Synthesis of Nitrogen and Boron Co-doped Carbon Dots for Picric Acid Detection
WANG Yingte,YANG Yujie,DUAN Rong,ZHANG Yuanyuan,WANG Yunxia
(1.School of Chemistry and Chemical Engineering,Shanxi University,Taiyuan 030006,China;2.Shanxi Lüshanyuan Liquor Co.,Ltd.,Taiyuan 030063,China)
Abstract:To improve sensitivity and selectivity of picric acid(PA)detection by carbon dots(CDs),the quantum yield(QY)of the synthesized CDs was increased via co-doping ethylenediamine and sodium tetraphenylboron as nitrogen(N)and boron(B)dopants to modify the CDs surfaces.The QY of the obtained N,B-CDs was higher up to 75.6% which was obviously improved by compari‐son to our previous reported N-CDs synthesized by mandelic acid as carbon sources and ethylenediamine as nitrogen sources.The surface morphology and optical properties of N,B-CDs were investigated.The consequences revealed that N,B-CDs were spherical structure,and the average diameter was 3.6 nm.The optimal emission and excitation wavelength of the synthesized N,B-CDs were 442 nm and 342 nm,accompanied by bright blue fluorescence.The fourier transform infrared spectroscopy showed that there were abundant hydrophilic groups on N,B-CDs surface,which made N,B-CDs have good water solubility.A strategy for selectively and sensitively detecting PA by the synthesized N,B-CDs was based on PA quenching the FL intensity of CDs via a possible fluorescence resonance energy transfer mechanism and inner filter effect.Under the optimum conditions of experiment,the N,B-CDs fluores‐cence intensity showed a great linear correlation with the PA concentration between 0.1 μmol·L-1to 24.7 μmol·L-1,and the limit of detection was 0.019 μmol·L-1(S/N=3).In addition,the N,B-CDs were employed to detect PA of real water samples and satisfacto‐ry results were obtained.The recoveries ranged from 95.80% to 103.10%.
Key words:picric acid;citric acid;co-doped carbon dots;ethylenediamine;sodium tetraphenylboron;fluorescence quenching
1 Introduction
Picric acid(PA),named 2,4,6-trinitrophenol(TNP),is a toxic,non-biodegradable and highly ex‐plosive nitro aromatic organic compound with poten‐tial threat to life[1-2].PA has good water solubility and is widely used in military,dye and pharmaceutical industries[3-4].Trace amount of PA released into the environment will cause serious soil and water pollu‐tion,which will endanger the health of plants,animals and human body[5].Long-term intake can cause con‐tact dermatitis,respiratory injury,liver function dam‐age and even lead to chronic poisoning[6-8].The stan‐dards for drinking water quality of China(GB5749-2006)have a clear limit on the PA concentration with 0.001 mg·L-1[9].Therefore,it is significant to ex‐plore an accurate,highly-sensitive,and highly-selec‐tive trace analysis of PA detection method.
Carbon dots(CDs)which acted as fluorescencebased sensors are proved to be very sensitive to PA and have been widely used to detect PA in recent years[10-14].However,the detection linear range,sensi‐tivity and selectivity still need to be improved be‐cause the chemical compounds which have similar structures to PA often interfere in selectivity of the de‐tection.Therefore,various methods such as surface modification and heteroatom doping have been used to increase the sensitivity and selectivity of CDs in de‐tecting PA.In recent years,hetero-atom doping has become a widely used method to effectively regulate the fluorescence properties of luminescent materials and adjust surface defects[15-16].Fortunately,fluores‐cence properties of CDs could be improved by heteroatom doping[17-19].Since sulfur(S),phosphorous(P),nitrogen(N)and boron(B)have similar atomic radii to carbon atoms,they were often used as doped atoms to modify the optical properties of CDs by improving the quantum yield(QY)of CDs[20-23].Ju et al.[24]pre‐pared the P-CDs by utilizing sodium pyrophosphate and β-cyclodextrin as the precursors for detection of PA.A linear response was obtained for PA from 0.1 μmol·L-1to 10 μmol·L-1and the limit of detection(LOD)was 0.082 μmol·L-1,based on the inner filter effect(IFE)between PA and P-CDs.In our previous study[14],we used mandelic acid as carbon sources and ethylenediamine as N sources to synthesize NCDs through the green hydrothermal method,and the QY reached up to 41.4%.N-CDs were used to detect PA,showing a good linear relationship at0.5 μmol·L-1~30 μmol·L-1(LOD was 0.041 μmol·L-1).Although single doping described above improved the fluores‐cence properties of CDs with a certain extent,co-dop‐ing was proved to be better.In order to improve the sensitive and selective detection of PA,we proposed to synthesis co-doping CDs on the condition of our previous study of single doping N-CDs[14].Better se‐lectivity and sensitivity were expected compared to our previous study.
