小胶质细胞在慢性间歇低氧相关认知障碍中的作用*
徐家欢, 王玮
小胶质细胞在慢性间歇低氧相关认知障碍中的作用*
徐家欢, 王玮△
(中国医科大学附属第一医院呼吸与危重症医学科,辽宁 沈阳 110001)
慢性间歇低氧;认知障碍;小胶质细胞
慢性间歇低氧(chronic intermittent hypoxia, CIH)是阻塞性睡眠呼吸暂停(obstructive sleep apnea, OSA)的重要病理生理学特征,参与OSA相关并发症的发生发展[1]。认知障碍是OSA常见的并发症之一,其患病率高达30%以上[2],严重影响患者的生活质量。因此,探讨CIH导致认知障碍的具体机制,为临床提供新的治疗思路十分重要。小胶质细胞作为中枢神经系统的免疫细胞,在维持中枢神经系统稳态中起重要作用。研究显示,CIH可影响小胶质细胞表型及功能,后者进一步参与认知障碍的发生发展,但具体机制仍不十分清楚[3-4]。本文就小胶质细胞在CIH相关认知障碍中的作用及机制进行综述。
1 CIH引起认知障碍的主要表现
CIH是指OSA患者在夜间睡眠过程中,由于气道反复塌陷引起呼吸暂停或低通气而导致的缺氧和复氧反复交替,是OSA患者重要的病理生理学特征之一。早在2001年,Gozal等[1]就提出CIH可引起认知障碍。他们观察到低氧2周后(10%与21%的氧浓度每90 s或每30 min交替,12 h/d),大鼠在水迷宫中寻找平台的潜伏期和路线长度均较常氧组明显延长。此后,越来越多的研究采用不同的间歇低氧模式及行为学检测方法证实CIH引起大/小鼠长期或短期的空间、工作记忆等方面的损害[5-6]。病理学结果提示,CIH可引起大/小鼠海马、大脑额顶叶皮质层等学习及记忆相关区域损伤,主要表现为神经元凋亡、坏死,突触异常,细胞结构改变等[7-8]。但CIH引起认知障碍的具体机制目前仍不十分清楚。
2 小胶质细胞对认知功能的双向影响
近年来,越来越多的研究证实小胶质细胞在神经退行性疾病中发挥重要作用[9-10]。小胶质细胞是脑内定居的免疫细胞群。正常条件下,小胶质细胞以静止状态存在,其主要功能是检测病原体和宿主衍生的配体,包括病原体相关的分子模式和危险相关的分子模式,维持中枢神经系统的稳态[11]。当病原体入侵时,小胶质细胞被激活,并分化成M1型(促炎型)和M2型(抗炎型)细胞,参与疾病的发生发展[12]。