In situ Autogenous Magnetic Resonance Imaging and Thermal Imaging of Cancer Using Hydrogen Peroxide Activated Nanoparticles
JIA Xiao-dan,WANG Yue*,JIANG Xiu-e,2*
(1.State Key Laboratory of Electroanalytical Chemistry,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China;2.University of Science and Technology of China,Hefei 230026,China)
Abstract:Early accurate detection of malignancy tumor plays a key role in reducing cancer mortality.Current detection strategies mainly rely on the imaging technologies.However,single-modality biomedical image cannot provide enough information due to its inherent defects.Therefore,the dual-modal biomedical imaging strategies which can provide more available information for precise cancer diagnosing,have become a hotspot for research.Among the biomedical imaging methods applied in clinical,magnetic resonance imaging(MRI)is considered to be an effective method for clinicopathological diagnosis due to its high three-dimensional(3D)spatial resolution and high soft tissue resolution,but it has also been plagued by the long inspection time and expensive inspection fee.Whereas,thermal imaging is an efficient and minimally invasive method for biomedical imaging,which could offset the above deficiencies of MRI.Most importantly,it is convenient and economical.Furthermore,the development of multifunctional nanoparticles brings more possibilities for the establishment of novel tumor diagnostic platforms,which is expected to have a greater impact in the field of biomedical imaging.Thus,a kind of highly biocompatible multifunctional nanoparticles(CuSSe NPs)were prepared by a facile one-pot hydrothermal method for tumor MRI/thermal imaging in this study.The diameter of Cu SSe NPs is about 100 nm,and it exhibited an excellent dispersity in aqueous solution.However,the CuSSe NPs did not exhibited the best MRI capability due to the existence of diamagnetic Cu(Ⅰ),but i n v i v o experiments demonstrated that the CuSSe NPs could specifically respond to the excess hydrogen peroxide(H2O2)at the tumor site so as to realize the conversion of diamagnetic Cu(Ⅰ)to paramagnetic Cu(Ⅱ),acting as in situ autogenous MRI agents for visualizing and diagnosing the location of cancer.Moreover,attributing to the intensive absorption in the second near-infrared(NIR-Ⅱ)region,CuSSe NPs exhibited as a concentration-and laser-power-dependent photothermal imaging agent with considerable photothermal conversion effects in the NIR-Ⅱregion.The photothermal conversion efficiency of CuSSe NPs was calculated to be-48%under the illumination of NIR-Ⅱ laser(1 064 nm).Both i n v i v o and i n v i v o experiments demonstrated that CuSSe NPs have good thermal imaging capabilities for real-time cancer monitoring.Furthermore,the blood analysis on the mice injected with or without CuSSe NPs were evaluated after 3 and 25 days.The results showed that CuSSe NPs were highly biocompatible without acute and long-term toxicity.Consequently,the constructed copper-based multifunctional NPs have been demonstrated to be a potential nanoprobe for dual-modal imaging with high tumor contrast in clinical cancer theranostics.
Key words:H2O2-activatable;in situ autogenous MRI;thermal imaging;NIR-Ⅱregion;cancer diagnosis
Cancer is still one of the most destructive diseases with high mortality to humanity,and researchers have made great efforts to develop accurate,efficient and rapid cancer diagnosis strategies for more effective treatment[1].Many imaging methods have been explored,such as positron emission tomography(PET),fluorescence imaging,magnetic resonance imaging(MRI),photoacoustic imaging(PAI)and X-ray computed tomography(CT).However,no one modality can offers a perfect imaging,and each has its own shortcomings.Since the conception of precision medicine was proposed,the multifunctional nanosystem which integrates multiple imaging modes has become a"star"in cancer theranostics[2-3].It can detect and track the occurrence and development of lesions in real time,thereby improving the accuracy of targeted therapy and providing a plan for subsequent treatment.Obviously,exploring a simple,intuitive and highly biocompatible multifunctional nanoimaging probe remains in high demand in cancer treatment[4-8].
