Time-course monitoring of in vitro biotransformation reaction via solid-phase microextraction-ambient mass spectrometry approaches
Karol Jaroch,Janusz Pawliszyn
a Department of Chemistry,University of Waterloo,Waterloo,N2L 3G1,Canada
bDepartment of Pharmacodynamics and Molecular Pharmacology,Faculty of Pharmacy,Collegium Medicum in Bydgoszcz,Nicolaus Copernicus University in Torun′ Poland,85090,Bydgoszcz,Poland
Keywords:
Solid-phase microextraction
Coated probe electrospray ionization
Coated blade spray
Combretastatin
Biotransformation
Prodrug activation
A B S T R A C T
The solid-phase microextraction technique quantifies analytes without considerably affecting the sample composition.Herein,a proof-of-concept study was conducted to demonstrate the use of coated probe electrospray ionization(coated-PESI)and coated blade spray(CBS)as ambient mass spectrometry approaches for monitoring drug biotransformation.The ability of these methods was investigated for monitoring the dephosphorylation of a prodrug,combretastatin A4 phosphate(CA4P),into its active form,combretastatin A4(CA4),in a cell culture medium supplemented with fetal bovine serum.The CBS spot analysis was modified to achieve the same extraction efficiency as protein precipitation and obtained results in 7 min.Because coated-PESI performs extraction without consuming any samples,it is the preferred technique in the case of a limited sample volume.Although coated-PESI only extracts small quantities of analytes,it uses the desorption solvent volume of 5-10 pL,resulting in high sensitivity,thus allowing the detection of compounds after only 1 min of extraction.The biotransformation of CA4P into CA4 via phosphatases occurs within the simple matrix,and the proposed sample preparation techniques are suitable for monitoring the biotransformation.
1.Introduction
Drug development processes are often challenged by the poor solubility of newly synthesized chemicals in water.This issue can be effectively resolved using a phosphate group to modify the chemical moiety of these compounds via esterification because phosphate bonds can be easily cleaved by enzymes(i.e.,phosphatases),which are abundant in living organisms[1].
The present study aims to monitor the biotransformation of a prodrug,combretastatin A4 phosphate(CA4P)(Fig.1A),into its active form,combretastatin A4(CA4)(Fig.1B),in a cell culture medium comprising the Dulbecco's modified Eagle's medium(DMEM)supplemented with 10% fetal bovine serum(FBS).This simple matrix was selected based on previous findings,where only CA4 was observed after administering CA4P into the cell culture of non-small cell lung cancer(A549),even within a medium control group where no cells were seeded[2].Thus,phosphate-bond cleavage was caused by FBS.A subsequent human-based clinical trial showed that the half-life of CA4P ranges from 0.084 to 2.12 h,with a mean value of 0.47 h[3].
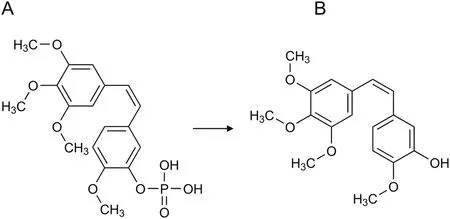
Fig.1.Biotransformation of(A)combretastatin A4 phosphate(CA4P)to(B)combretastatin A4(CA4).
Solid-phase microextraction(SPME)is based on establishing an equilibrium state between the concentration of the target analyte(s)in a sample and the extraction phase in the device[4,5].By optimizing the type and dimensions(i.e.,length and thickness)of the coating,SPME can be modified to perform nonexhaustive extraction,where only a negligible portion of the target compound(s)is removed from the sample.Furthermore,SPME,as a nonexhaustive,nondestructive,and minimally invasive sample preparation technique,allows multiple sequential extractions from the same sample over time,thus facilitating the time profiling of biochemical trends.This eliminates the need for large numbers of sampling in parallel,which considerably decreases the overall test costs[6].