研究显示,小胶质细胞对认知功能的影响是双向的。一方面,小胶质细胞对认知功能起保护作用。基因组学研究提示,小胶质细胞特异性基因突变如髓样细胞Ⅱ型触发受体基因,脾焦点形成病毒前病毒整合癌基因等,可导致小胶质细胞对β淀粉样蛋白的清除能力及对细胞碎片的吞噬能力下降,进而导致神经元的损伤,最终发生认知障碍[13-14];在脑创伤的小鼠模型中,外源性脑室内注入小胶质细胞后有利于神经元存活,改善小鼠脑创伤后认知功能[15]。此外,小胶质细胞也是其他神经保护性药物发挥作用的重要靶点。研究显示贝沙罗汀通过视黄醇X受体激活过氧化物酶体增殖物激活受体γ依赖性通路促进脑出血小鼠血肿吸收,改善认知功能;而在小胶质细胞被消耗的小鼠中,贝沙罗汀的神经保护作用消失[16]。另一方面,小胶质细胞也可能对认知功能起负面影响。在腹腔注射脂多糖后,小鼠海马区激活的小胶质细胞明显增多,小鼠认知功能受损;而给予黄芩苷治疗后,小鼠海马区反应性小胶质细胞及炎症因子减少,小鼠认知功能改善,提示过渡激活的小胶质细胞对中枢神经系统造成损伤[17]。
3 CIH及其相关认知障碍与小胶质细胞的相互关系
CIH作为外源性刺激,影响小胶质细胞的功能状态,后者又通过一系列机制参与CIH相关认知障碍的形成。
3.1 CIH对小胶质细胞的影响
3.1.1CIH参与小胶质细胞的激活及表型转化研究提示,与常氧组大/小鼠比较,CIH组大/小鼠海马区激活的小胶质细胞明显增多,且以M1型小胶质细胞为主[3, 18-19]。此外,其他模型提示,与单纯糖尿病比较,合并CIH的糖尿病模型组小鼠海马区M1型激活小胶质细胞明显增多,而M2型激活小胶质细胞减少[4]。体外细胞实验在间歇低氧8 h或18 h后,获得同样的结果[3-4]。CIH可能通过以下机制参与小胶质细胞激活与表型转化:(1)间歇低氧可以直接引起中枢神经系统内的氧化应激,产生大量的活性氧或炎症因子[1]。一方面,活性氧或者炎症因子可以直接作用于小胶质细胞,通过激活还原型烟酰胺腺嘌呤二核苷酸磷酸氧化酶等,从而促使小胶质进一步产生炎症因子发挥第一道防线的作用[20];另一方面,活性氧导致神经元及其他胶质细胞损伤产生损伤相关分子模式,后者可与小胶质细胞表面的多种受体例如Toll样受体4、髓样细胞Ⅱ型触发受体2等结合,进而激活下游通路,发挥小胶质细胞功能[21]。(2)CIH可引起外周循环中炎症因子增加。当CIH时,血脑屏障通透性明显增加[22],部分外周炎症因子例如白细胞介素1(interleukin-1, IL-1)可透过血脑屏障进入中枢神经系统,促进小胶质细胞的激活并释放炎症因子。