Among the above mentioned diagnostic imaging methods,MRI is considered to be an effective method for clinical pathological diagnosis with its merits of 3D spatial resolution and high soft tissue resolution[9-10].Through shortening the relaxation time of water protons in imaging tissue area,the exogenous contrast agents can further improve imaging sensitivity[11].Some contrast agents have been reported,such as superparamagnetic iron oxide nanoparticles[12-13],FeCo/GC nanocrystal[14]and complex of single-crystal iron oxide nanoparticles and transferrin(Tf-MION)[15].Owing to the signal are always detectable both in tumor and normal tissue,most of the reported MRI contrast agents show bad target-to-background ratios.Therefore,exploring MRI contrast agents which can selectively enhance the imaging signal of tumor site in response to the tumor microenvironment(TME)will be a promising strategy.Due to the 3D orbitals of Cu(Ⅰ)are completely filled,Cu(Ⅰ)-based materials exhibit diamagnetism that can’t be employed to MRI contrast agents.However,they can be oxidized by excess hydrogen peroxide(H2O2)in TME,and the obtained Cu(Ⅱ)has good T1-MRI capability because of its paramagnetic[16-18].Therefore,the Cu(Ⅰ)-based nanoparticles can be the candidates for in situ autogenous MRI.Nevertheless,the long inspection time and expensive inspection fee of MRI may hinder its application in accurate cancer diagnosis[19].Multi-modal imaging that merges several imaging methods can integrate the advantages of each imaging technology while avoiding their defects,thus becoming an ideal demand[20-21].
Attributing to the outstanding tissue penetration of near-infrared(NIR)light,NIR photothermal nanoparticles have received enough attention in cancer theranostics[22-24].It can convert NIR irradiation to heat at the tumor site to produce mild high temperature,and the radiant energy can be recorded by an infrared thermal imager to form a thermal image for real-time monitoring the treatment.Thermal imaging is an efficient and minimally invasive biological imaging method that can continuously monitor the real-time temperature changes of the lesion during cancer treatment,and provide an intuitive data basis for experimental research,analysis and evaluation. It is convenient,fast,sensitive and economical,which can complement MRI well.
In this study,we synthesized the CuSSe NPs dual-modal probe through one-pot hydrothermal method for cancer bioimaging(Fig.1).We used different techniques to comprehensively characterize the structure,morphology,composition,imaging performance and biocompatibility of the synthesized CuSSe NPs.Firstly,Cu(Ⅰ)in CuSSe NPs can be oxidized by excess H2O2in the TME to generate paramagnetic Cu(Ⅱ),which can be applied to the in situ autogenous MRI contrast agent and improve the signal ratio of tumor tissue.Secondly,because of the intensive absorption in the NIR-Ⅱregion of the CuSSe NPs,it shows considerable photothermal conversion performance,and has excellent photothermal imaging capability for real-time monitoring cancer treatment.We have systematically studied the application of this functional nanoplatform in dual-modal MRI/thermal imaging of biology.
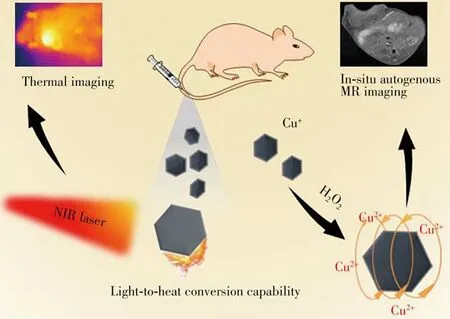
Fig.1 Sketch map of the H2O2-activatable CuSSe NPs for in situ autogenous MRI and thermal imaging of cancer
1 Experimental
1.1 Materials
Sodium hydroxide(NaOH)and copper sulfate pentahydrate(CuSO4·5H2O)were received from Xilong Science Co.,Ltd.,(Guangdong,China).Ascorbic acid(Vc),D-glucose,30%DL-tartaric acid anhydrous and thiourea were all obtained from Beijing Chemical Plant Co.,Ltd.,(Beijing,China).Selenium dioxide(SeO2)and polyvinylpyrrolidone(PVP,MW58 kDa)were all acquired from Sigma Aldrich Chemical Co.,Ltd.,(America).Ultra-pure water was used in the process of all the experiments.All chemical reagents were used directly after purchase without any purification.
1.2 Instruments
The morphology of nanoparticles was observed by transmission electronic microscope(TEM,H-600 electron microscope,Hitachi,Japan)and scanning electron microscope(SEM,S-4800 FE-SEM system,Hitachi,Japan).The dynamic laser scattering(DLS)data was obtained from ZEN3690 Zetasizer(Malvern,UK).We used the inductively coupled plasma atomic emission spectroscopy(ICP-AES,Thermo Fisher Scientific,USA)to determine the exact concentrations of metallic elements.X-ray photoelectron spectra(XPS)was measured through ESCALab220i-XL electron spectrometer(VG Scientific,UK).Powder X-ray diffraction(XRD)spectrum was gained by a D8 ADVANCE X-ray diffractometer.