Ambient mass spectrometry(AMS)eliminates the need for analyte separation(i.e.,by liquid or gas chromatography)and sample preparation,enabling samples to be directly introduced into the mass spectrometer.Despite these advantages,AMS is very “dirty”because interferences are introduced into instrumentation,leading to a need of frequent maintenance,is difficult to deploy on-site,and cannot yield a high throughput.To address these limitations,SPME has been introduced to supplement ambient approaches.To date,researchers have developed several notable approaches that directly couple SPME devices with analytical instrumentation,including direct analysis in real time,coated blade spray(CBS),a microfluidic open interface,and desorption electrospray ionization[5,7].Although such SPME-AMS employs similar extraction mechanisms,their ionization and detection mechanisms differ.For example,in CBS,a stainless steel blade coated with a thin layer of the extraction phase is first wetted with a desorption solution(10μL)and a strong electrical field is then applied to the tip of the blade to achieve electrospray ionization(ESI).Thereafter,ESI initiates desolvation,producing gaseous ions that are directly transferred to a mass spectrometer[8].In the classical application of probe ESI(PESI),a liquid sample is collected and introduced to the mass spectrometer using a needle.However,sample preparation is required to effectively quantify the components in biofluids.In traditional PESI combining of a sample with an organic solvent is the most commonly used approach[9].In our modified PESI approach(i.e.,coated-PESI),the probe is first coated with a sorbent to enable sampling via SPME.The coated probe is then placed in front of the mass spectrometer,and the desorption solution is collected from the reservoir.Thereafter,the probe rapidly moves to the ionization position where a high voltage is applied,enabling the simultaneous occurrence of electrospraying and desorption processes[10,11].Coated-PESI uses small probes and minimal quantities of the desorption solvent,facilitating multiple extractions from the same plate with no substantial target analyte depletion.Moreover,its high enrichment factor enhances sensitivity.
Thus,the main study objective was to assess the abilities of CBS and coated-PESI approaches for monitoring the biotransformation of CA4P into CA4 in one of the most commonly used cell culture media,i.e.,DMEM supplemented with 10% FBS.To this end,SPME in thin-film(CBS)and tip(coated-PESI)formats was employed as extraction and ambient ionization techniques.
2.Materials and methods
2.1.SPME probe manufacturing
The probes of coated-PESI were manufactured using five layers of 1.3-μm hydrophilic-lipophilic balance(HLB)particles(coating length:2 mm).The CBS devices were manufactured using a single layer of 5-μm HLB particles and an underlayer of polyacrylonitrilebased glue(coating lengths:10 and 15 mm).A detailed information of the synthesis and coating protocols for the 1.3-μm and 5-μm HLB particles were described by previous studies[8,12,13].
2.2.Sample preparation
Before use,the coated-PESI and CBS devices were preconditioned overnight in a MeOH:water mixture(1:1,V/V).Sampling was performed in 1 mL of DMEM supplemented with 10% FBS preheated to 37°C using the following procedures:for coated-PESI,the probe was directly immersed in the sample for 1 min;for CBS,10μL of the sample was applied to the CBS device,followed by 2.5μL of acetonitrile(ACN);and for protein precipitation(PPT),10μL of the sample was used(described in detail in the subsequent text).A small amount of ACN during CBS spot analysis was added,as previous findings have demonstrated its effectiveness in disrupting the protein binding of drugs[8],and CBS spot extraction was performed for 5 min.Before and after the extraction,the coated-PESI and CBS devices were immersed in liquid chromatography-mass spectrometry(LC-MS)-grade water for 5 s to remove any residual organic solvent or nonspecifically bonded matrix component,respectively.The PPT protocol was adopted from the literature[14].Briefly,10μL of the sample was transferred to an Eppendorf tube prefilled with 10μL of cold ACN and then vortexed.Thereafter,70μL of an ACN:water mixture(7:1,V/V)was added,followed by centrifugation at 4,000 r/min for 5 min.Next,the sample was transferred into an autosampler amber vial.All used sample preparation techniques consume a small portion of the sample(in fact,the required sample volume for coated-PESI is negligible and only 10μL is required for each CBS and PPT),the sampling was repeated five times using the same reaction tube,thus achieving a time-course monitoring was possible.The samples used for the time-course monitoring were obtained at 5,10,20,30,and 45 min after the medium supplemented with FBS was spiked with CA4P and internal standard(IS)and shaken for 5 s.The initial extraction rates of the SPME-based techniques were determined under the following desorption conditions:a solvent comprising the ACN:water mixture(4:1,V/V);desorption solvent volume of 100 and 250μL for coated-PESI and CBS,respectively;time of 2 h;and shaking at 1,000 r/min in an orbital shaker.
