Deciphering bioactive compounds of complex natural products by tandem mass spectral molecular networking combined with an aggregation-induced emission based probe
Zhenzhong Yng,Jun Li,Xuechun Chen,Xioping Zho,Yi Wng ,*
aPharmaceutical Informatics Institute,College of Pharmaceutical Sciences,Zhejiang University,Hangzhou,310058,China
bCollege of Preclinical Medicine,Zhejiang Chinese Medical University,Hangzhou,310053,China
Keywords:
Bioactive profile
Bioactive molecular network
Fangjihuangqi decoction
Angiotensin converting enzyme inhibitors
A B S T R A C T
Natural products are great treasure troves for the discovery of bioactive components.Current bioassay guided fractionation for identification of bioactive components is time-and workload-consuming.In this study,we proposed a robust and convenient strategy for deciphering the bioactive profile of natural products by mass spectral molecular networking combined with rapid bioassay.As a proof-of-concept,the strategy was applied to identify angiotensin converting enzyme(ACE)inhibitors of Fangjihuangqi decoction(FJHQD),a traditional medicine clinically used for the treatment of heart failure.The chemical profile of FJHQD was comprehensively revealed with the assistance of tandem mass spectral molecular networking,and a total of 165 compounds were identified.With characterized constituents,potential clinical applications of FJHQD were predicted by Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine,and a range of cardiovascular related diseases were significantly enriched.ACE inhibitory activities of FJHQD and its constituents were then investigated with an aggregation-induced emission based fluorescent probe.FJHQD exhibited excellent ACE inhibitory effects,and a bioactive molecular network was established to elucidate the ACE inhibitory profile of constituents in FJHQD.This bioactive molecular network provided a panoramic view of FJHQD's ACE inhibitory activities,which demonstrated that flavones from Astragali Radix and Glycyrrhizae Radix et Rhizoma,saponins from Astragali Radix,and sesquiterpenoids from Atractylodis Macrocephalae Rhizoma were principal components responsible for this effect of FJHQD.Among them,four novel ACE inhibitors were the first to be reported.Our study indicated that the proposed strategy offers a useful approach to uncover the bioactive profile of traditional medicines and provides a pragmatic workflow for exploring bioactive components.
1.Introduction
Botanical drugs and traditional medicines are great treasure troves[1,2],and thus provide rich resources for discovering bioactive components and developing novel drugs[3-5].Many efforts have been made in screening for bioactive compounds from complex mixtures[6,7],although it is very common that loss of activity or failure occurs during the process of bioassay guided fractionation[8].In addition,the active constituents identified might not play the key role in the therapeutic effects of traditional medicines due to low concentrations.
Benefiting from the rapid development of analytical techniques,the advanced high-performance liquid chromatography(HPLC)coupled with mass spectrometry(MS)[9-11]or nuclear magnetic resonance[12]is now available for the phytochemists,and identification of the chemical constituents and elucidation of the chemical profile of traditional medicines are no longer significant difficulties[13,14].In contrast,the bioactive investigation of the traditional medicines remains sporadic and fragmented.Contemporary pharmacological researches have been mainly carried out on traditional medicines as a whole or the major components.
Deciphering the bioactive profile of traditional medicines is a pivotal process to reveal the key components responsible for the pharmacological activities,which will provide the basis for the quality control and secondary development of these medicines[15].Efforts have been made in some traditional medicines or botanical drugs[16,17].In previous studies,our group had developed a bioactive chemical markers based strategy for the rational quality assessment and chemistry,manufacturing,and controls research of botanical drugs derived from Panax notoginseng(Burk.)F.H.Chen[5,18-20].For traditional medicines with complex composition,the aforementioned method might be relatively labor-intensive and time-consuming.Thus,a more robust and convenient method is urgently needed.
The prevalence of heart failure,especially chronic heart failure,poses a substantial burden worldwide,owing to the progressive aging of population[21].Renin-angiotensin-aldosterone system(RAAS)plays a pivotal role in heart failure[22].Angiotensin converting enzyme(ACE)is one of the key targets in RAAS,and ACE inhibitors used in daily practice have been known to reduce morbidity and mortality in patients with heart failure[23].Although ACE inhibitors are generally well tolerated,the side effects of ACE inhibitors,such as cough,angioedema,hyperkalemia,and functional renal insufficiency[24-26],could not be ignored.Alternative ACE inhibitors with fewer side effects have constantly been sought in clinic.