We prepared water-soluble N,B-CDs by utilizing citric acid(CA)as carbon source,ethylenediamine and sodium tetraphenylboron as N and B dopants(Scheme 1).The QY of N,B-CD was higher up to 75.6% by co-doping effect which was obviously in‐creased in comparison to 41.4% in our previous study[14].The obtained N,B-CDs were more sensitive and selective to PA than our previous N-CDs.A good linear correlation with a lower detection limit was ob‐tained.Especially,the selectivity was obviously im‐proved.Additionally,the method of using assynthe‐sized N,B-CDs to detect PA had been successfully em‐ployed to detect real water samples.
2 Experimental Section
2.1 Materials
CA,ethylenediamine,sodium tetraphenylborate((C6H5)4BNa)and PA were obtained from Beijing Chemical Industry Co.,Ltd.(Beijing,China).The chemicals of our experiments were analytically pure level and had not been further purified.The water used in the experiments was the ultra-pure water(Mil‐lipore and Billerica,USA)with an electrical resis‐tance of 18.2 MΩ·cm-1.The dialysis bag we used had a molecular weight cutoff of 1 000 Da(Shanghai Chemical Reagent Company,China).Other chemical material used in the experiment can be found in the S1 of Supporting Information.
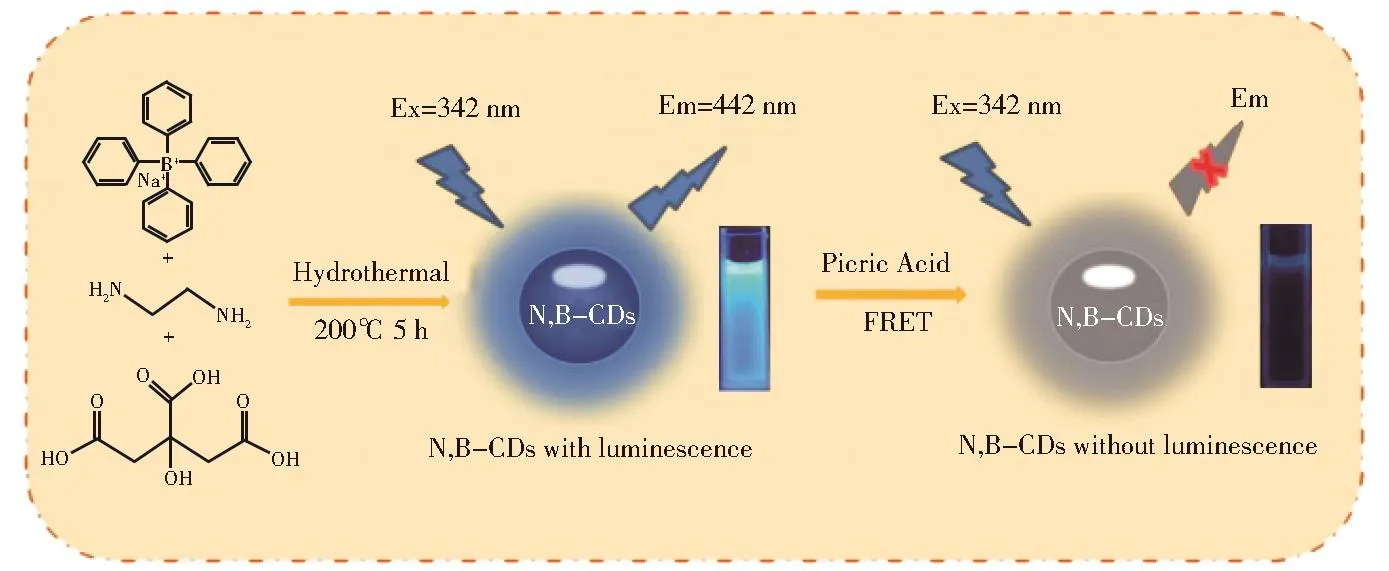
Scheme 1 Diagrammatic sketch of N,B-CDs synthesis and fluorescence sensing
2.2 Preparation of N,B-CDs
0.096 g CA(0.5 mmol)and 0.171 g sodium tet‐raphenylborate(0.5 mmol)were dissolved in 30 mL ultrapure water at ambient temperature and sonicated;followed by adding 67 μL ethylenediamine(0.5 mmol)and shaking.The ratio of the amount of substance of CA,ethylenediamine,and sodium tetraphenylborate was 1∶1∶1.Then the mixture was transferred to 50 mL polytetrafluoroethylene autoclave for heating 5 h at 200℃.After the solution was naturally cooled to ambient temperature,it was dialyzed and purified for 24 h to eliminate the nonreactive molecules.After that,the solution in the dialysis bag was collected and lyophilized to obtain the dried N,B-CDs powder,which was saved at 4℃ for following study.Charac‐terizations of the synthesized N,B-CDs were ex‐pressed in the S2 of Supporting Information.
2.3 Detection of PA