1.3 Synthesis of CuSSe NPs
We synthesized CuSSe NPs by using a simple one-pot hydrothermal method and strictly managing reaction time and temperature.The specific experimental steps are as follows:30%DL-tartaric acid anhydrous(0.8 mmol),PVP(0.016 6 mmol)and CuSO4(0.2 mmol)were dissolved in 160 mL ultra-pure water and stirred in an oil bath at 50℃.Then NaOH(4.4 mmol)was added to the above solution after 5 minutes and the color of the solution became light blue in an instant.Five minutes later,Vc(0.8 mmol)andD-glucose(0.2 mmol)were added to the above solution quickly,and the light blue aqueous solution quickly formed the light-yellow turbid liquid.After 5 minutes,quickly add thiourea(0.116 mmol)and SeO2(0.123 mmol)to the system,and then the reaction temperature was quickly adjusted to 120℃,and stirred about 10 minutes.Later,Vc(0.48 mmol)was added into the above solution and reacted for 6 hours.Finally,the products were collected through centrifugal washing.
1.4 Photothermal properties of CuSSe NPs
The photothermal performance of CuSSe NPs was examined in this way:1 mL of CuSSe NPs solutions with different concentrations([CuSSe NPs]=0,200,300,400,500μg·mL-1)was put into a 3 mL quartz cell and then irradiated upon the 1 064 nm laser(1.0 W·cm-2)for 10 minutes.We recorded the temperature state of the solution every 30 seconds with a digital thermometer.As for the thermal performance under different power,1 mL of CuSSe NPs solutions at the concentration of 500μg·mL-1was put into the quartz cell and then irradiated upon a 1 064 nm laser under different power(0.25,0.50,0.75,1.0 W·cm-2)for 10 minutes.The temperature state of the solution was also recorded by a digital thermometer every 30 seconds.
The photothermal conversion efficiency(η)of CuSSe NPs was worked out according to the method established in the former report[25].Briefly,1 mL of CuSSe NPs solution in quartz cell was irradiated with a 1 064 nm laser for 10 minutes.After turning off the laser,the temperature change was recorded during the cooling of the solution.
According to the following formula,we could obtain the value ofη:

h S(mW·℃-1):heat passing through the surface of the container per unit time;Ts(℃):the temperature of solution after heating for 10 minutes;Tr(℃):room temperature;QDis(mW):the heat absorbed by the container wall from the laser;I(mW):laser power density;A1064:the absorbance of CuSSe NPs at 1 064 nm.
The photothermal stability of CuSSe NPs was evaluated by four laser switch cycles.In brief,1 mL of CuSSe NPs solution was irradiated upon the 1 064 nm laser for 10 minutes;then the laser was turned off.This operation was repeated four times.
1.5 Animal model
All animal experiments complied with the regulations of the Animal Ethics Committee of the First Hospital of Jilin University(SYXK(Ji)20180006).We established the xenograft uterine cervical cancer cells(U14)tumor models on female Kunming mice(weighing 20-25 g,6 weeks).100μL of U14 cells dispersed in normal saline was subcutaneously injection in the right axilla area of the mice.When the tumor grew to around 250 mm3,the mice could be used for tumor imaging.
1.6 Thermal imaging of CuSSe NPs
The tumor-bearing mice were administrated with PBS and CuSSe NPs solution(20 mg·kg-1)via tail vein.At 8 h and 12 h after injection,we used infrared thermal imaging camera to record the thermal imaging under irradiation of 1 064 nm laser at 1.0 W·cm-2.
1.7 MRI of CuSSe NPs
As fori n vit roMRI,different concentrations of CuSSe NPs aqueous solutions(0.4,0.5,0.8,1.0,1.25,1.6 mol/L)were used to acquire MR imaging by MRI scanner.Thein vivoMR imaging was performed by 1 T MRI scanner at different intervals(0,8,12 h)after injection of CuSSe NPs into tumor-bearing mice intratumor or via tail vein.
1.8 Examinations of in vivo blood parameters
After mice injected with CuSSe NPs(20 mg·kg-1)for 3 and 25 days,the blood drawn from the eye socket was collected.The healthy mice without any treatment were used as control.Then,the blood parameters were measured by an automatic blood analyzer.