Fangjihuangqi decoction(FJHQD)is traditionally used in the treatment of chronic heart failure in China[27,28].Several studies have been performed to explore the main chemical constituents in FJHQD[29,30],whereas a more comprehensive chemical analysis was warranted.Moreover,the bioactive profile and the key constituents responsible for the therapeutic effects of FJHQD remained unclear.
In the present study,a novel strategy was proposed for deciphering the bioactive profile of traditional medicines.As a proof-ofconcept,the strategy was applied to FJHQD.The comprehensive chemical structural characterization of constituents in FJHQD was performed with the aid of tandem mass spectral molecular networking[31].The potential clinical applications of FJHQD were predicted by Bioinformatics Analysis Tool for Molecular mechANism of traditional Chinese medicine(BATMAN-TCM)with these constituents identified,and cardiovascular diseases were significantly enriched.One of the key therapeutic targets of cardiovascular diseases,ACE,was then employed for the further bioassay.The ACE inhibitory activities of FJHQD were determined by an aggregation-induced emission based fluorescent probe[32],and a bioactive molecular network was established.Utilizing this bioactive molecular network,the ACE inhibitory profile of FJHQD,as well as the constituents which might play significant roles in therapeutic effects,was elucidated(Fig.1).

Fig.1.The flowchart of the bioactive molecular network establishment.BATMAN-TCM:Bioinformatics Analysis Tool for Molecular mechANism of traditional Chinese medicine;ACE:angiotensin converting enzyme;S:serine;D:aspartic acid;K:lysine;P:proline.
2.Materials and methods
2.1.Chemicals and reagents
Stephaniae Tetrandrae Radix and Atractylodis Macrocephalae Rhizoma were collected from Zhejiang Province,China.Astragali Radix was collected from the Inner Mongolia Autonomous Region,China.Glycyrrhizae Radix et Rhizoma was collected from the Xinjiang Uygur Autonomous Region,China.Zingiberis Rhizoma Recens was collected from Yunnan Province,China.Jujubae Fructus was collected from Hebei Province,China.These plant materials were morphologically authenticated by Dr. Zhenzhong Yang.The voucher specimens were deposited in Pharmaceutical Informatics Institute,Zhejiang University(Hangzhou,China).
Licochalcone A, glycyrol, atractylon, fangchinoline, tetrandrine,astragaloside II, liquiritin apioside, isoliquiritin apioside, isocorydine, prunin, calycosin, formononetin, and isoliquiritin werepurchased from Shanghai Yuanye Bio-Technology Co., Ltd.(Shanghai, China). Atractylenolide I, atractylenolide II, atractylenolide III, calycosin-7-O-glucoside, liquiritigenin, liquiritin, isoliquiritigenin, and [6]-gingerol were purchased from ShanghaiRonghe Pharmaceutical Technology Development Co., Ltd.(Shanghai, China). Schaftoside, neoliquiritin, and neoisoliquiritinwere purchased from Shanghai PureOne Biotechnology Co., Ltd.(Shanghai, China). Licochalcone B and glycyrrhizic acid were purchased from Chengdu Must Bio-Technology Co., Ltd. (Chengdu,China). Astragaloside IV and ononin were obtained from ZhongxinPharmaceuticals (Tianjin, China). The purities of all referencestandards were greater than 97%.
Acetonitrile(HPLC grade)and methanol(HPLC grade)were purchased from Merck(Darmstadt,Germany).Formic acid(HPLC grade)was purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.(Shanghai,China).ACE was purchased from Sigma-Aldrich(St.Louis,MO,USA).TPE-SDKP was synthesized through solid phase peptide synthesis,as described in our previous publication[32].Deionized water was prepared using a Milli-Q system(Millipore,Bedford,MA,USA).All the other chemicals and solvents were of analytical grade.
2.2.Sample preparation
According to the original record in Jin Gui Yao Lue,Stephaniae Tetrandrae Radix,Astragali Radix,Atractylodis Macrocephalae Rhizoma,Glycyrrhizae Radix et Rhizoma,Zingiberis Rhizoma Recens,and Jujubae Fructus were mixed at a ratio of 60:75:45:30:45:36,and then extracted twice with water(1:8,m/V)for 1 h under reflux.The extract was combined and concentrated under reduced pressure to syrup,and then re-suspended in CH3OH:H2O(50:50,V:V)at a concentration of 0.4 g raw materials per milliliter.After centrifugation at 5,000 g for 10 min,the supernatant was used for further analysis.