50 μL of the synthesized N,B-CDs(80.6 g·L-1)solution was diluted 40 times with water,and the fluo‐rescence intensity was measured,and then PA was added with different concentrations.After incubating for 2 min at ambient temperature and fixing the excitation wave‐length was 342 nm,the respective fluorescence emission spectra were recorded for PA detection.
For the selectivity of PA detection,20 μL of met‐al ion aqueous solution of 0.01 mol·L-1(Hg2+,Pb2+,Cr3+,Mn2+,Cu2+,Al3+,Fe3+,Fe2+,Na+,K+,Li+,Mg2+,Ni2+,Ag+,Ba2+,Cd2+,Zn2+,Co2+),or 20 μL of a small molecular substance with a concentration of 0.01 mol·L-1(benzyl alcohol,BA,acetone,PHE,o-NT,4-NP,o-AP,m-NBA,o-NBA,ethanol,AN,SA,2,4-di‐chlorophenol,DNP,m-HBA,p-AP,NB,PA)were add‐ed into the diluted N,B-CDs aqueous solution.After incubating for 2 min at ambient temperature,the fluo‐rescence intensity of mixed solution was recorded with the excitation wavelength at 342 nm.All the above tests were measured in parallel for three times.
3 Results and Discussion
3.1 Characterization
The chemical composition and surface morpholo‐gy of synthesized N,B-CDs were systematically char‐acterized through TEM,FT-IR spectrometry,XRD,Raman spectroscopy and XPS.These interrelated re‐sults and discussions were as follows.As shown in Fig.1(a)and Fig.1(c)studied by TEM,N,B-CDs were well dispersed from one another in aqueous solu‐tion and possessed a typical spherical structure with a diameter of(3.6±0.5)nm.The high-resolution TEM picture clearly revealed from Fig.1(b)that the lattice spacing of N,B-CDs was 0.346 nm,which was matched well with the(002)plane of graphitic car‐bon[25-26].

Fig.1 (a)TEM image,(b)high-resolution TEM picture,(c)particle size distribution diagram and(d)the FT-IR spectrogram of synthesized N,B-CDs
As described in Fig.1(d),the surface functional groups of N,B-CDs were investigated by FT-IR spec‐trum.The N,B-CDs mainly contained chemical bonds including O-H or N-H(3 241 cm-1),C=C(1 653 cm-1),N-H(1 540 cm-1),C-H(1 356 cm-1),C-N(1 049 cm-1)and B-O(1 473 cm-1)[27].These all demonstrated that there are oxygen-containing functional groups on the N,B-CDs surface,which greatly increased the water solubility of N,B-CDs.In addition,B-C stretching vibration was found at 1 154 cm-1,indicating that the B element was suc‐cessfully doped into the CDs.These abundant hydro‐philic functional groups not only improve water solu‐bility of N,B-CDs,but also take a significant impact on the detection of PA as label-free fluorescent probes.