2 Results and discussion
2.1 Characterization of CuSSe NPs
The preparation of CuSSe NPs is shown in Fig.2.The uniform CuSSe NPs were synthesized by a hydrothermal method.The TEM image and SEM image showed that the synthesized CuSSe NPs were monodispersed,and the diameter was about 100 nm(Fig.3A-C).Energy-dispersive spectrometry(EDS)spectrum demonstrated the existence of the elements Cu,Se,S,C and O(Fig.3D).The powder X-ray diffraction(XRD)spectrum confirmed that the as-synthesized CuSSe NPs were bulk cubic structure and the positions of the diffraction peaks were close to those of the binary bulk cubic Cu2S(JCPDS No.84-1770)and Cu1.8Se(JCPDS No.88-2046)[26].As shown in Fig.3E,they only shift to the lower 2θvery slightly and the profile resembles the diffraction peak of a binary compound.Moreover,the strong and sharp diffraction peaks in the XRD spectrum also verified that CuSSe NPs possess good crystallinity.The elements of Cu 2p,S 2p,Se 3d,C 1s and O 1s in CuSSe NPs were characterized by X-ray photoelectron spectra(XPS)(Fig.3F-I).The presence of C 1s and O 1s were mainly assigned to PVP.The existence of Cu 2p3/2peak at 931.9 eV and Cu 2p1/2peak at 951.7 eV revealed the presence of Cu+[27-29].The binding energy of the S 2p at 160.38 eV indicating the existence of S2-in the CuSSe NPs[30-31].Similarly,the Se 3d peak at 54.09 eV was assigned to Se2-[32].These results proved the successful synthesis of CuSSe NPs.

Fig.2 Schematic diagram of the preparation of CuSSe NPs
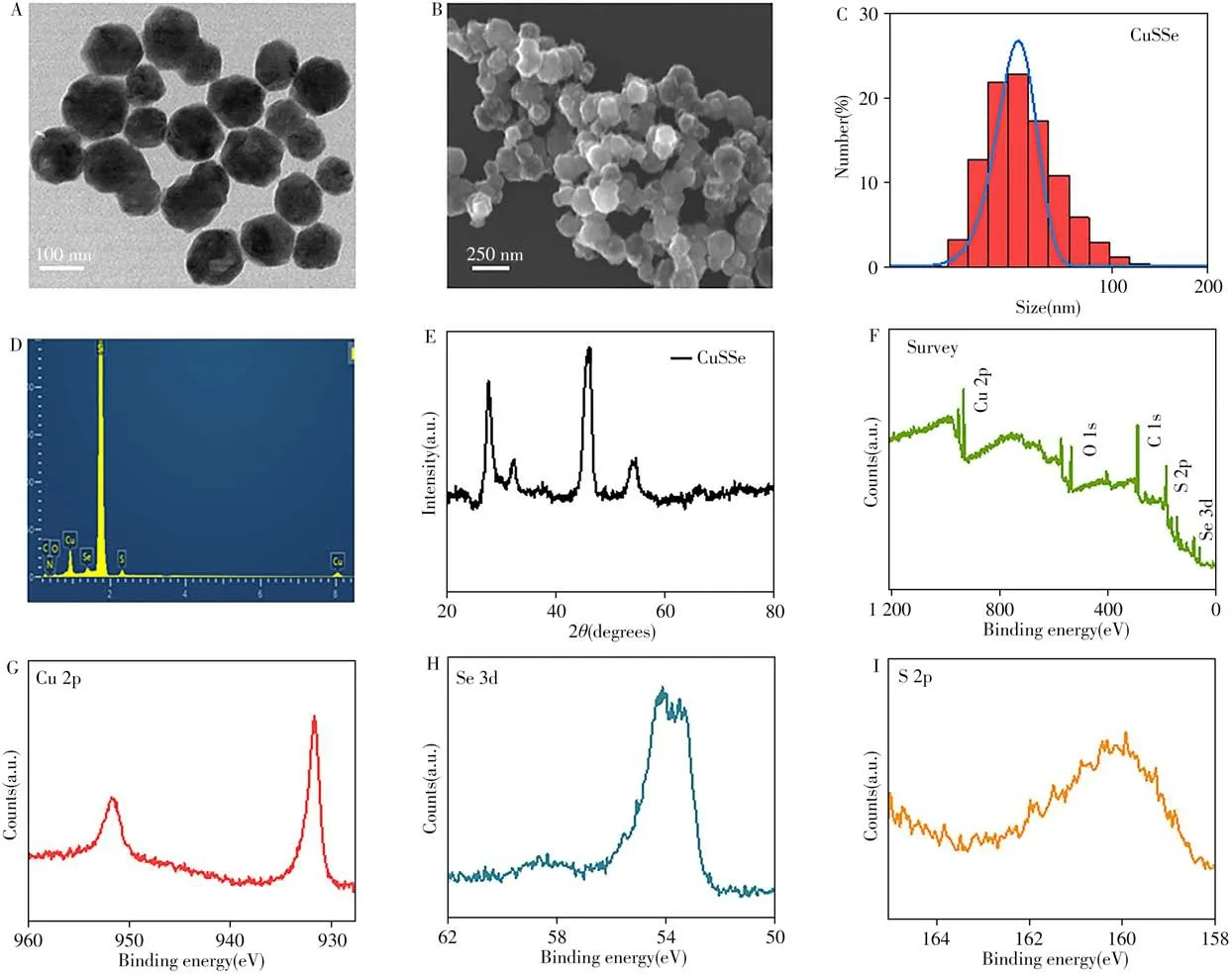
Fig.3 Characterization results of CuSSe NPs
2.2 Photothermal properties of CuSSe NPs