3.1.2CIH参与小胶质细胞的增殖与凋亡Liu等[23]给予BV-2小胶质细胞8 h的间歇低氧后(1% O2与21% O2每400 s交替1次),观察到间歇低氧组小胶质细胞数量明显低于常氧组,与Gong等[24]的研究结果相似,提示间歇低氧影响小胶质细胞的增殖。进一步机制研究认为,间歇低氧通过启动多个 P53 相关通路抑制细胞周期蛋白 D1 和细胞周期蛋白 E2 的表达,阻断细胞周期转变并减弱小胶质细胞的增殖能力[23]。此外,Lin等[3]的细胞实验提示间歇低氧(1% O210 min/21% O25 min交替,共18 h)可促进BV-2小胶质细胞凋亡,苍术酮通过作用于沉默调节蛋白3减少炎症因子释放,可以减轻间歇低氧引起的小胶质细胞凋亡。Gong等[24]的BV-2细胞实验同样提示,与常氧组比较,间歇低氧组小胶质细胞凋亡增多,生松素通过线粒体自噬相关蛋白通路减轻炎症反应改善细胞凋亡。因此,CIH可能通过诱导炎症反应导致小胶质细胞凋亡,但具体分子机制仍需进一步研究。
CIH对小胶质细胞影响的总结见图1。
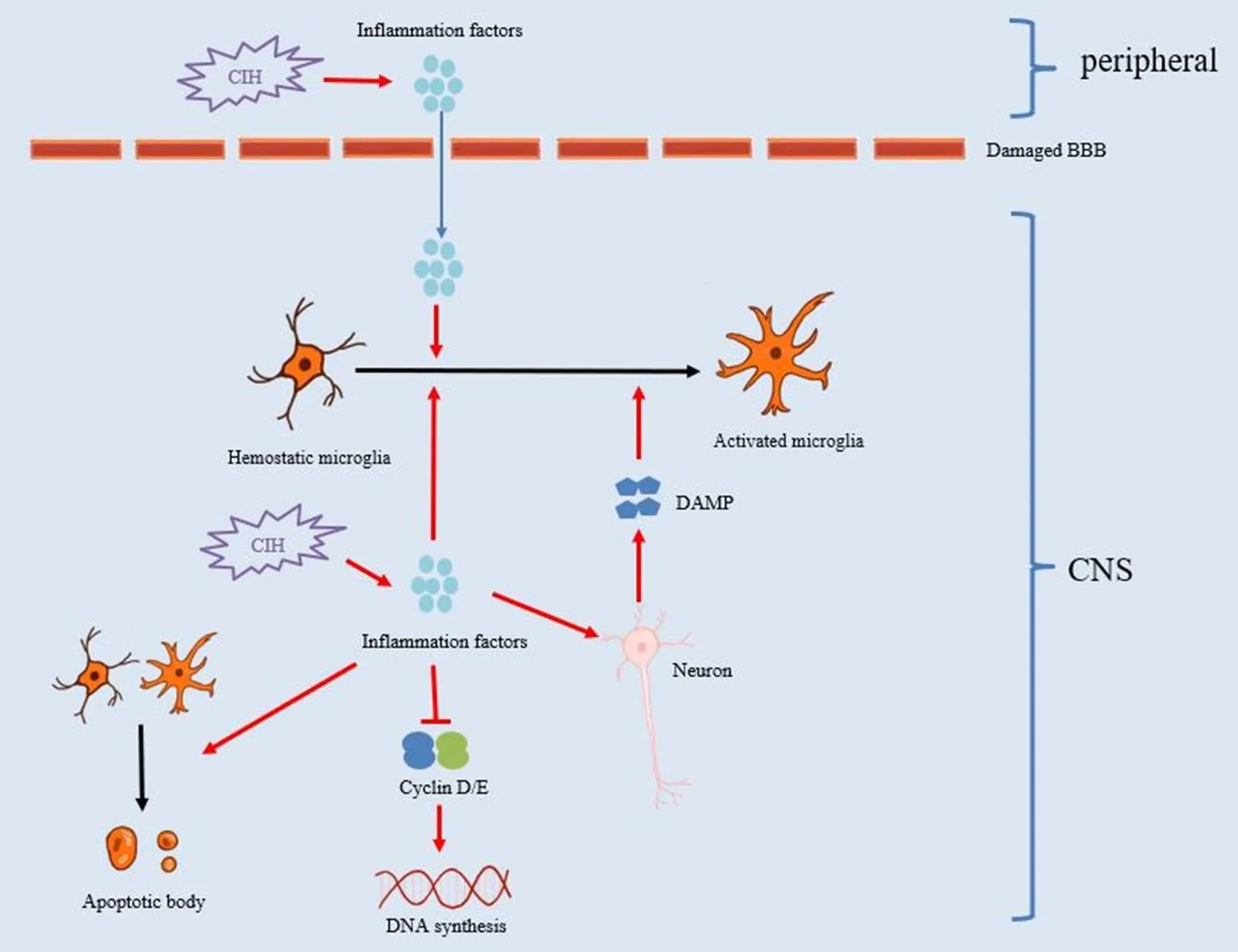
Figure 1.The effect of chronic intermittent hypoxia (CIH) on microglia. The red arrows represent cause-effect relationship. The black arrows represent the changes of the phenomena. The blue arrow represents the inflammation factors crossing the blood-brain barrier (BBB). CNS: central nervous system; DAMP: damage-associated molecular patterns.