XRD and Raman spectroscopy were used to characterize solid samples of the synthesized N,BCDs.In Fig.2(a),the XRD pattern showed a broad diffraction peak at 2θ=22.9°,corresponding to the(002)diffractive surface of graphite,which was con‐sistent with the information obtained from the TEM image.The N,B-CDs Raman spectrum(Fig.2(b))showed two characteristic peaks,the crystalline G band at 1 532 cm-1and the disordered D-band of car‐bon material at 1 338 cm-1,which indicated that the graphite defect center of sp2was present in the amor‐phous carbon of sp3.

Fig.2 (a)XRD pattern and(b)Raman spectrum of N,B-CDs
XPS analysis was employed to study the elemen‐tal composition and chemical environments of the synthesized N,B-CDs.The XPS elemental analysis showed that there are mainly four elements C,O,N and B in the synthesized N,B-CDs.As shown in Fig.3(a),the four peaks 285.6 eV(C 1s),532.6 eV(O1s),402.6 eV(N 1s)and 191.9 eV(B 1s)could be clearly seen[27].The XPS spectra of C1s(Fig.3(b))showed four peaks at 287.36 eV,285.77 eV,284.65 eV and 283.90 eV belonged to functional groups of C=O,C-N,C=C/C-C and C-B.Two character‐istic peaks at 400.22 eV and 399.31 eV were found in the N 1s spectrum(Fig.3(c)),which were related to N-H and N-C[28].At the same time,the B 1s XPS spectrum(Fig.3(d))had two different peaks at 191.7 eV(B-C)and 192.1 eV(B-O)[29].In summary,the experiment results of XPS and FT-IR spectrosco‐py showed that N,B-CDs may has several groups such as-COOH,-OH,-C-B-and-NH2,and the synthesized CDs were successfully doped with N and B elements,which improved the QY of CDs to some extent.

Fig.3 XPS(a)full spectra,(b)C1s,(c)N1s,and(d)B1s of the N,B-CDs
3.2 Optical Properties
The optical properties of the N,B-CDs were stud‐ied by measuring fluorescence and UV-vis absorption spectra.The UV-vis spectrum of N,B-CDs exhibited two characteristic absorption bands at 370 nm and 263 nm in Fig.4(a).The absorption band appeared at 263 nm possible represent the n-π*transition of C=O,while another absorption peak at 370 nm might belong to the π-π*transition of C=C bond.The fluo‐rescence spectra of N,B-CDs were also recorded in Fig.4(a),showing that the maximum emission wave‐length was 438 nm and the excitation wavelength was 345 nm.The insets showed that the N,B-CDs aque‐ous solution was yellow under visible light(left).It could be clearly seen that the solution under the UV light of 365 nm(right)presented light blue,which was sufficient to prove the high QY of the synthesized N,B-CDs.
Notably,Fig.4(b)showed that the correspond‐ing fluorescence emission bands of N,B-CDs did not exhibit obvious shift as the excitation wavelengths changed from 360 nm to 400 nm,reflecting that the synthesized N,B-CDs had a distinctively excitation-in‐dependent fluorescence property.The QY of the syn‐thesized N,B-CDs was determined to be 75.6% ac‐cording to the calculation method of QY in the sup‐porting information S3,which was obviously higher than that of boron-free doped QY(41.4%)in our pre‐vious study[14].

