A three-dimensional matrix system containing melatonin and neural stem cells repairs damage from traumatic brain injury in rats
Xuan-Yu Fang, Da-Wei Zhao, Chao Zhang, Hong-Fei Ge, Xu-Yang Zhang,Feng-Chun Zhao, Yi-Bin Jiang, Hua Feng, Rong Hu
Abstract Brain lesions can cause neural stem cells to activate, proliferate, differentiate, and migrate to the injured area.However, after traumatic brain injury, brain tissue defects and microenvironment changes greatly affect the survival and growth of neural stem cells; the resulting reduction in the number of neural stem cells impedes effective repair of the injured area.Melatonin can promote the survival, proliferation, and differentiation of neural stem cells under adverse conditions such as oxidative stress or hypoxia that can occur after traumatic brain injury.Therefore, we investigated the therapeutic effects of melatonin combined with neural stem cells on traumatic brain injury in rats.First, in vitro studies confirmed that melatonin promoted the survival of neural stem cells deprived of oxygen and glucose.Then, we established a three-dimensional Matrigel-based transplantation system containing melatonin and neural stem cells and then used it to treat traumatic brain injury in rats.We found that treatment with the Matrigel system containing melatonin and neural stem cells decreased brain lesion volume, increased the number of surviving neurons, and improved recovery of neurological function compared with treatment with Matrigel alone, neural stem cells alone, Matrigel and neural stem cells combined, and Matrigel and melatonin combined.Our findings suggest that the three-dimensional Matrigelbased transplantation system containing melatonin and neural stem cells is a potential treatment for traumatic brain injury.
Key Words: cell therapy; magnetic resonance imaging; Matrigel; melatonin; neural stem cells; neurological function recovery; three-dimensional transplantation; traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and disability from brain damage (Taylor et al., 2017) The predominant interventions used in many countries and recommended in the treatment guidelines for the management of TBI are early detection and evacuation of intracranial hematoma, decompressive craniectomy as indicated, and mannitol dehydration (Carney et al., 2017; Marehbian et al., 2017; Huang et al., 2020).These treatment modalities have significantly improved the survival rate of patients when performed in the early stages of TBI.However, neurological dysfunctions, including hemiplegia, aphasia, and cognitive impairment, are obstacles for patients who need to remain self-sufficient and integrated into society.Therefore, the development and testing of new drugs and strategies remains a global priority for scientists and clinicians.Nanomaterials have been studied as a new treatment for TBI; some are used to inactivate reactive oxygen species to reduce toxicity that causes neuron injury, and others are used to deliver small interfering RNAs that knock down expression of the proapoptotic protein caspase 3 to protect neurons after TBI (Reddy et al.,2008; Bitner et al., 2012; Singhal et al., 2013; Yoo et al., 2017; Di Pietro et al., 2020).Unfortunately, neuroprotective agents tested in animals as interventions for TBI have so far proven ineffective in clinical trials (Green and Shuaib, 2006; Kaur et al., 2013; Neuhaus et al., 2017).Innovative ideas for the use of neural stem cells (NSCs) in the treatment of TBI continue to be developed (Gazalah et al., 2016; Jiang et al., 2019).
Endogenous NSCs can be activated and migrate to the area of TBI to participate in neural repair.NSCs are cells that have the ability to self-renew and differentiate into other subtypes of neural cells, including neurons,astrocytes, and oligodendrocytes (Crane and Trainor, 2006; Ahmed, 2009).However, several issues prevent endogenous NSCs from being exploited for TBI treatments.First, there may be too few endogenous NSCs to fully repair a TBI.Second, a series of cascade events, including inflammatory reactions, increase oxidative stress and calcium overload, which do not favor the survival of NSCs.Finally, it is difficult for NSCs to survive and persist in structurally damaged brain tissue (Zibara et al., 2019).Specific external biomaterials or drugs may allow NSCs to maintain stemness and integrate into host neural tissue (Shi et al., 2012; Gupta et al., 2013; Wang et al., 2019).The transplantation of exogenous NSCs in a suitable three-dimensional scaffold that enhances cells’ stress resistance may be an appropriate way to solve these problems.