2.3.LC-MS
Chromatography separation was achieved on a Kinetex®F5 LC column(100 mm × 3.0 mm,2.6 μm,100 Å;Phenomenex,Torrance,CA,USA)using ACN+0.1% formic acid(FA)and water+0.1% FA as mobile phases B and A,respectively.The following gradient was used for chromatography:0-1 min at 10%B;1-3.3 min increase to 95%B;3.3-4.3 min of hold at 95%B;4.3-6 min of reduction back to 10%B.
2.4.AMS
For AMS,the desorption solution for coated-PESI comprised an isopropyl alcohol(IPA):water(different mixture ratios were used,see Section 3.1)mixture and 0.1% FA,whereas a solution of a MeOH:water mixture(19:1,V/V)and 0.1% FA was used for CBS.The coated-PESI run was performed in cycles of 0.5 min with sample pick-up and ionization time of 50 and 200 ms,respectively.Further,10μL of desorption solution was spiked into a conventional PESI sample plate(Figs.2A and C).For the CBS device,AMS was performed by mounting the device in front of the mass spectrometer using an in-house three-dimensional-printed adapter(Ultimaker 3,Ultimaker,Mississauga,Canada)that was connected to the contact closure with a cable(Figs.2B and D).Analysis was performed by pipetting 10μL of desorption solution onto the blades,followed by 20 s of desorption.Thereafter,the voltage was increased to 4 kV to create a spray cone.The total ion current(TIC)area for CA4P and CA4 was normalized using colchicine(Col)as an IS because it is not prone to dephosphorylation(unlike deuterated standards),and its structure is similar to that of combretastatin.Additionally,Col has been employed as an IS for CA4 and CA4P in a previous study[15].Except CA4 and CA4P,which were purchased from Toronto Research Chemicals(Toronto,Canada),all the other chemicals were of LC-MS grade and purchased from Sigma-Aldrich(Oakville,Canada).The multiple reaction monitoring transitions were as follows:Col m/z 400.1>358.2 and m/z 400.1>310.1;CA4 m/z 317.1>286.1 and m/z 317.1>131.5;and CA4P m/z 419.0>339.1 and m/z 419.0>386.0.