Fig.4 (a)Fluorescence excitation/emission spectra and UV-vis spectrum of the N,B-CDs.Insets:The left picture was N,B-CDs solution under visible light,the right was at 365 nm UV lamp.(b)Fluorescence emission spectra with excitation wavelength dependence of N,B-CDs(350 nm-400 nm)
3.3 Influences of External Environments
In order to further optimize the experimental con‐ditions,the effects of different ionic strength and pH on N,B-CDs fluorescence were investigated.Fig.5(a)showed that fluorescence intensity(F)of N,BCDs under different pH conditions was normalized to the maximum fluorescence intensity(F0,pH=11).The fluorescence intensity decreased at pH<3 and pH=12,which was attributed to the surface groups of N,B-CDs,such as amino and carboxyl groups,proton‐ating in acidic solutions and deprotonating in alkaline solution.There was almost no change at pH 4-11,demonstrating good stability in the broad pH range.Therefore,the following experiments were conducted under neutral conditions.Subsequently,the influence of different ionic strengths was studied on N,B-CDs fluorescence intensity.In Fig.5(b),F and F0repre‐sented the N,B-CDs fluorescence intensities with and without NaCl in the solution,respectively.As the in‐creasing of NaCl concentration even the concentra‐tion reached 1.0 mol·L-1,the N,B-CDs fluorescence intensity decreased slightly,which revealed the N,BCDs possessed good resistance to salt solution.

Fig.5 Effects of(a)pH and(b)ion strength on fluorescence of N,B-CDs
In different time periods after adding PA,the variation of N,B-CDs solution fluorescence intensity was studied in Fig.S1.As we could see that the fluo‐rescence intensity of the system reduced to the mini‐mum within 20 second after the PA was added,and re‐mained basically stable for longer time.Therefore,2 minutes was chosen to be the optimal reaction time for N,B-CDs and PA systems.
The selectivity of synthetic N,B-CDs to PA was evaluated by investigating the effects of different in‐terfering substance on N,B-CDs fluorescence intensi‐ty.In Fig.6(a),the N,B-CDs fluorescence intensity solution was significantly reduced with the addition of PA,while the addition of other metal ions did not give rise to notable changes.Other organics were al‐so considered and did not cause significant fluores‐cence quenching(Fig.6(b)).In conclusion,it was de‐duced that PA detection by N,B-CDs was not affected by metal ions and organics,and the method had good selectivity.
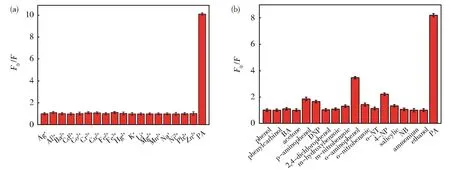
Fig.6 Influences of different(a)cations and(b)organics on the fluorescence intensity of N,B-CDs separately
3.4 Detection of PA
The correlation between PA concentration and N,B-CDs fluorescence intensity was recorded at the opti‐mum conditions of experiment.As we can see from Fig.7(a),the N,B-CDs fluorescence intensity de‐creased steadily as the PA concentration increased,ac‐companied by a slight red shift of the maximum emis‐sion peak(from 442 nm to 457 nm).According to Fig.7(b),on plotting the relative fluorescence intensity(F0/F)against the PA concentration,a good linear rela‐tionship(R2=0.992)for PA quantification was ob‐tained in the range 0.1 μmol·L-1-24.7 μmol·L-1.The linear fitting equation was F0/F=1.009+0.045[PA](μmol·L-1),and the corresponding LOD was 0.019 μmol·L-1(S/N=3).It could be seen that the concen‐tration of PA can be quantitatively determined within a certain range according to the obtained linear regres‐sion equation.