Apart from the lack of structural support in injured brain tissue, we must also consider how to protect NSCs from the hostile microenvironment post-TBI.Therefore, we focused on investigation of the endogenous hormonemelatonin (MEL), which is synthesized and secreted by the pineal gland.Its major physiological function is to regulate biological rhythm (Chu et al.,2016).MEL can regulate the physiological activities of cells by binding to MEL receptors 1 and 2.The binding of MEL to these receptors activates Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2, Ras/Raf,and mitogen-activated protein kinases/extracellular signal-regulated kinase signal pathways (Chern et al., 2012).In the nervous system, it has been shown that MEL can promote the survival, proliferation, and differentiation of NSCs under adverse conditions such as oxidative stress or hypoxia (Shu et al., 2016;Zhang et al., 2017; Chen et al., 2020).
We sought to explore the effect of transplanting a three-dimensional system consisting of NSCs, Matrigel (MTX), and MEL into brain lesions using a TBI model in rats.In this system, MTX provides the three-dimensional support structure, and MEL enhances stress resistance and promotes the survival of NSCs.
Materials and Methods
Animals
Five pregnant Sprague-Dawley rats with embryos at embryonic day (E)13.5 and 60 adult male Sprague-Dawley rats (4 weeks, weight 250 ± 19 g,specific-pathogen-free) were obtained from the Animal Center of the Army Medical University, Chongqing, China (license No.SCXK (Yu) 20170002).All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Army Medical University.For consistency, we used male rats for ourin vivostudy.All the male rats were housed in a temperaturecontrolled room with a 12-hour light/dark cycle.They were fed a standard diet and provided with water until 12 hours before surgery.All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
Neural stem cell isolation and culture
NSCs were isolated from Sprague-Dawley rat embryos at E13.5.The pregnant rats were sacrificed after inhalation anesthesia with 2% isoflurane (RWD Life Science Co., Shenzhen, China)/air mixture (1-2 L/min).The embryos were removed from the uteri, and brain tissue was obtained from the cerebral cortex near the forehead; the brain tissue was dissociated with 0.25% trypsinethylenediamine tetraacetic acid (Thermo Fisher Scientific, Waltham, MA,USA), and trypsin treatment was stopped by the addition of 10% fetal bovine serum (Thermo Fisher Scientific) diluted with Dulbecco’s modified Eagle medium (DMEM)/F12 (Thermo Fisher Scientific).The cells were washed twice with DMEM/F12 and resuspended with complete NSC culture medium, which consists of DMEM/F12, 2% B-27 supplement (Thermo Fisher Scientific), 20 ng/mL basic fibroblast growth factor (Peprotech, Cranbury, NJ, USA), and 20 ng/mL epidermal growth factor (Peprotech).The cells were seeded into 75-cm2flasks, incubated in a humidified atmosphere with 5% CO2and 95% air at 37°C.Every 2-3 days, half of the medium was substituted with fresh NSC medium.After 7-10 days, NSCs were cultured as neurospheres (80-100µm in diameter), collected, and centrifuged at 300 ×gfor 5 minutes.The cell samples were enzymatically treated with Accutase (Innovative Cell Technologies, San Diego, CA, USA) and mechanically dissociated to give singlecell suspensions.Samples were further expanded using NSC medium into 75-cm2flasks under the same conditions described above.
Neural stem cell identification and differentiation
NSCs were identified by plating neurospheres into 15-mm-diameter glassbottom dishes (Wuxi NEST Biotechnology Co., Wuxi, China) coated with poly-L-ornithine (10 µg/mL, MilliporeSigma, Burlington, MA, USA) and incubated for 6 hours in complete NSC culture medium.Then the neurospheres were fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton X-100 for 30 minutes, and blocked with 5% bovine serum albumin for 1 hour.The neurospheres were incubated at 4°C overnight with the primary antibodies for nestin (mouse, 1:100, Santa Cruz Biotechnology, Dallas, TX, USA, Cat# sc-23927, RRID: AB_627994) or SOX2 (rabbit, 1:100, Wuhan Sanying, Wuhan,China, Cat# 11064-1-AP, RRID: AB_2195801).Then, the secondary antibodies donkey anti-rabbit IgG-CFL 555 (1:100, Santa Cruz Biotechnology, Cat# sc-362271, RRID: AB_10989578) and donkey anti-mouse IgG-CFL 488 (1:100,Santa Cruz Biotechnology, Cat# sc-362258, RRID: AB_11014318) were added for 2 hours at 37°C.Images were captured with an LSM 780 confocal microscope (Zeiss, Oberkochen, Germany).