2.3.UPLC-Q-TOF-MS analysis
The analysis of FJHQD was performed on a UPLC(Waters,Milford,MA,USA)equipped with a Triple TOF 5600+mass spectrometer(AB SCIEX,Framingham,MA,USA).Chromatographic separation was carried out at 30°C on a CORTECS C18column(4.6 mm×100 mm,2.7μm)with mobile phase A(0.1% formic acid-water)and mobile phase B(100% acetonitrile).The flow rate was 0.7mL/min.The elution conditions were optimized as follows:0 min,2%B;4 min,5%B;10min,12%B;15min,15%B;20min,20%B;25min,25%B;30min,33%B;35 min,45%B;45 min,70%B;50min,100%B.The parameters for negative and positive modes were as follows:curtain gas,30psi;Gas1(N2),50 psi;Gas 2(N2),50 psi;ion spray voltage,5.5 kV(positive mode)and-4.5 kV(negative mode);collision energy,10 V;declustering potential,100 V;temperature,600°C(positive mode)and 550°C(negative mode);scan range,m/z100-2000.A volume of 5μL was injected into the system for the analysis.
2.4.Molecular networking
A molecular network of the constituents in FJHQD was created following the online workflow at Global Natural Products Social Molecular Networking(GNPS,http://gnps.ucsd.edu).The data were clustered with a precursor ion mass tolerance of 0.02 Da and a fragment ion mass tolerance of 0.02 Da.A network was created,where the edges were filtered to have a cosine score above 0.7.The molecular network was visualized in Cytoscape(version 3.6.1).
2.5.Prediction of potential clinical applications for FJHQD
BATMAN-TCM(http://bionet.ncpsb.org/batman-tcm/index.php)[33],an online bioinformatics analysis platform for molecular mechanism of traditional Chinese medicines,was applied for the prediction of the potential clinical applications for FJHQD.The list of constituents identified in FJHQD was submitted for the analysis.The score cutoff of the target prediction was set at 20,while the adjusted P-value for the disease enrichment was 0.05.
2.6.ACE inhibition assay
A novel method using aggregation-induced emission based fluorescent probe had been established previously[32],which was adopted to assess the ACE inhibitory activities of FJHQD and related constituents.
Sample solution was incubated with TPE-SDKP(50μM)and ACE enzyme(12.5 mU/mL)for 2 h at 37°C,and then ZnCl2solution(final concentration of 3 mM)was added and incubated for 1 h.The fluorescence intensity was measured with excitation wavelength of 320 nm and emission wavelength of 470 nm on Tecan infinite M1000 PRO(Tecan Group Ltd.,M¨annedorf,Switzerland).
The ACE inhibitory activity was calculated with the Eq.(1):
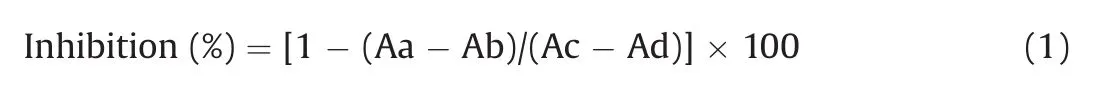
where Aa is the fluorescence intensity with test sample and enzyme,Ab is the fluorescence intensity with test samples but without enzyme,Ac is the fluorescence intensity with enzyme but without test sample,and Ad is the fluorescence intensity without test samples and enzyme.
3.Results and discussion
3.1.Chemical profile identification of FJHQD
The extract of FJHQD was prepared and then analyzed with UPLC-Q-TOF-MS.The base peak chromatograms of FJHQD are shown in Fig.2.