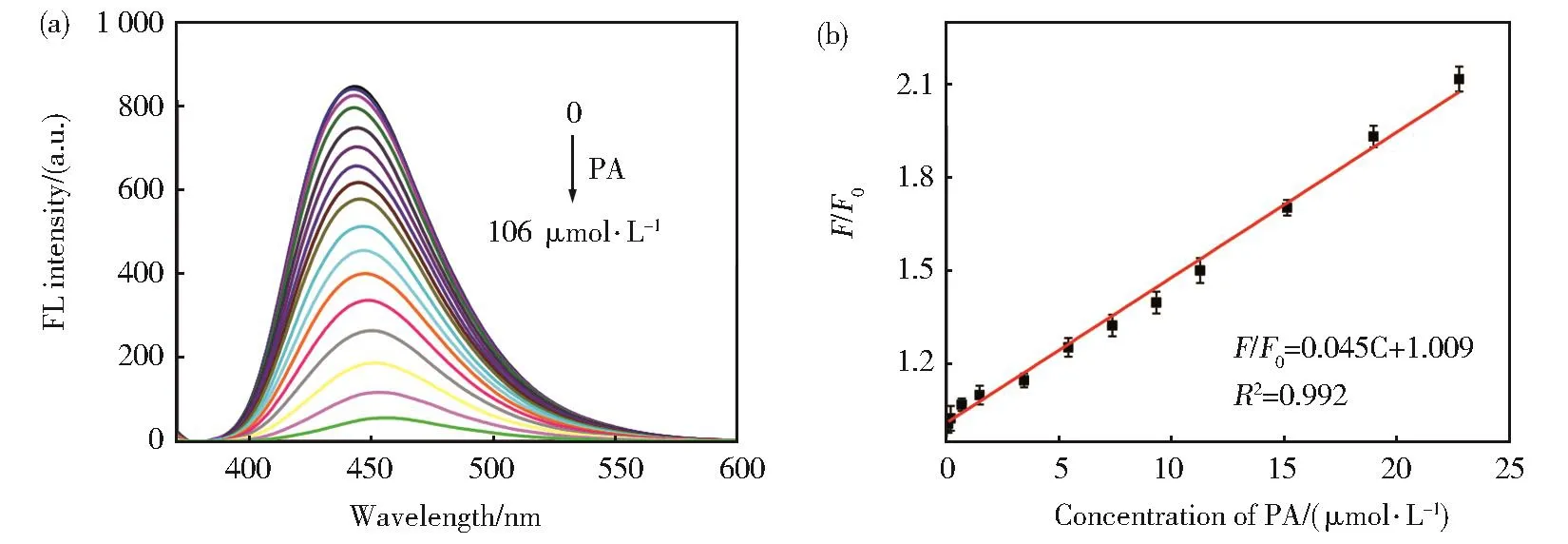
Fig.7 (a)N,B-CDs fluorescence intensity was recorded after adding different concentrations of PA(0.00,0.099,0.700,0.990,1.48,3.46,5.42,7.38,9.33,11.3,15.1,19.0,22.8,29.5,39.1,53.5,72.6,106 μmol·L-1).(b)Plots of the relative fluorescence intensity(F0/F)against the PA concentration
3.5 Reaction Mechanism
To further speculate the mechanism,the fluores‐cence lifetime and the relevant UV-visible absorption spectra were studied.The UV-vis absorption spec‐trum of PA had a clear overlap with the fluorescence emission and excitation peak of N,B-CDs in Fig.8,which indicated that IFE effect existed.The UV ab‐sorption spectrum of N,B-CDs did not show a new ab‐sorption peak after the addition of PA from Fig.9(a),indicating that there was no formation of a non-fluo‐rescent ground state complex[30].The average fluores‐cence lifetime of N,B-CDs by adding 50 μmol·L-1PA(14.28 ns)and 100 μmol·L-1(13.07 ns)was shorter than that without PA(14.93 ns)from Fig.9(b),which indicated that the fluorescence quenching of N,B-CD caused by PA may be due to the fluorescence resonance energy transfer(FRET).These results were consistent with previous literature reports[10].The possible reason was that N,B-CDs acted as elec‐tron donors,and PA acted as an electron acceptor,causing energy transfer.Therefore,PA induced N,BCD fluorescence quenching can be attributed to two possible procedures:IFE and FRET.