More neurospheres were collected, centrifuged, and mechanically dissociated into single-cell suspensions to verify the differentiation ability of NSCs.Briefly, 1 × 105NSCs were plated into poly-L-ornithine-coated 15-mm-diameter glass-bottom dishes in differentiation medium composed of DMEM/F-12, 2% B-27 supplement, and 1% glutaMAX (Thermo Fisher Scientific).After 7 days, the cells were washed twice in 10 mM phosphatebuffered saline (pH 7.4; Boster Biological Technology, Wuhan, China), fixed for 30 minutes in 4% paraformaldehyde, permeabilized with 0.3% Triton X-100 for 30 minutes, and blocked with 5% bovine serum albumin for 1 hour before immunocytochemistry.The cells were incubated overnight at 4°C with the primary antibodies oligodendrocyte transcription factor 2 (Olig2;mouse, 1:200, Proteintech Group Inc., Cat# 66513-1-Ig, RRID: AB_2881876),doublecortin (DCX; rabbit, 1:200, Proteintech Group Inc., Cat# 13925-1-AP, RRID: AB_2088476), glial fibrillary acidic protein (GFAP; rabbit, 1:1000,Abcam, Cambridge, UK, Cat# ab7260, RRID: AB_305808), or microtubuleassociated protein 2 (MAP2; rabbit, 1:500, Proteintech Group Inc., Cat#17490-1-AP, RRID: AB_2137880).The cells were washed three times with 10 mM phosphate-buffered saline and incubated with donkey anti-rabbit IgG-CFL 555 (1:100, Santa Cruz Biotechnology, Cat# sc-362271, RRID: AB_10989578)or donkey anti-mouse IgG-CFL 488 (1:100, Santa Cruz Biotechnology, Cat# sc-362258, RRID: AB_11014318) for 2 hours at 37°C.After three washes with 10 mM phosphate-buffered saline, the cells were incubated with the nuclear dye 4′,6-diamidino-2-phenylindole for 10 minutes.Images were captured using an LSM 780 confocal microscope (Zeiss).
Oxygen and glucose deprivation and melatonin treatment5
Passage 3 NSCs (1 × 10) were plated into poly-L-ornithine-coated six-well plates.Oxygen and glucose deprivation (OGD) was carried out after 7 days as described (Page et al., 2016).Cells were incubated in glucose-free Earle’s Balanced Salt Solution (Thermo Fisher Scientific) with 100 µM cobalt chloride(Chongqing Chuandong Chemical [Group] Co.Ltd., Chongqing, China) for 2,4, or 6 hours.MEL (MilliporeSigma) was added into the culture medium of NSCs at 6.25, 12.5, 25, 50, or 100 µM 30 minutes before and during OGD to determine the effect of MEL on neuroprotection.
Neural stem cell viability assay
NSC viability was assessed using the Cell Counting Kit-8 in accordance with the manufacturer’s instructions (Beyotime Biotechnology, Shanghai, China).Triplicate wells in 96-well plates seeded with 1 × 105NSCs were used for each dosage group and for the untreated control group; 10 µL of solution from the Cell Counting Kit-8 was added to each well.After 2 hours of incubation at 37°C, the absorbance was measured at a wavelength of 450 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).The quantity of the formazan product is directly proportional to the number of living cells in the culture; the NSC viability results are presented as a percentage, being the absorbance of treated cells relative to untreated control cells.
In vivo traumatic brain injury model and treatment
The rats were inhalation anesthetized with 2% isoflurane/air mixture (1-2 L/min).The fur of the head was shaved, and the rats were immobilized on the brain stereotactic apparatus (RWD Life Science Co.) in the prone position.The head area was sterilized with povidone iodine.A midline incision was conducted to expose the skull.The periosteum was removed by application of 3% hydrogen peroxide solution until the cranial suture was clearly exposed.A hole of approximately 3 mm × 3 mm was made using a dental drill with the following coordinates: anterior-posterior +1-4 mm and medial-lateral +1-4 mm, relative to bregma.The dura was carefully cut using microscissors to avoid damage to blood vessels.A cavity lesion of approximately 3 mm × 3 mm × 3 mm was made in the M1 and M2 motor cortex regions according to the rat brain stereotactic atlas of Paxinos and Watson (Paxinos and Watson,2007).The bleeding was stopped, and the incision was sutured.