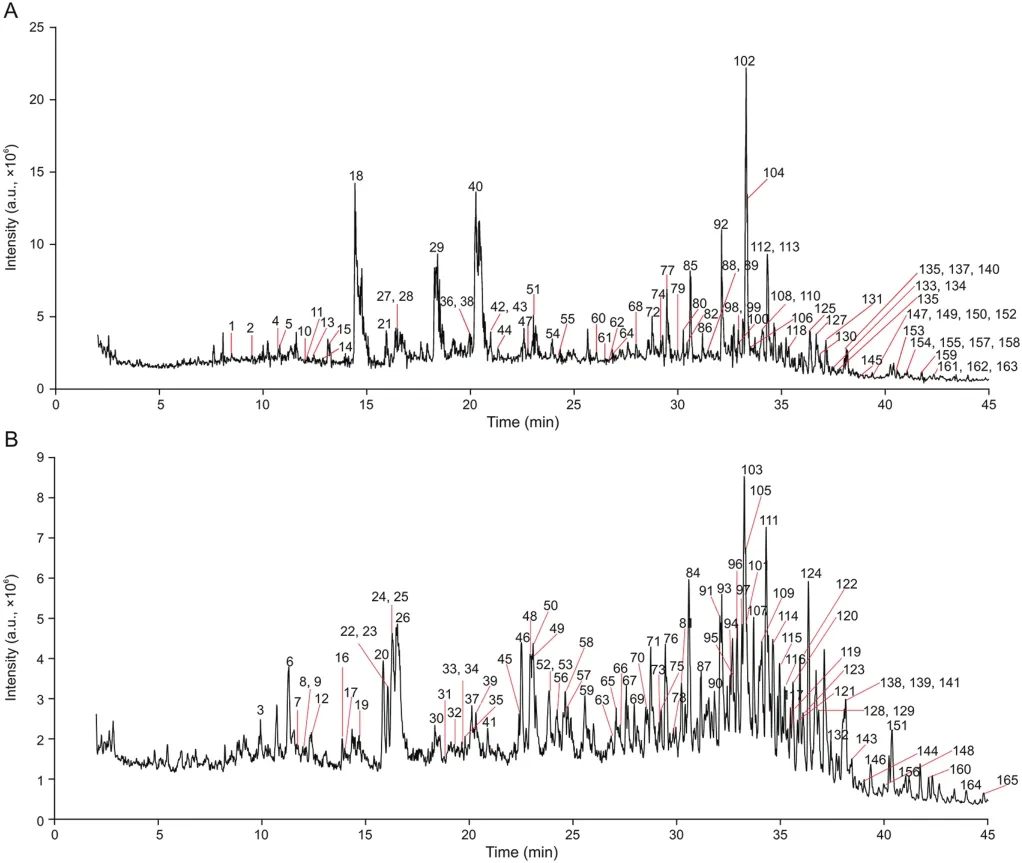
Fig.2.Base peak chromatograms of Fangjihuangqi decoction(FJHQD)obtained by UPLC-Q-TOF-MS in both(A)positive and(B)negative ion modes.Peaks are numbered according to Table S1.
GNPS is an open-access knowledgebase,which provides a highthroughput platform for the characterization of natural products[31].Following the online workflow in GNPS,a molecular network of the constituents in FJHQD was created(Fig.3),which could facilitate dereplication.Fig.4 and Figs.S1-S5 show the detailed view of the representative clusters of the molecular network.Totally,there were 86 clusters(node≥2)and 372 single nodes in this molecular network.

Fig.3.Molecular network of the constituents in FJHQD.Node size indicated the peak area in UPLC-Q-TOF-MS;the node with blue circle was the one identified using reference substance;and node color indicated the chemical type of the compound(blue:triterpenoid;red:flavanone;orange:isoflavone;purple:sesquiterpenoid;yellow:chalcone;green:alkaloid;grey:others).