The NIR light absorption feature renders the CuSSe NPs with thermal imaging property.We firstly studied its light-to-heat conversion ability.The temperature changes of water,and CuSSe NPs solution at different concentrations(200-500μg·mL-1)and power(0.25-1.0 W·cm-2)upon the 1 064 nm laser were monitored by a digital thermometer for 10 minutes.As shown in Fig.4A and 4B,we found that the photothermal performance of CuSSe NPs depended on the concentration and laser-power.The temperature of the CuSSe NPs(500μg·mL-1)solution could increase to 62.3℃within 10 minutes upon the laser irradiation at 1.0 W·cm-2.Further,the photothermal effect of CuSSe NPs solution was unaffected after four times of on/off irradiation cycles,demonstrating the good photothermal stability of CuSSe NPs(Fig.4C).Theηof the CuSSe NPs was measured upon 1 064 nm laser irradiation(1.0 W·cm-2).The CuSSe NPs solution(500μg·mL-1)was irradiated for 10 minutes,and then turned off the laser.The steep cooling curve of the CuSSe NPs solution suggested the excellent light-to-heat conversion performance of the CuSSe NPs,which was shown in Fig.4D.According to the previously reported method[25],theηof CuSSe NPs was calculated to be-48%.Then we further studied thein vitrothermal imaging capability of CuSSe NPs.The temperature of CuSSe NPs solution irradiated under 1 064 nm laser at different power was recorded every two minutes by infrared thermal imaging camera,and the excellenti n vi t rothermal imaging capability of CuSSe NPs was observed in Fig.4E.
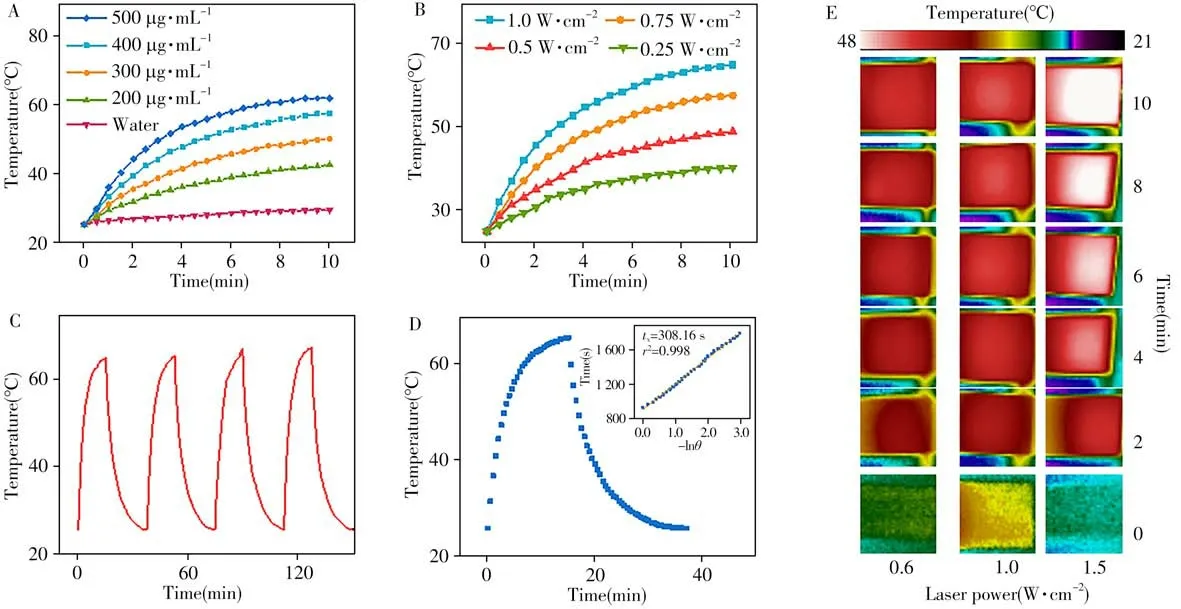
Fig.4 Photothermal properties of CuSSe NPs
2.3 I n vivo photothermal imaging
Encouraged by the excellent photothermal performance of CuSSe NPsi n vitro,thein vivothermal imaging of CuSSe NPs was further explored.A thermal imager was applied to monitor the immediate temperature of tumor area after injection of CuSSe NPs for 0 h,8 h and 12 h.As shown in Fig.5,the accumulating ratio of CuSSe NPs in the tumor site reached the highest at 8 h,which helped us to better grasp the time point of MRI and optimize the dosage of CuSSe NPs.