Sixty rats were used in the TBI study; these rats were randomly divided into five experimental groups with 12 animals in each group.1) MTX: MTX(Matrigel diluted 1:3 in the cell culture medium; BD Biosciences, San Jose,CA, USA) was added into the lesion sites; 2) NSC: NSCs cultured in complete NSC culture medium were transplanted into lesion cavities in the M1 and M2 motor cortices; 3) MTX + NSC: NSCs cultured in liquid MTXin vitrowere transplanted into lesion cavities in the M1 and M2 motor cortices; 4) MTX+ MEL: 25 µM MEL in liquid MTX was transplanted into lesion cavities in the M1 and M2 motor cortices; and 5) MTX + NSC + MEL: NSCs cultured in liquid MTX and 25 µM MEL were transplanted into lesion cavities in the M1 and M2 motor cortices.To prepare the NSCs for transplantation, the cells were collected from culture medium by centrifugation, dissociated using Accutase,resuspended at 1 × 108cells/mL, and labeled with CellTrackerTMCM-Dil Dye(Thermo Fisher Scientific).The cells were mixed with MTX or MEL, if required,and 1 × 106NSCs were transplanted into each rat brain lesion, if required.The transplantation procedure is shown in Figure 1.
Behavioral evaluation
The modified neurological severity score (mNSS) (Chen et al., 2001) was determined immediately after treatment and on days 3, 7, and 14 after treatment by an investigator who was blinded to the experimental groups.Other indicators for the animal groupings were not blinded.The mNSS includes motor, sensory, reflex, and balance tests and has a range of 0-18 points.A score of 18 points implies severe neurological deficit and a score of 0 implies normal performance, 13-18 points implies severe injury, 7-12 points implies moderate injury, and 1-6 points implies mild injury.The details of the mNSS are shown in Additional Table 1.
Magnetic resonance image examination
Thein vivolesions were monitored using a Bruker 7.0T small-animal magnetic resonance image scanner (Bruker Corporation, Billerica, MA, USA) on day 14 after treatment.Proper positioning and image quality were verified by initially obtaining a scout image.T2-weighted images (T2WIs) were acquired using a TurboRARE-T2 WI scanning sequence.The imaging parameters used were as follows: repetition time/echo time, 4000 ms/45 ms; field of vision, 25 mm ×25 mm; and slice thickness, 0.5 mm.The open-source software 3D Slicer 4.10.2(Fedorov et al., 2012) was used to analyze high-signal areas to determine the volume of the damaged site.
Histological examination
On day 14 after treatment, the rats were sacrificed after inhalation anesthesia with 2% isoflurane/air mixture (1-2 L/min) and then were perfused transcardially with 100 mL cold 0.9% normal saline followed by 100 mL 4% paraformaldehyde (adjusted pH 7.4; Boster Biological Technology).Brain samples were removed and fixed in 10% neutral buffered formalin,dehydrated, and embedded in paraffin.Sections were cut at a thickness of 5 µm.The wax was removed by immersion of sections in xylene twice for 10 minutes each time.The sections were rehydrated by immersion in 100%ethanol, 100% ethanol, 90% ethanol, 80% ethanol, and then 70% ethanol,each for 5 minutes.Then the sections were washed in water, immersed in filtered Harris hematoxylin for 10 seconds, and washed until the water was clear.Sections were immersed in eosin stain for 30 seconds and washed again.Samples were examined and imaged with an optical microscope (IX71;Olympus Corporation, Tokyo, Japan).