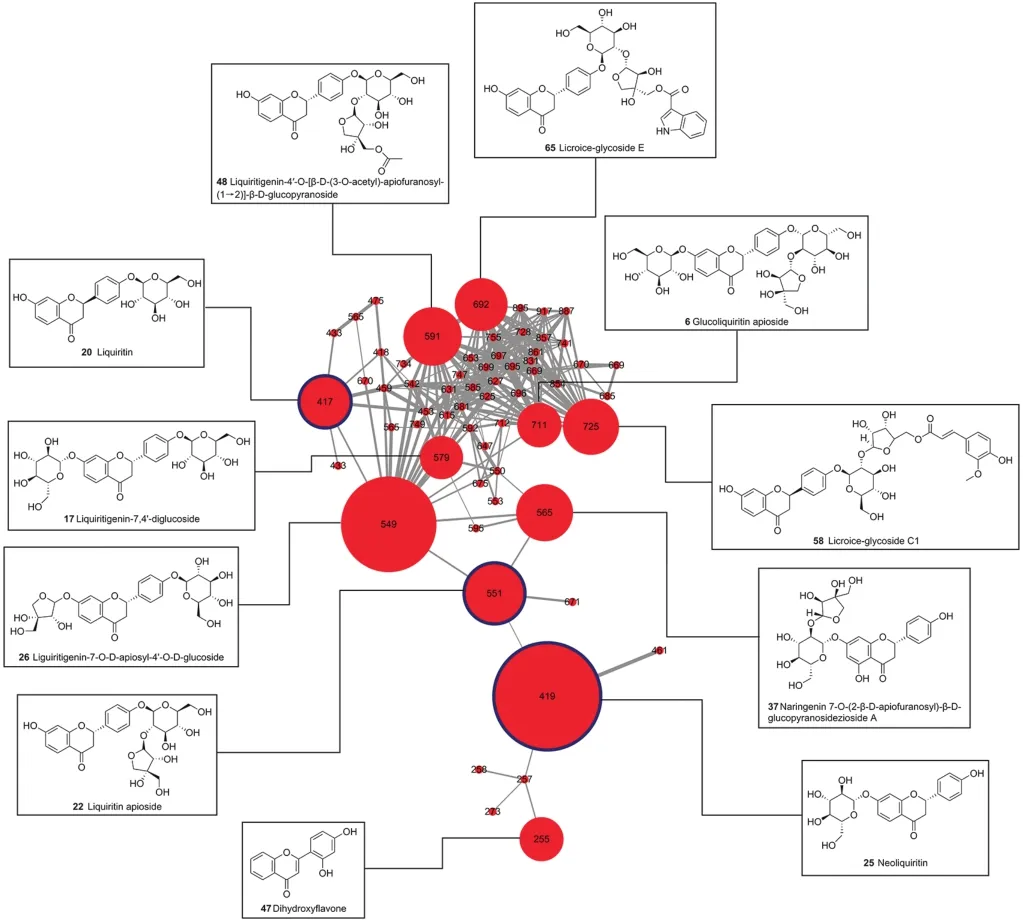
Fig.4.Detailed view of the representative cluster(Cluster 2)of the molecular network.
The high resolution MS data provided the accurate molecular weight,which enabled the characterization of the molecular formula.The MS/MS data could offer more structural information,which would be helpful for analyzing the chemical structures of these constituents.Compared with the reference standards and the data in literatures[20,34-62],the constituents in FJHQD were characterized.Besides,the established molecular network was very helpful in chemical characterization,especially for the constituents with similar chemical structures,which greatly accelerated the dereplication process.Herein,Cluster 2 was taken as an example to illustrate how molecular network assisted constituent characterization.In Cluster 2(Fig.4),the node of ion at m/z 417 showed a quasi-molecular ion[M-H]-at m/z 417.1203 that corresponded to the molecular formula of C21H22O9(-1.7 ppm).Its MS/MS data possessed fragment ions at m/z 255,135,and 119,corresponding to the aglycone,and the aglycone's products of retro-Diels-Alder reaction.Compared with the retention time and MS data of reference standard and literatures[40,41,43],this node was unambiguously identified as liquiritin.Adjacent to the node of liquiritin,the nodes of ions at m/z 579 and m/z 549 exhibited high MS/MS spectral similarity,but with 162 and 132 Da difference in quasi-molecular ion.This indicated that these two nodes shared the same aglycone,but with one more hexosyl group and pentosyl group,respectively.These two nodes were tentatively identified as liquiritigenin-7,4'-diglucoside and liguiritigenin-7-O-D-apiosyl-4-O-glucoside.The node of ion at m/z 591 was located adjacent to the node of liguiritigenin-7-O-D-apiosyl-4-O-glucoside,and exhibited high MS/MS spectral similarity with 16 Da difference in quasi-molecular ion and fragment ions.This indicated that there might be one more hydroxyl group in the node of ion at m/z 591,compared with liguiritigenin-7-O-D-apiosyl-4-O-glucoside.Based on literature,this node was tentatively identified as naringenin 7-O-(2-β-D-apiofuranosyl)-β-D-glucopyranoside. Similarly, other nodes in the cluster were characterized.