3.2 小胶质细胞参与CIH相关认知障碍的机制
3.2.1小胶质细胞的保护作用下降小胶质细胞具有吞噬和清除神经毒性物质,对认知功能起保护作用。β-淀粉样蛋白可激活小胶质细胞表面的髓样细胞Ⅱ型触发受体2,并激活下游的哺乳动物雷帕霉素靶蛋白通路促进小胶质细胞对细胞碎片及β淀粉样蛋白的清除,并在淀粉样斑块周围聚集产生屏障功能减少对周围神经元的影响,起神经保护作用[25-26]。CIH一方面直接引起β淀粉样物质生成增多,另一方面其还可引起海马区神经炎症反应促使小胶质细胞凋亡增加或增殖减少,对吞噬和清除毒性物质的作用减弱[3,24,27]。这引起β淀粉样物质在中枢神经系统中的产生与清除失平衡,导致海马区β淀粉样物质堆积,从而导致认知障碍的发生。此外,M2型小胶质细胞具有抗炎作用,具有组织修复作用。在CIH作用下,M2型小胶质细胞激活相关分子减少,导致小胶质细胞M2型转化受抑制[3],小胶质细胞抗炎及组织修复作用减弱,脑组织过度损伤,导致认知障碍。
3.2.2小胶质细胞的损伤作用增强
3.2.2.1 小胶质细胞介导的神经炎症反应 小胶质细胞介导的神经炎症反应在认知障碍中具有重要作用。CIH作为外源性刺激,促进小胶质细胞向M1细胞转化,并激活相应炎症通路导致大量炎症因子释放,引起神经炎症反应,进而导致认知障碍[28-30]。那么小胶质细胞介导的神经炎症反应是如何引起认知障碍的呢?可能与下列机制有关:(1)神经元损伤:神经元是神经系统最基本的结构和功能单位,海马区或额顶叶皮质层神经元损伤可引起认知障碍。研究显示,在BV-2小胶质细胞与HT-22神经元共培养体系中,与常氧组比较,间歇低氧组炎症因子、神经元凋亡明显增加[31]。进一步动物实验观察到,CIH引起小鼠海马区小胶质细胞M1型激活并释放IL-1β、IL-6、肿瘤坏死因子α等多种损伤性炎症因子,引起神经元凋亡及死亡,造成小鼠认知功能障碍;当给予外源性应用药物促进小胶质细胞向M2型转化,减少损伤性炎症因子释放,增加IL-10、IL-13等保护性炎症因子,减少神经元损伤,小鼠认知功能改善[3,18-19]。此外,神经炎症反应还能抑制神经干细胞的增殖,促进其凋亡,并能显著降低其分化成神经元,尤其是胆碱能神经元的能力,从而使功能性神经元减少,引起认知障碍[32]。上述结果提示小胶质细胞介导的神经炎症反应通过引起神经元凋亡或抑制神经元分化参与间歇低氧引起的认知障碍。(2)血脑屏障受损:血脑屏障是由血管内皮细胞,周细胞及星形胶质细胞组成,在维持脑内的稳态及对外源性刺激后调节脑组织局部代谢需求方面具有重要作用[33]。近年来,研究认为血脑屏障受损参与多个神经退行性疾病的发生发展[34-35],可能的机制如下:脑能量代谢异常:血脑屏障功能受损时,其表面的葡萄糖转运蛋白数量减少或重新分布,导致葡萄糖转运减少,脑能量代谢不足,引起脑组织受损,进而引起认知障碍[35]。