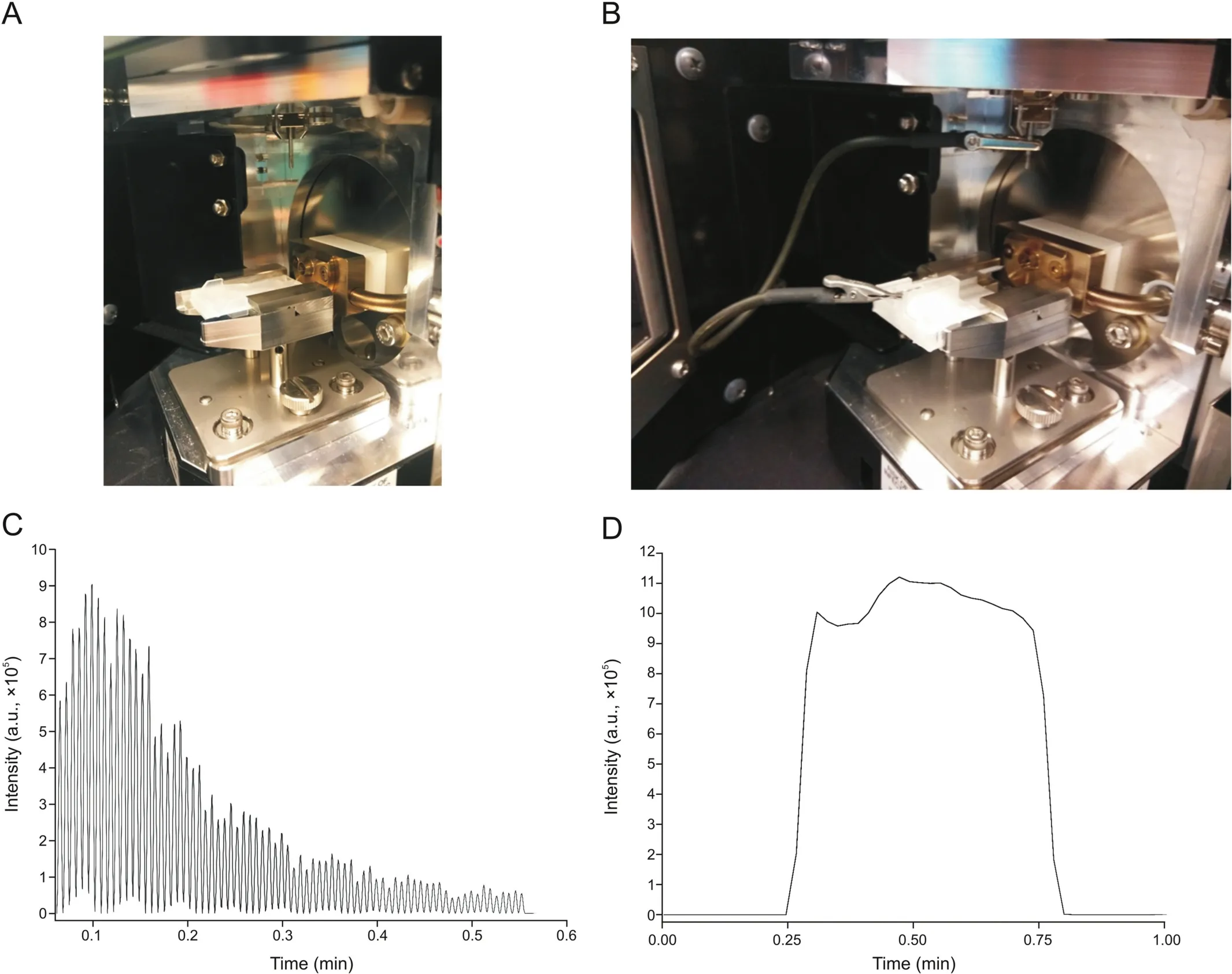
Fig.2.(A)Coated-probe electrospray ionization(coated-PESI)and(B)coated blade spray(CBS)devices,and typical views of(C)coated-PESI and(D)CBS analyses.
3.Results and discussion
3.1.Optimization of coated-PESI-AMS protocol
The desorption solution for the coated-PESI-AMS experiments was optimized by analyzing the following compositions:IPA:water mixture(1:9,V/V)and 0.1% FA,IPA:water mixture(1:3,V/V)and 0.1% FA,IPA:water mixture(1:1,V/V)and 0.1% FA,and IPA:water mixture(3:1,V/V)and 0.1% FA.Results showed that the solution comprising the IPA:water mixture(1:1,V/V)and 0.1% FA yielded the highest area counts for all tested compounds;thus,this solution was selected for subsequent analyses(Fig.3).Because the probe of coated-PESI was immersed in the solvent only for milliseconds,the desorption/ionization efficiency of coated-PESI depended not only on the desorption capacity of the selected solvent.In addition to the desorption rate,the desorption solvent should be easily lifted up by the probe of coated-PESI.Therefore,the surface tension,wettability,and viscosity of the desorption solution,as well as the target analyte solubility,should be considered[10,11].