Immunofluorescence
On day 14 after treatment, the rats were sacrificed after inhalation anesthesia with 2% isoflurane/air mixture (1-2 L/min).The brain tissues of rats were obtained and fixed using 4% paraformaldehyde (Boster Biological Technology).The brain tissues were covered with Tissue-Tek® O.C.T.Compound (Sakura Finetek, Torrance, CA, USA) and frozen on a cutting plate at -20°C.Cryosections were sliced using a CM1860 UV cryostat (Leica Biosystems, Nussloch, Germany) for histological analysis.Sections were cut at a thickness of 10 µm and stained at 4°C for 12 hours with nestin antibodies as an NSC marker (mouse, 1:100, Santa Cruz Biotechnology, Cat# sc-23927,RRID: AB_627994) and GFAP antibodies as an astrocyte marker (rabbit, 1:500,Abcam, Cat# ab7260, RRID: AB_305808).Donkey anti-mouse IgG-CFL 488(1:500, Santa Cruz Biotechnology, Cat# sc-362258, RRID: AB_11014318),donkey anti-rabbit IgG-CFL 488 (1:500, Santa Cruz Biotechnology, Cat# sc-362261, RRID: AB_10989100), mouse anti-rabbit IgG-CFL 647 (1:500, Santa Cruz Biotechnology, Cat# sc-516251), and goat anti-mouse IgG H&L (Alexa Fluor® 647, 1:500, Abcam, Cat# ab150115, RRID: AB_2687948) were used as secondary antibodies for incubation at 37°C for 2 hours.4′,6-Diamidino-2-phenylindole was used to stain nuclei to evaluate NSC survival, proliferation,and differentiation in the injected grafts.A Zeiss LSM 780 confocal microscope was used to observe and count CM-Dil-positive transplanted cells.
Statistical analysis
No statistical methods were used to predetermine sample sizes.All experiments were repeated at least in triplicate.No animals or data points were excluded from analysis.Data were analyzed using GraphPad Prism version 6.02 for Windows (GraphPad Software, San Diego, CA, USA; www.graphpad.com) and are presented as the mean ± standard deviation (SD).Intergroup differences for mNSS were evaluated by two-way analysis of variance followed by Tukey’s multiple comparisons test when the overall difference was significant.The other intergroup comparisons were evaluated by one-way analysis of variance followed by Tukey’s multiple comparisons test or Dunnett’s multiple comparisons test when the overall difference was significant.APvalue less than 0.05 was considered statistically significant.
Results
Identification of cultured neural stem cells
The neurospheres were positive for expression of nestin and SOX2, specific markers of NSCs, using immunocytochemistry; this confirmed the cells we isolated were NSCs (Figure 2).We also confirmed the ability of the NSCs to differentiate.NSCs were cultured in differentiation medium and were shown to express the differentiation markers DCX and MAP2 for neurons, Olig2 for oligodendrocytes, and GFAP for astrocytes (Figure 3).
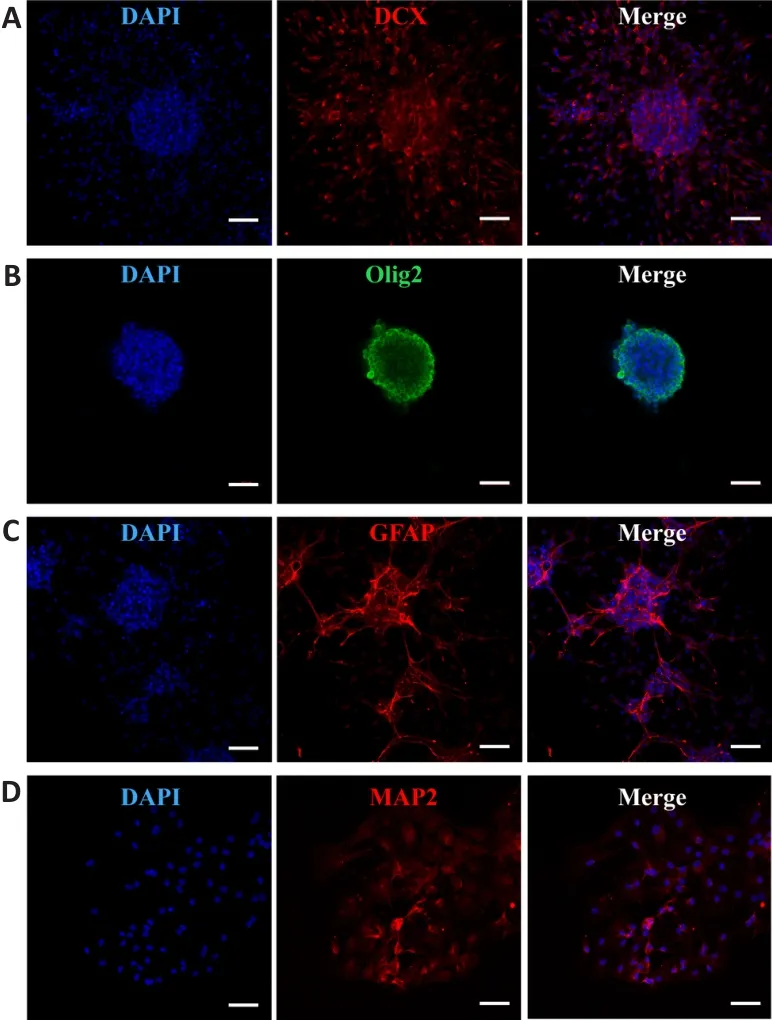
Figure 3 | Neural stem cell differentiation was identified by immunofluorescence.