脑内神经毒性物质增多:在缺血性脑卒中模型中,小鼠血脑屏障受损,腺苷三磷酸转运蛋白表达下调,导致神经毒性物质在脑内聚积,损伤正常脑功能;当外源性用药恢复蛋白表达后,脑功能恢复[36]。CIH可引起血脑屏障受损,通透性增加,参与认知障碍的发生[22]。那么,CIH是如何引起血脑屏障损伤参与认知障碍的呢?研究认为激活的小胶质细胞在血脑屏障受损中具有重要的作用[37],当抑制小胶质细胞激活后,血脑屏障受损减轻[38]。在缺血再灌注模型中,小鼠海马区小胶质细胞激活,引起神经炎症反应及血脑屏障受损,进而导致小鼠认知障碍;给予小鼠具有抗炎作用的药物治疗后,小鼠海马区小胶质细胞激活减少,神经炎症反应减轻,血脑屏障功能改善,最终认知障碍缓解[39]。因此,CIH通过激活小胶质细胞并介导神经炎症反应,后者引起血脑屏障损伤进而导致认知障碍。
3.2.2.2 小胶质细胞依赖性突触吞噬 突触减少影响神经冲动在神经元之间的传递,是神经退行性疾病认知障碍的重要机制之一[40]。在神经系统的生长发育过程中,小胶质细胞通过补体途径参与突触的修饰,维持正常的神经系统功能[41]。在缺血缺氧等状态下,小胶质细胞介导的突触吞噬被异常激活,引起突触减少及相应功能损伤[42]。研究显示,在阿尔茨海默病及缺血再灌注小鼠模型中,海马区突触补体C1q与C3异常激活并与小胶质细胞的补体C3受体接合,启动小胶质细胞依赖性突触吞噬,从而造成突触减少及突触功能障碍,影响小鼠认知功能;当敲除小胶质细胞C3受体或应用特异性C3受体抑制剂后,突触减少及突触功能障碍得到改善,小鼠认知功能恢复[43-44]。CIH可以诱导补体C3激活或减少补体抑制剂CD59的表达,从而导致循环中激活的补体增加[45-46],进而引起损伤。因此,CIH可能通过补体途径引起小胶质细胞依赖性突触吞噬,从而参与认知障碍形成,具体机制仍需进一步研究。
小胶质细胞参与CIH相关认知障碍机制的总结见图2。
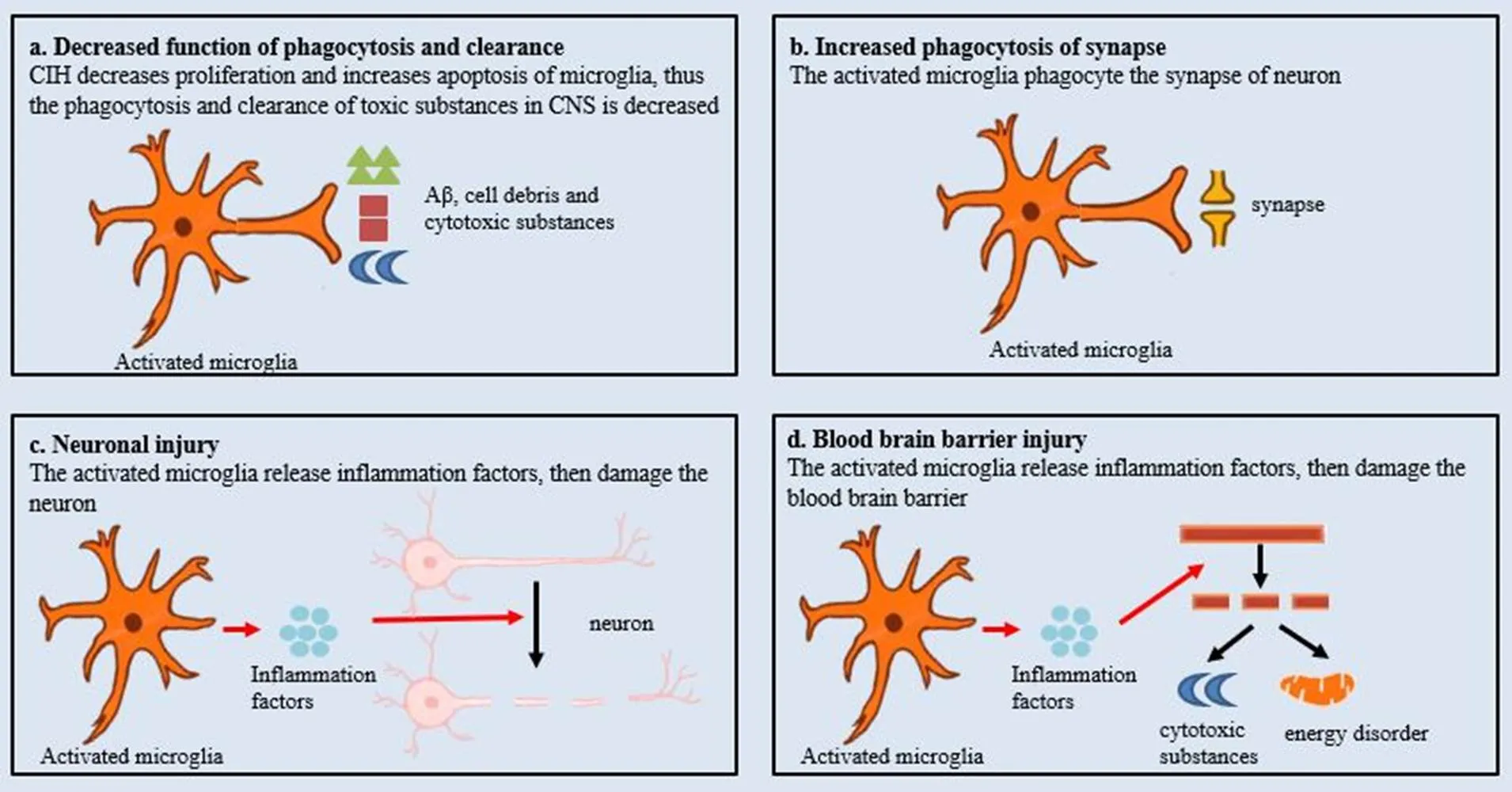
Figure 2.The role of microglia and its underlying mechanism in chronic intermittent hypoxia (CIH)-related cognitive impairment. The red arrows represent cause-effect relationship. The black arrows represent the changes of the phenomena. CNS: central nervous system.