In the network,the compounds with similar chemical skeleton tended to assemble in a cluster.As shown in Fig.S1,Cluster 1 was mainly constituted by nodes of triterpenoids.Furthermore,there were two kinds of triterpenoids in Cluster 1.On the top right,there were cycloartanes,i.e.,astragaloside I,II,and IV,which were a kind of tetracyclic triterpenoid and one of the main bioactive chemical types in Astragali Radix.The residual ones mainly belonged to oleananes,a type of pentacyclic triterpenoid,including glycyrrhizic acid,licorice saponin A3,and licorice saponin G2.These oleananes were mainly derived from Glycyrrhizae Radix et Rhizoma.The most nodes in Cluster 2 were flavanones(Fig.4),which were mainly from Glycyrrhizae Radixet Rhizoma.Three clusters,i.e.,Cluster 3,Cluster 6,and Cluster 11,were all mainly constituted by isoflavones(Fig.S2).Astragali Radix and Glycyrrhizae Radix et Rhizoma were the main origins of these isoflavones.Sesquiterpenoids were characteristic chemicals from Atractylodis Macrocephalae Rhizoma in FJHQD,which were mainly gathered in Cluster 4(Fig.S3).Chalcones in this formula were mostly derived from Glycyrrhizae Radix et Rhizoma,and two of them were congregated in Cluster 14(Fig.S4).Benzyltetrahydroisoquinolines were the characteristic constituents of Stephaniae Tetrandrae Radix.Most of these alkaloids were single nodes in this molecular network,such as tetrandrine,fanchinoline,and isocorydine,while two of them,i.e.,N-methylcoclaurine and coclaurine,were assembled in Cluster 15(Fig.S5).
Combining the manual identification and the assistance of tandem mass spectral molecular networking,the chemical profile of FJHQD was comprehensively investigated.Totally,165 compounds in this formula were identified(Table S1[20,34-62])and the chemical structures are shown in Fig.S6.
3.2.Predicting potential clinical applications of FJHQD
TCMs are usually prescribed for particular “patterns,”called“Zheng”in Chinese,which represent a specific functional state[63,64].The modern medicines are developed for certain diseases.It is necessary to link the TCMs to their related diseases[33].The disease associated genes and proteins could be targeted by some constituents in TCMs,which is the basis for the therapeutic effects of TCMs to corresponding diseases.With the identification of the constituents in FJHQD,BATMAN-TCM was used to predict the targets of these constituents,which was further adopted to enrich Therapeutic Target Database(TTD)[65]disease phenotypes.The disease phenotypes,shown in Fig.5A,were significantly enriched by the predicted targets.Of note,about 1/3 of these disease phenotypes were related to cardiovascular disease,such as hypertension,atherosclerosis,heart failure,etc.This result was consistent with the clinical usage of FJHQD.Thus,the bioactivity associated with the treatment of cardiovascular disease was further investigated.
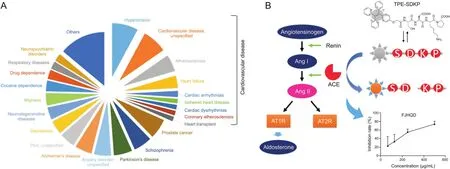
Fig.5.Prediction of FJHQD's clinical applications and the related bioassay performance.(A)Prediction of potential clinical applications of FJHQD by BATMAN-TCM.(B)ACE is a key component of renin-angiotensin-aldosterone system,which was selected as the target for the bioassay using an aggregation-induced emission fluorescent probe,and the ACE inhibitory activity of FJHQD was tested.
3.3.ACE inhibitory activities
RAAS is a hormonal system that plays a pivotal role in the homeostatic control of the cardiovascular system[66].Inhibition of RAAS can effectively alleviate the morbidity and mortality of patients with heart failure[67].ACE can cleave angiotensin I(Ang I)to generate angiotensin II(Ang II),which is a key component of RAAS(Fig.5B)[68].Inhibiting ACE is an effective way to prevent or reverse organ damage in cardiovascular diseases.
Within a model based on aggregation-induced emission fluorescent probe,FJHQD exhibited a superior dose-dependent ACE inhibitory activity(Fig.5B),with an IC50of 202.16±90.61μg/mL.Then,the constituents in FJHQD were explored for their ACE inhibitory activities.The bioactivities of these constituents were mapped to the molecular network,and the bioactive molecular network was established.As shown in Fig.6,the node color indicates the ACE inhibitory activities of the constituents,and the node size presents constituents’relative contents in FJHQD.It provided a panoramic view of the ACE inhibitory activity of this medicine.From this bioactive molecular network,it could be easily revealed that the flavones from Astragali Radix and Glycyrrhizae Radix et Rhizoma,the saponins from Astragali Radix,and the sesquiterpenoids from Atractylodis Macrocephalae Rhizoma were the main ACE inhibitory components in FJHQD.Among these constituents,licochalcone B,isoliquiritin apioside,liquiritin apioside,and atractylenolide III were reported for their ACE inhibitory activities for the first time.