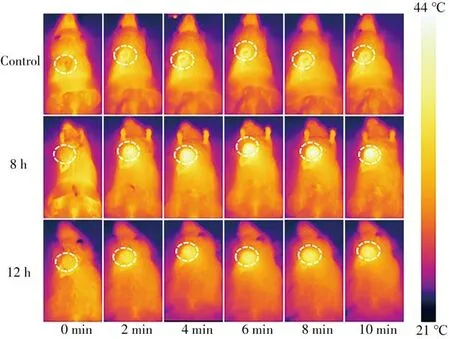
Fig.5 In vivo photothermal imaging of CuSSe NPs
2.4 MRI properties of CuSSe NPs
Furthermore,MRI scanner was used to evaluate the capacity of the CuSSe NPs as a contrast agent for T1-weighted MRI.Surprisingly,although the T1-MRI signal could not be controlled by varying the Cu concentration in CuSSe NPs solution,the MRI signal became significantly stronger upon H2O2treatment(Fig.6A).This is mainly due to the 3d orbitals of Cu(Ⅰ)are completely filled(3d orbitals:3d104s0),and diamagnetic Cu(Ⅰ)has no imaging capability.Once exposed to the high H2O2concentration in TME,it could be oxidized to the paramagnetic Cu(Ⅱ)(3d orbitals:3d9),the oscillating satellite peaks in the 939-945 eV region[33]in XPS spectrum indeed indicated the presence of Cu(Ⅱ)in the CuSSe NPs after H2O2treatment(Fig.6B).Due to the valence state transition characteristics of the Cu(Ⅰ),CuSSe NPs could be used as a TME responsive T1-MRI contrast agent.By plotting the relationship between the MRI signals value and the concentrations of CuSSe NPs,longitudinal relaxation rate(r1)was calculated to be 0.7 mmol·L-1·s-1(Fig.6C).Next,the in situ autogenous MRI capability of CuSSe NPs was proved throughin vi voexperiments.As exhibited in Fig.6D-E,contrasted with the no signal of control group,the T1-MRI signal of the tumor was significantly increased after the intratumor injection or tail vein injection.Moreover,the signal reached its maximum at 8 h,which corresponding to thermal imaging(Fig.6F).These results indicated that CuSSe NPs could be applied to in situ autogenous MRI with enhanced MRI signal of target site,which also offer a new idea for other variable valence elements-based contrast agents.