CIH作为OSA的重要病理生理学特征,参与OSA患者认知障碍的发生发展。小胶质细胞是中枢神经系统中重要的免疫细胞,受CIH的影响,参与相关并发症的发生发展,但目前机制尚不十分清楚。明确小胶质细胞在CIH相关认知障碍中的作用机制,可通过上调其神经保护作用、减弱其神经损伤作用而CIH引起的认知障碍,为临床治疗OSA相关认知障碍提供参考资料。
[1] Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat[J]. J Neurosci, 2001, 21(7):2442-2450.
[2] Xu J, Qin Z, Li W, et al. Effects of somatotropic axis on cognitive dysfunction of obstructive sleep apnea[J]. Sleep Breath, 2020, 24(1):175-182.
[3] Lin Y, Liu X, Tan D, et al. Atractylon treatment prevents sleep-disordered breathing-induced cognitive dysfunction by suppression of chronic intermittent hypoxia-induced M1 microglial activation[J]. Biosci Rep, 2020, 40(6):BSR20192800.
[4] Shi Y, Guo X, Zhang J, et al. DNA binding protein HMGB1 secreted by activated microglia promotes the apoptosis of hippocampal neurons in diabetes complicated with OSA[J]. Brain Behav Immun, 2018, 73:482-492.
[5] Patil SS, Sunyer B, Höger H, et al. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze[J]. Behav Brain Res, 2009, 198(1):58-68.
[6] Gozal D, Khalyfa A, Qiao Z, et al. Temporal trajectories of novel object recognition performance in mice exposed to intermittent hypoxia[J]. Eur Respir J, 2017, 50(6):1701456.
[7] Guo X, Shi Y, Du P, et al. HMGB1/TLR4 promotes apoptosis and reduces autophagy of hippocampal neurons in diabetes combined with OSA[J]. Life Sci, 2019, 239:117020.
[8] Douglas RM, Ryu J, Kanaan A, et al. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction[J]. Am J Physiol Cell Physiol, 2010, 298(6):C1594-C1602.
[9] Rangaraju S, Dammer EB, Raza SA, et al. Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer's disease[J]. Mol Neurodegener, 2018, 13(1):24.
[10] George S, Rey NL, Tyson T, et al. Microglia affect α-synuclein cell-to-cell transfer in a mouse model of Parkinson's disease [J]. Mol Neurodegener, 2019, 14(1):34.
[11] Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages[J]. Science, 2010, 330(6005):841-845.
[12] Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury[J]. Nat Neurosci, 2005, 8(6):752-758.
[13] Pimenova AA, Herbinet M, Gupta I, et al. Alzheimer's-associated PU.1 expression levels regulate microglial inflammatory response[J]. Neurobiol Dis, 2021, 148:105217.
[14] McQuade A, Kang YJ, Hasselmann J, et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer's disease[J]. Nat Commun, 2020, 11(1):5370.
[15] Willis EF, MacDonald KPA, Nguyen QH, et al. Repopulating microglia promote brain repair in an IL-6-dependent manner[J]. Cell, 2020, 180(5):833-846.e16.
[16] Xu C, Chen H, Zhou S, et al. Pharmacological activation of RXR-α promotes hematoma absorption via a PPAR-γ-dependent pathway after intracerebral hemorrhage[J]. Neurosci Bull, 2021, 37(10):1412-1426.