Licochalcone A and licochalcone B showed excellent ACE inhibitory activities(Fig.6),with IC50values of 6.89±0.54μM and 2.32±0.33μM,respectively.Another chalcone from Glycyrrhizae Radix et Rhizoma,echinatin,had also been reported to exhibit potent ACE inhibitory activity of 72.52% at 100μM[69].Other chalcones,such as isoliquiritin and isoliquiritigenin,did not show ACE inhibitory activity(inhibition rate of less than 15% at 50μM).The methoxy group at C-2 of ring B of chalcones might be important for the activities,while the hydroxy group at C-2’of ring A might decrease this effect.Compared with ignorable ACE inhibitory effects of isoliquiritin and liquiritin,neoisoliquiritin and neoliquiritin possessed moderate activities,with 21.92%±5.96% and 22.50%±11.77% inhibition at 50μM,respectively.It meant that the glucosyl group at ring A could benefit the inhibitory activities.
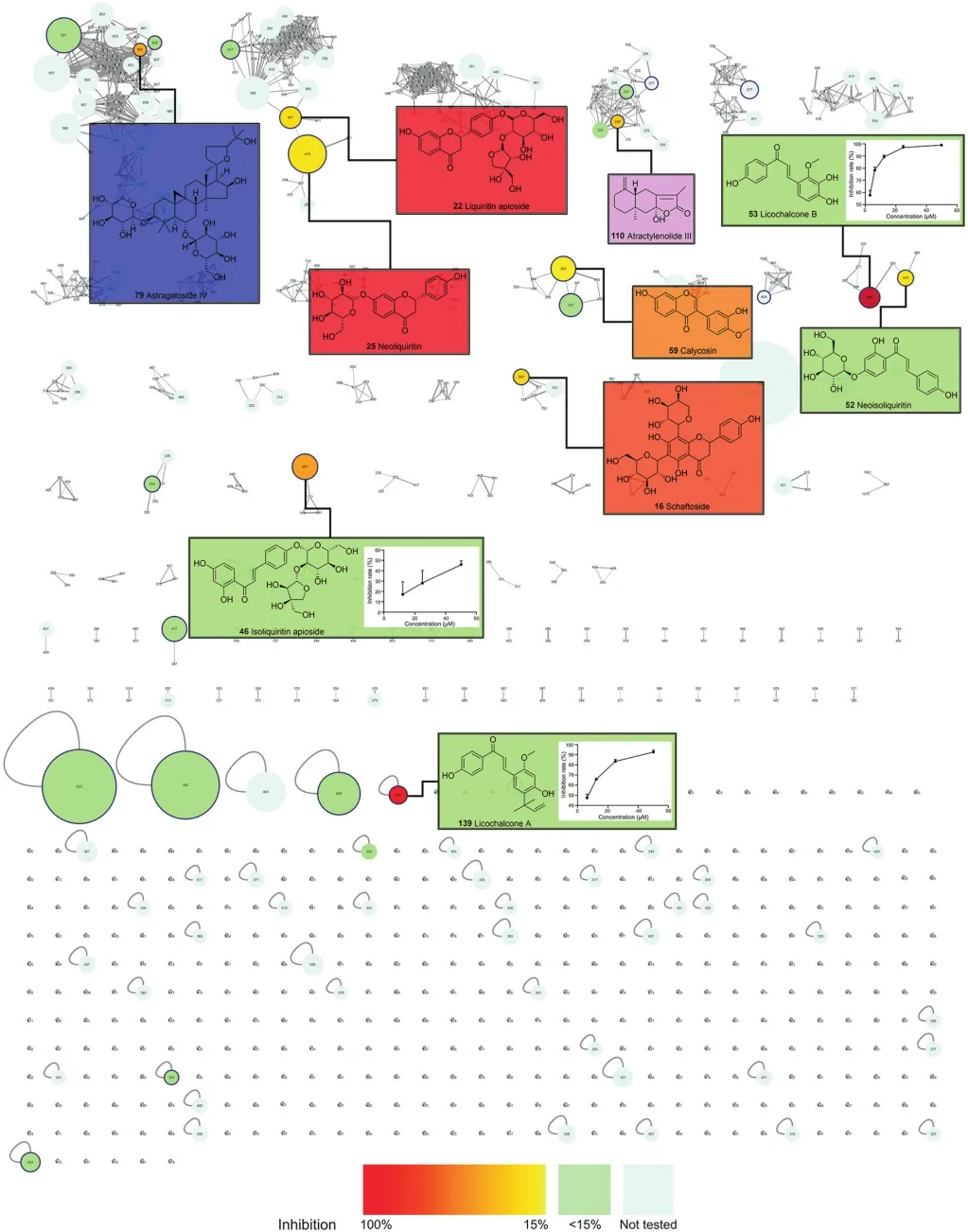
Fig.6.Bioactive molecular network of constituents in FJHQD.Node color indicated the ACE inhibitory activities of the compounds;node size indicated the peak area in UPLCQ-TOF-MS.Dose-response curves of the constituents with superior ACE inhibitory activity were presented.