Fig.8 UV-vis absorption spectrogram of PA and the N,B-CDs fluorescence spectrogram

Fig.9 (a)Absorption of N,B-CDs,PA and PA+N,B-CDs.(b)Fluorescence lifetime curve of the N,B-CDs under different concentrations of PA
It should be noted that the structures and proper‐ties of nitroaromatic compounds are very similar.Ta‐ble S1 lists the information of the compound's struc‐tures similar to PA and some small molecular organ‐ics,such as p-aminophenol(p-AP),m-hydroxybenzo‐ic acid(m-HBA),2,4-dinitrophenol(DNP),2,4-di‐chlorophenol,o-nitrobenzoic acid(o-NBA),4-nitro‐phenol(4-NP),m-nitrobenzoic acid(m-NBA),phenol(PHE),o-aminophenol(o-AP),etc.These com‐pounds with similar structures to PA often interfere in the selectivity of PA determination.Therefore,the ab‐sorption spectra of these structurally similar com‐pounds were determined.It can be found from the Fig.10 that the UV-visible spectra of several com‐pounds in the range of 200 nm-600 nm were differ‐ent.Although the spectra of p-AP,o-AP,DNP,4-NP and PA had obvious absorption bands after 400 nm,which overlapped the fluorescence emission peaks of N,B-CDs(400 nm-600 nm),they had particularly small interference and did not affect the detection of PA by N,B-CDs.The rest similar structure com‐pounds did not interfere in the selectivity of the PA de‐tection for the UV absorption bands did not overlap the fluorescence emission peaks at all.The results above were consistent with the observations in Fig.6.

Fig.10 Fluorescence spectra N,B-CDs and UV absorption of other different analytes.(a)DNP,2,4-dichlorophenol,4-NP,PA,PHE,p-AP,m-HBA,m-NBA,o-AP,o-NBA.(b)Phenylcarbinol,BA,acetone,o-NT,SA,NB,AN,PA,acetic acid
3.6 Comparison of PA Methods
Using N,B-CDs as a fluorescent probe for detect‐ing PA,we comprehensively compared the water-solu‐ble N,B-CDs from literatures by aspects of sensitivity and selectivity.Carbon source,mechanism,linear range,LOD and QY,the specific results were dis‐played in Table S2.The comparison in the table showed that LOD of our synthesized N,B-CDs was relatively low and the linear range was wide.Es‐pecially compared with our previously synthesized N-CDs[14],the linear range of N,B-CDs was 0.1 μmol·L-1-24.7 μmol·L-1which was better than the previously linear range(0.5 μmol·L-1-30 μmol·L-1),and the LOD of N,B-CDs was 0.019 μmol·L-1which was significantly lower than the 0.041 μmol·L-1of the N-CDs.From the QY aspect,the QY of the synthe‐sized N,B-CDs was higher up to 75.6% which was ob‐viously higher than the previous N-CDs(41.4%).Ascan be seen from the table, the co-doping by N and B sig‐nificantly improved QY of CDs,resulting in a lower LOD and a wider linear range,which showed obvious progress and advantages compared with the reported methods and our previous study.

Table 1 Application of PA determination in real water samples
3.7 Actual sample detection
The PA of tap water samples was tested to assess the reliability of the designed detection method.Be‐cause the presence of PA was not detected in real wa‐ter samples with the prepared N,B-CDs,the standard sample recoveries were carried on the samples.The re‐sults were shown in Table 1,recoveries of the known amounts of PA in water samples were obtained in the range of 95.80%-103.10% and the relative stan‐dard deviations(RSD)were lower than 3.05%.All of results suggested that the proposed strategy was poten‐tially applicable for PA detection in practical samples.
4 Conclusion
In this paper,a simple and economical hydrother‐mal process was utilized to synthesize N,B-CDs by employing CA as carbon source,sodium tetraphenyl‐borate as B source and ethylenediamine as N source,and the higher QY of 75.63% was got.The N,B-CDs successfully detected PA with high selectivity and sen‐sitivity,and a great linear relationship for PA quantifi‐cation was obtained,ranging from 0.1 μmol·L-1to 24.7 μmol·L-1(LOD was 0.019 μmol·L-1,S/N=3).From the quantitative measurement of PA concen‐tration in the actual water sample of tap water,the re‐covery rate was between 95.80% and 103.10%(RSD was 1.53%-3.05%),which proved the practical application of synthetic N,B-CDs.Furthermore,the fluorescence quenching between N,B-CDs and PA can be attributed to two mechanisms:FRET,which N,B-CDs acted as electron donors and PA acted as an electron acceptor,and IFE.Co-doping CDs showed obvious progress and advantages for detection PA by a significantly higher QY and a lower LOD compared with the reported methods and our previous study.

