflicts of interest:The authors declare that there is no conflict of interests.
Financial support:This work was supported by a grant from the National Natural Science Foundation of China, No. 81671671; and Clinical Research Center for Medical Imaging in Hunan Province of China, No.2020SK4001 (both to JL).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Abraham Martin Muñoz, Achucarro - Basque Center for Neuroscience, Spain; Hiroki Kato, Osaka University, Japan.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Genes for RNA-binding proteins involved in neuralspecific functions and diseases are downregulated in Rubinstein-Taybi iNeurons
- Research advances on how metformin improves memory impairment in “chemobrain”
- Dendritic spine density changes and homeostatic synaptic scaling: a meta-analysis of animal studies
- Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions
- Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease
- Growth differentiation factor 5: a neurotrophic factor with neuroprotective potential in Parkinson’s disease