Calycosin from Astragali Radix possessed an inhibitory activity of 19.04%±1.32% at 50μM,which was consistent with those of previous reports[70].However,the activities of other flavones from Astragali Radix,like ononin and calycosin-7-O-glucoside,were less than calycosin.Astragaloside IV possessed an inhibitory rate of 42.44%±4.59%,which was also reported in other literature[70,71].Astragaloside IV is one of the main active constituents in Astragali Radix for the treatment of cardiovascular and cerebrovascular diseases.The therapeutic mechanisms of astragaloside IV include antithrombosis,immune regulation,anti-oxidation,and vasodilation through ACE inhibition[71].Astragaloside II was also tested for the ACE inhibitory activities.Different from astragaloside IV,astragaloside II did not show unambiguous ACE inhibitory activities.Thus,acetylation of the xylose group might be disadvantageous to the ACE inhibitory activities.
The ACE inhibitory activities of sesquiterpenoids were rarely reported.In FJHQD,there were several sesquiterpenoids derived from Atractylodis Macrocephalae Rhizoma.Atractylenolide III exhibited an inhibitory rate of 33.26%±11.31%,while atractylenolide I and atractylenolide II did not.The hydroxyl group of these sesquiterpenoids might benefit the ACE inhibitory activities.
4.Conclusions
A novel strategy for deciphering the bioactive profile of natural products was proposed and applied to FJHQD to confirm its utility.With the assistance of tandem mass spectral molecular networking,the chemical profile of FJHQD was comprehensively investigated,and 165 compounds in this formula were identified.Based on this chemical profile,a range of cardiovascular diseases were considered as FJHQD's potential clinical applications using BATMAN-TCM.FJHQD exhibited convincing ACE inhibitory activities in a model based on aggregation-induced emission fluorescent probe.Through the bioactive molecular networking,the ACE inhibitory profile of the constituents in FJHQD and the main bioactive constituents responsible for the therapeutic effects were worked out.Four novel ACE inhibitors were found for the first time.This study might provide a workflow for bioactive profile disclosure and major bioactive components discovery,which is significant for quality control and new drug development.
CRediT author statement
Zhenzhong Yang:Conceptualization,Methodology,Investigation,Writing-Original draft preparation,Writing-Reviewing and Editing;Jun Li:Investigation,Writing-Original draft preparation;Xuechun Chen:Investigation;Xiaoping Zhao:Conceptualization,Supervision;Yi Wang:Conceptualization,Writing-Reviewing and Editing,Supervision.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by the National Key R&D Program of China(Grant No.:2018YFC1704502),the National Natural Science Foundation of China(Grant No.:81603268),and the National Natural Science Foundation of China(Grant No.:81822047).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.11.007.
 Journal of Pharmaceutical Analysis2022年1期
Journal of Pharmaceutical Analysis2022年1期
- Journal of Pharmaceutical Analysis的其它文章
- Time-course monitoring of in vitro biotransformation reaction via solid-phase microextraction-ambient mass spectrometry approaches
- Erythrocyte sphingolipid species as biomarkers of Alzheimer's disease
- Biological analysis of an innovative biodegradable antibiotic eluting bioactive glass/gypsum composite bone cement for treating experimental chronic MRSA osteomyelitis
- Global characterization of modifications to the charge isomers of IgG antibody
- Pharmacokinetics,distribution,and excretion of sodium oligomannate,a recently approved anti-Alzheimer's disease drug in China
- An integrated strategy for comprehensive characterization of metabolites and metabolic profiles of bufadienolides from Venenum Bufonis in rats