[17] Li Y, Liu T, Li Y,et al. Baicalin ameliorates cognitive impairment and protects microglia from LPS-induced neuroinflammation via the SIRT1/HMGB1 pathway[J]. Oxid Med Cell Longev, 2020, 2020:4751349.
[18] Dong P, Zhao J, Li N,et al. Sevoflurane exaggerates cognitive decline in a rat model of chronic intermittent hypoxia by aggravating microglia-mediated neuroinflammation via downregulation of PPAR-γ in the hippocampus[J]. Behav Brain Res, 2018, 347:325-331.
[19] Zhang X, Li N, Lu L, et al. Pioglitazone prevents sevofluraneinduced neuroinflammation and cognitive decline in a rat model of chronic intermittent hypoxia by upregulating hippocampal PPARγ[J]. Mol Med Rep, 2019, 19(5):3815-3822.
[20] Spranger M, Kiprianova I, Krempien S, et al. Reoxygenation increases the release of reactive oxygen intermediates in murine microglia[J]. J Cereb Blood Flow Metab, 1998, 18(6):670-674.
[21] Zhang J, Song N, Liu Y, et al. Inhibits β-amyloid-induced inflammation and oxidative stress in BV-2 cells via suppressing TLR4/NF-κBsignaling pathway and activating Nrf2/HO-1 signaling pathway[J]. Neurochem Res, 2021, 46(3):638-647.
[22] Zolotoff C, Voirin AC, Puech C, et al. Intermittent hypoxia and its impact on Nrf2/HIF-1α expression and ABC transporters: anhuman blood-brain barrier model study[J]. Cell Physiol Biochem, 2020, 54(6):1231-1248.
[23] Liu S, Wang Z, Xu B, et al. Intermittent hypoxia reduces microglia proliferation and induces DNA damage[J]. Iran J Basic Med Sci, 2016, 19(5):497-502.
[24] Gong LJ, Wang XY, Gu WY, et al. Pinocembrin ameliorates intermittent hypoxia-induced neuroinflammation through BNIP3-dependent mitophagy in a murine model of sleep apnea[J]. J Neuroinflammation, 2020, 17(1):337.
[25] Yeh FL, Wang Y, Tom I, et al. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia[J]. Neuron, 2016, 91(2):328-340.
[26] Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer's disease[J]. Cell, 2017, 169(7):1276-1290.e17.
[27] Liguori C, Mercuri NB, Nuccetelli M, et al. Obstructive sleep apnea may induce orexinergic system and cerebral β-amyloid metabolism dysregulation: is it a further proof for Alzheimer's disease risk?[J]. Sleep Med, 2019, 56:171-176.
[28] Wang H, Yang T, Sun J, et al. SENP1 modulates microglia-mediated neuroinflammation toward intermittent hypoxia-induced cognitive decline through the de-SUMOylation of NEMO[J]. J Cell Mol Med, 2021, 25(14):6841-6854.
[29] Liu S, Sun JY, Ren LP, et al. Propofol attenuates intermittent hypoxia induced up-regulation of proinflammatory cytokines in microglia through inhibiting the activation of NF-Bκ/p38 MAPK signaling[J]. Folia Neuropathol, 2017, 55(2):124-131.
[30] Wu X, Gong L, Xie L, et al. NLRP3 deficiency protects against intermittent hypoxia-induced neuroinflammation and mitochondrial ROS by promoting the PINK1-Parkin pathway of mitophagy in a murine model of sleep apnea[J]. Front Immunol, 2021, 12:628168.
[31] Wang H, Xiong W, Hang S, et al. Depletion of SENP1-mediated PPARγ SUMOylation exaggerates intermittent hypoxia-induced cognitive decline by aggravating microglia-mediated neuroinflammation[J]. Aging (Albany NY), 2021, 13(11):15240-15254.
[32] 韦美丹, 林继宗, 朱宁, 等.Aβ1-42作用的小胶质细胞对体外培养的神经干细胞生存的影响[J]. 中国病理生理杂志, 2012, 28(4):683-688.