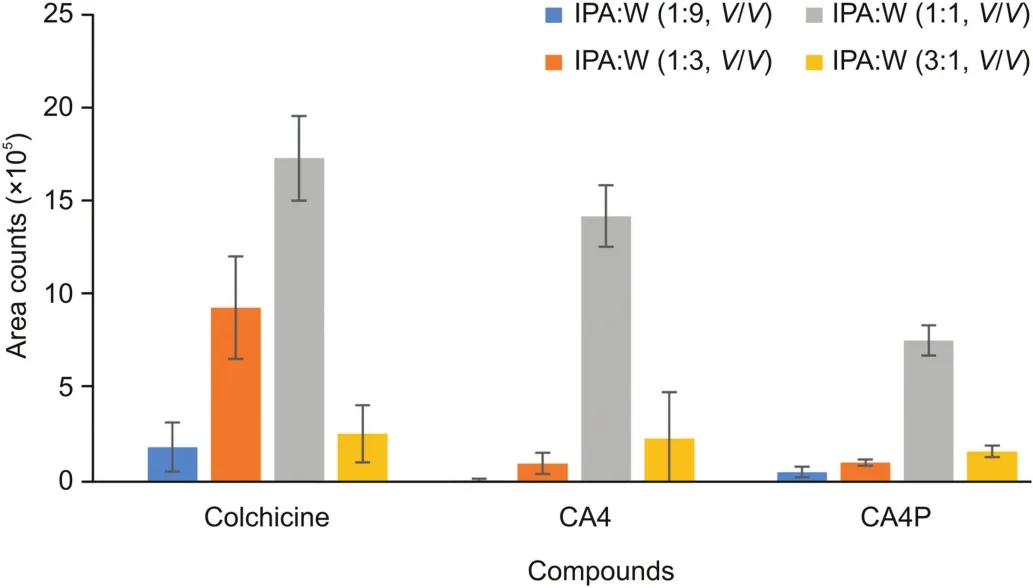
Fig.3.Optimization of the desorption solvent for coated-PESI experiments.(Blue)IPA:water(1:9,V/V)+0.1% FA;(red)IPA:water(1:3,V/V)+0.1% FA;(green)IPA:water(1:1,V/V)+0.1% FA;and(purple)IPA:water(3:1,V/V)+0.1% FA.IPA:isopropanol;FA:formic acid.Columns represent the mean values obtained from at least three replicates,and whiskers represent the standard deviations.
3.2.Optimization of CBS spot extraction
The CBS spot extraction was modified to yield the same extraction efficiency as the PPT approach.To maximize the quantity of compounds extracted using the CBS coating,spot-analysis experiments were conducted using two coating lengths(10 and 15 mm).The CBS devices were quickly rinsed with water after sampling,and the analytes were then desorbed and injected into the LC-MS device.As expected,the coating with the longer length yielded a higher response(blue and orange columns in Fig.4)because an additional amount of compounds was extracted.Note that the same matrix was sampled for 1 min using the coated-PESI device,followed by desorption in a 100-μL desorption solution using the same parameters as those used for CBS-LC.The quantity of compounds extracted using coated-PESI-LC was extremely low,and no peaks were detected.These findings confirm that this approach can extract compounds with negligible analyte depletion.Thus,extractions using coated-PESI enable multiple and uncomplicated analyses of a single small sample without the depletion of the target or the analyte-protein binding.