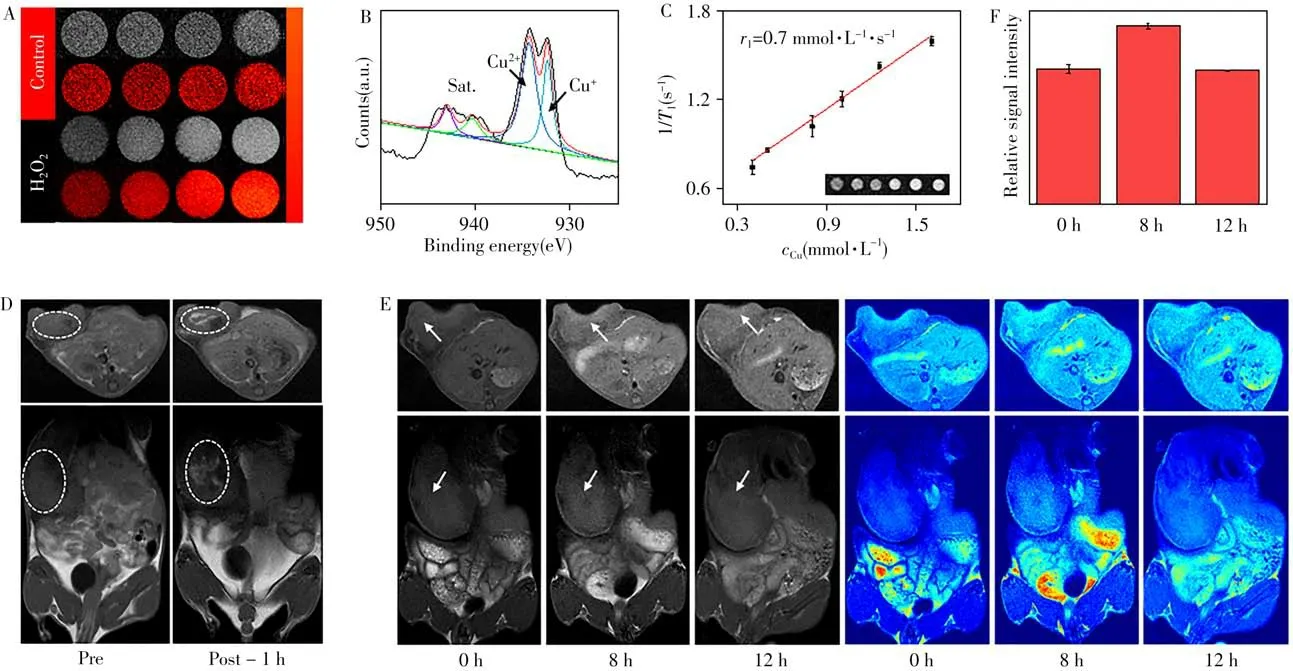
Fig.6 MRI characteristics of the CuSSe NPs
2.5 Biocompatibility of CuSSe NPs
Encouraged by the above excellent bioimaging performance,the biological safety of CuSSe NPs was also studied.The blood was collected from mice administrated with CuSSe NPs or the control group at the 3rdand 25thday.The blood analysis parameters showed that no obvious differences between control group and CuSSe NPs group(Fig.7).These results proved that the CuSSe NPs were highly biocompatible without acute and long-term toxicity.

Fig.7 Liver function and renal function indexes of the mice after intravenous injection of CuSSe NPs at the 3rd day(A),25th day(B)and the mice in control group
3 Conclusions
In summary,the highly biocompatible Cu(Ⅰ)-based contrast agent(CuSSe NPs)for MRI/thermal imaging of tumor was developed for the first time.The results demonstrated that CuSSe NPs were able to generate Cu(Ⅱ)with paramagnetic in response to high concentration of H2O2in tumor site,which made it a promising T1contrast agent for in situ autogenous MRI.Furthermore,CuSSe NPs were a concentrationdependent photothermal imaging agent with considerable photothermal conversion effects in the NIR-Ⅱregion,thus it could be employed to monitor the cancer treatment in real-time.Taken together,a uniquely multifunctional nanoplatform was constructed to realize effective dual-modal imaging of tumor.We believe these observations could bring new thinking and directions for the development of new muti-modal imaging contrast agents for in situ real-time diagnosis of tumor.
- 分析测试学报的其它文章
- 阿尔兹海默症生物标志物和早期诊断新技术
- 40周年刊庆 引 言
- 微管纸喷雾质谱法快速筛查血液中5种强极性毒物
- 基于核酸分子光开关的闭管可视化环介导等温扩增检测方法
- Detection of Broad Spectrum Bacteria Using a FITC-Lysozyme and Positively Charged AuNPs Constructed FRET Platform
- Detection of Biomarker Protein PDGF-BB in Esophageal Squamous Cell Carcinoma Using a DNA Biosensor Based on Enzyme Cycle Amplification