Wei M, Lin J, Zhu N, et al. Effects of Aβ1-42-induced microglia cells on survival of neural stem cells[J]. Chin J Pathophysiol, 2012, 28(4):683-688.
[33] Harik SI, Hritz MA, LaManna JC. Hypoxia-induced brain angiogenesis in the adult rat[J]. J Physiol, 1995, 485(Pt 2):525-530.
[34] Hainsworth AH, Minett T, Andoh J, et al. Neuropathology of white matter lesions, blood-brain barrier dysfunction, and dementia[J]. Stroke, 2017, 48(10):2799-2804.
[35] Vázquez-Rosa E, Shin MK, Dhar M, et al. P7C3-A20 treatment one year after TBI in mice repairs the blood-brain barrier, arrests chronic neurodegeneration, and restores cognition[J]. Proc Natl Acad Sci U S A, 2020, 117(44):27667-27675.
[36] ElAli A, Hermann DM. Liver X receptor activation enhances blood-brain barrier integrity in the ischemic brain and increases the abundance of ATP-binding cassette transporters ABCB1 and ABCC1 on brain capillary cells[J]. Brain Pathol, 2012, 22(2):175-187.
[37] Jolivel V, Bicker F, BinameF, et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra[J]. Acta Neuropathol, 2015, 129(2):279-295.
[38] Yenari MA, Xu L, Tang XN, et al. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocyclineand[J]. Stroke, 2006, 37(4):1087-1093.
[39] Liu T, Zhang T, Yu H, et al. Adjudin protects against cerebral ischemia reperfusion injury by inhibition of neuroinflammation and blood-brain barrier disruption[J]. J Neuroinflammation, 2014, 11:107.
[40] Zhao J, Fu Y, Yamazaki Y, et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer's disease patient iPSC-derived cerebral organoids[J]. Nat Commun, 2020, 11(1):5540.
[41] Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner[J]. Neuron, 2012, 74(4):691-705.
[42] 闵颖俊, 赵俊雄, 彭行, 等. 小胶质细胞参与新生儿缺氧缺血性脑损伤所致突触异常的研究[J]. 中国病理生理杂志, 2021, 37(3):385-392.
Min Y, Zhao J, Peng X, et al. Microglia cells are involved in synaptic abnormalities caused by hypoxic-ischemic brain damage in neonates[J]. Chin J Pathophysiol, 2021, 37(3):385-392.
[43] Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models[J]. Science, 2016, 352(6286):712-716.
[44] Alawieh AM, Langley EF, Feng W, et al. Complement-dependent synaptic uptake and cognitive decline after stroke and reperfusion therapy[J]. J Neurosci, 2020, 40(20):4042-4058.
[45] Fu J, Guo F, Chen C, et al. C1 inhibitor-mediated myocardial protection from chronic intermittent hypoxia-induced injury[J]. Exp Ther Med, 2016, 12(4):2208-2214.
[46] Emin M, Wang G, Castagna F, et al. Increased internalization of complement inhibitor CD59 may contribute to endothelial inflammation in obstructive sleep apnea[J]. Sci Transl Med, 2016, 8(320):320ra1.
The role of microglia in chronic intermittent hypoxia-related cognitive impairment
XU Jia-huan, WANG Wei△
(,,110001,)
Chronic intermittent hypoxia (CIH) is an important pathophysiological feature of obstructive sleep apnea, which is involved in the occurrence and development of cognitive impairment. As the immune cells in the central nervous system, microglia plays an important role in the pathological processes of neurodegenerative diseases. This article reviews the effect of CIH on microglia including its activation, phenotypic transformation, proliferation, and apoptosis. The role of microglia in CIH-related cognitive impairment includes the decrease in protective effect and the increase in harmful effect caused by the neuroinflammation and synaptic phagocytosis of microglia.
Chronic intermittent hypoxia; Cognitive impairment; Microglia
R563; R363.2
A
10.3969/j.issn.1000-4718.2022.03.023
1000-4718(2022)03-0566-06
2021-12-23
2022-01-14
[基金项目]国家自然科学基金资助项目(No. 81670085)
Tel: 024-83282532; E-mail: wwbycmu@126.com
(责任编辑:林白霜,罗森)