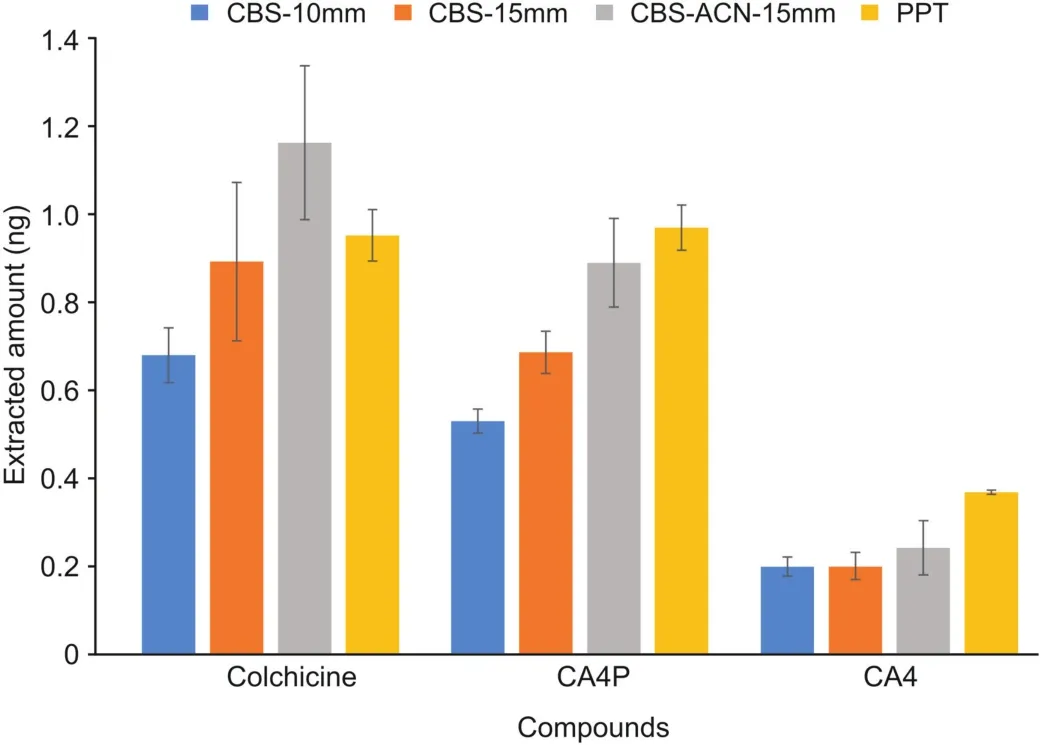
Fig.4.Comparison of the quantities of analytes extracted via CBS spot analysis with the addition of acetonitrile(ACN)as the matrix modifier and protein precipitation(PPT),followed by liquid chromatography(LC).(Blue)device with a coating length of 10 mm,(orange)device with a coating length of 15 mm,(gray)device with a coating length of 15 mm and extraction performed by adding ACN,(yellow)PPT.Columns represent the mean values obtained from at least three replicates,and whiskers represent the standard deviations.
Furthermore,we evaluated the effect of adding an organic solvent to the sample(gray column in Fig.4).The addition of ACN induced the release of compounds from their protein-bonded form to their free fraction form.This result is desirable because microextraction approaches can only extract components in their free form[8],and lower binding affinities facilitate higher extraction efficiency.Moreover,such modification may stop enzymatic reactions that simultaneously occur with the extraction,which last for 5 min.
No significant difference was observed between CBS-LC and PPT-LC approaches(gray and yellow columns in Fig.4),indicating that these techniques can be used interchangeably.Thus,the CBSAMS approach yielded the same results as the PPT-LC approach but CBS-AMS results were available within a min(s)after sampling.
3.3.Time-course monitoring of the biotransformation of CA4P into CA4
The biotransformation of CA4P into CA4 was investigated using four approaches.First,coated-PESI-AMS was employed as a nonsample-consuming microextraction technique.Moreover,three low-sample-consuming(10μL per sample)methods,including modified CBS-CAN spot analysis followed by AMS,modified CBSACN method followed by LC,and PPT approach followed by LC,were employed.To determine the sensitivity/specificity of these methods,the results of blank samples(unspiked cell culture medium supplemented with FBS)were analyzed and compared with those obtained during metabolism reactions[16].For the LC approaches(both CBS and PPT),we compared the data at retention time of the target compounds.For the AMS approaches(both coated-PESI and CBS),we compared the area counts.The signal-tonoise ratio(SNR)for each compound was calculated at 5-and 45-min time intervals to ensure that CA4 and CA4P were present at both the maximum and minimum levels.As shown in Table 1,the SNR value of the tested compounds was high.The proposed microextraction approaches were further validated using the classical approach(PPT),revealing the same pattern of biotransformation(yellow dots in Fig.5).
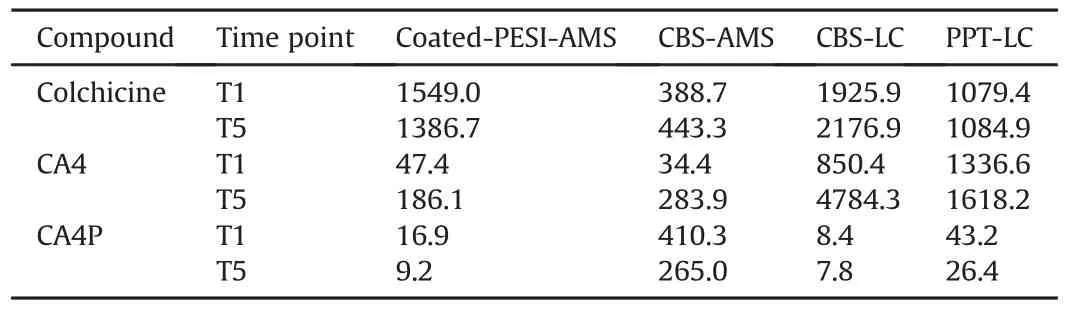
Table 1Signal-to-noise ratio values of colchicine,combretastatin A4(CA4)and CA4 phosphate(CA4P)among used methods.
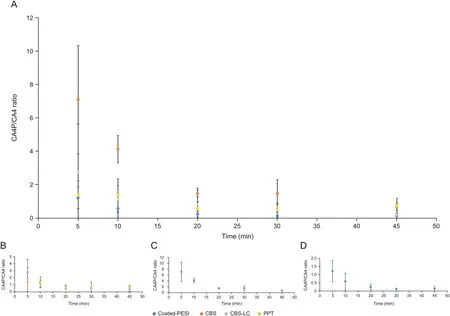
Fig.5.(A)Time-course monitoring of the biotransformation of CA4P to CA4.(B)Insert with LC-MS approaches:CBS and PPT.(C)Insert with spot analysis using CBS-AMS approach.(D)Insert with coated-PESI-AMS approach.(Blue)Coated-PESI-AMS,(orange)CBS-AMS,(gray)CBS followed by LC,(yellow)PPT followed by LC.The points represent the mean values obtained from at least three biological replicates,and whiskers represent the standard deviations.MS:mass spectrometry;AMS:ambient mass spectrometry.
The TIC areas of the target compounds were normalized using an IS,and the CA4P/CA4 ratio in each sample was calculated(Fig.5).All samples generally exhibited the same pattern,and the reaction lasted for 20 min(Fig.5A).Two of the LC approaches were very similar in terms of the absolute values of the CA4P/CA4 ratio(Fig.5B).These values were different for both AMS approaches(Figs.5C and D),which can be attributed to the difference in the way in which ions are introduced to the mass spectrometer.For instance,coated-PESI employs several short sprays in repeated cycles,whereas the CBS approach employs a more direct infusion approach,with a stable plum of electrospray introduced into the mass spectrometer inlet.Traditionally,time-course monitoring of enzymatic reactions are achieved by transferring a part of the sample into another vial,immediately followed by quenching.Herein,this approach was employed for the PPT-LC,CBS-LC,and CBS-AMS methods.Conversely,the coated-PESI approach was employed to monitor the reaction without enzyme denaturation.Thus,the lowest possible time of determination was required because the enzyme-based reaction occurred rapidly.To monitor the CA4P biotransformation,we performed sampling for 1 min to ensure that the change in the concentration of the compounds was minimized.Short sampling time is feasible with coated-PESI because desorption can occur with the use of a very small amount of organic solvent(5-10 pL)[17].The coated-PESI uses very small coating sizes;thus,it extracts small quantities of target analytes.However,the coated-PESI approach offers high sensitivity owing to the presence of high concentrations of target analytes due to small volume of desorption solution.This phenomenon can be referred to as “microdesorption,”and in this application,it allows compounds to be monitored after only 1 min of extraction.
4.Conclusions
From a biological viewpoint,it is important to determine whether soluble,phosphate prodrugs can be easily converted to their active forms in cell culture media(in vitro)and under preclinical settings(in vivo animal models),as well as in the clinical(human clinical trials)stages of drug development.Although sample preparation techniques are advancing at a rapid pace,PPT is still used for organic solvent extraction,followed by separation and detection.Herein,we found that SPME followed by AMS is similar to the classical approach.Moreover,both coated-PESI-AMS and CBS-AMS can rapidly monitor the biotransformation of phosphate prodrugs into their active forms in a cell culture medium,achieving results in approximately 2 and 7 min,respectively.Hence,both techniques can be used for fast screening to determine whether phosphate prodrugs of new chemical entities can successfully undergo this conversion.The results suggest that coated-PESI is effective for the time-course monitoring of simple biotransformation reactions because it achieves the same results as PPT-LC and is simpler and faster than the method of CBS followed by the addition of ACN.Furthermore,coated-PESI performs extractions without consuming any sample,making it a preferred technique in the case of limited sample volumes(e.g.,cell cultures cultivated in 96-well plates).The proposed AMS methods may fail if large numbers of compounds are monitored simultaneously.This is attributed to the limited capacity of mass spectrometers and the decrease in sensitivity in such a case.To the best of our knowledge,this study presents the use of a cell culture medium supplemented with FBS as a simple matrix for prodrug activation via a dephosphorylation reaction for the first time.Furthermore,the coated-PESI and CBS approaches can be employed for the rapid monitoring of more complex,than dephosphorylation,biotransformation reactions that are important during the in vitro stages of drug development(e.g.,phase I metabolism by liver microsomes or S9 fraction).In conclusion,the proposed methods can be used to investigate any biochemical or(bio)chemical reaction.
CRediT author statement
Karol Jaroch:Conceptualization,Methodology,Investigation,Writing-Original draft preparation,Formal analysis,Project administration,Visualization;Janusz Pawliszyn:Resources,Supervision,Conceptualization,Funding acquisition,Writing -Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by Shimadzu Scientific Instruments(Columbia,MD,USA)and Canada's National Science and Engineering Research Council-Industrial Research Chair(NSERC-IRC)program,grant number IRCPJ 184412-15.The authors thank Shimadzu Scientific Instruments and the Shimadzu Corporation(Kyoto,Japan)for providing the Shimadzu LCMS 8060 triple quadrupole MS,Shimadzu LC-30AD LC,DPiMS-8060,the PESI probes,and sample plates used in this study.The authors also acknowledge Milaan Thirukumaran for his assistance in PESI instrumentation and coated-PESI manufacturing,Varoon Singh for assistance in HLB particle synthesis,and Yohei Arao from the Shimadzu Corporation for his contributions in the CBS method development.
 Journal of Pharmaceutical Analysis2022年1期
Journal of Pharmaceutical Analysis2022年1期
- Journal of Pharmaceutical Analysis的其它文章
- Erythrocyte sphingolipid species as biomarkers of Alzheimer's disease
- Biological analysis of an innovative biodegradable antibiotic eluting bioactive glass/gypsum composite bone cement for treating experimental chronic MRSA osteomyelitis
- Global characterization of modifications to the charge isomers of IgG antibody
- Pharmacokinetics,distribution,and excretion of sodium oligomannate,a recently approved anti-Alzheimer's disease drug in China
- An integrated strategy for comprehensive characterization of metabolites and metabolic profiles of bufadienolides from Venenum Bufonis in rats
- Deciphering bioactive compounds of complex natural products by tandem mass spectral molecular networking combined with an aggregation-induced emission based probe