Melatonin promotes the viability of neural stem cells under oxygen and glucose deprivation
TBI can affect cell viabilityin vivo.To replicate TBIin vitro, we used the OGD model in NSCs.NSC viability gradually decreased with time after OGD (Figure 4).We selected 4 hours as the time point for the follow-up test because the damage to NSCs was too severe at 6 hours.MEL (0, 6.25, 12.5, 25, 50,or 100 µM) was added into the culture medium to treat NSCs 30 minutes before OGD conditions were introduced.After 4 hours of OGD, we tested the neuroprotective effect of MEL treatment.We found that NSC viability significantly increased as the concentration of MEL increased.MEL at 25 µM significantly enhanced the viability of NSCsin vitroand was neuroprotective.No additional protective effect was observed at 50 or 100 µM MEL (Figure 5).Therefore, 25 µM MEL was selected for further studiesin vivo.

Figure 4|Oxygen and glucose deprivation decreases neural stem cell viability.
A three-dimensional matrix system containing melatonin and neural stem cells reduces the volume of damage and ameliorates behavioral scores in rats with traumatic brain injury
We tested whether MEL promotes recovery of neurological functionsin vivoafter addition of MEL to three-dimensional systems composed of MTX and NSCs.Magnetic resonance imaging was performed on day 14 after treatment.T2WIs revealed the lesions in rats in the MTX + NSC + MEL group were markedly decreased compared with the other groups (P< 0.05; Figure 6A-F).The mNSS was used to assess the neurological recovery of all rats immediately after treatment and on days 3, 7, and 14 after treatment.The mNSS gradually decreased over time.The mNSS of the MTX + NSC + MEL group was significantly lower compared with the MTX (P< 0.01) and NSC (P< 0.01)groups on day 7 and 14 (Figure 6G).

Figure 6| Effect of the three-dimensional matrix system containing melatonin and neural stem cells on brain magnetic resonance imaging and the modified neurological severity score of rats with traumatic brain injury.
A three-dimensional matrix system containing melatonin and neural stem cells promotes retention and survival of neural stem cells in the lesions in rats with traumatic brain injury
Histological examinations were used to determine internal and peripheral changes to brain lesions after TBI.Hematoxylin and eosin staining revealed persisting MTX and cells within the matrix network in rats that received MEL and non-MEL treatments (MTX + NSC and MTX + NSC + MEL groups).An established sustaining network structure inside the lesion was evidence that cell growth and differentiation had occurred.There was significantly more residual MTX in the MTX + NSC + MEL group compared with the other groups,and the results of cell counting also showed the most cell survival in the lesions treated with MTX + NSC + MEL (Figure 7).
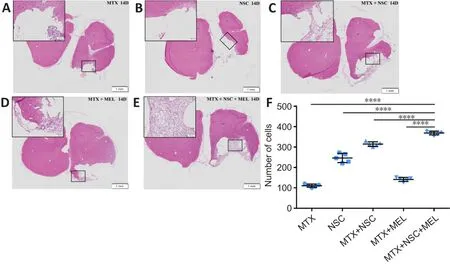
Figure 7| Effect of the three-dimensional matrix system containing melatonin and neural stem cells (NSCs) on pathological injury in the brains of rats with traumatic brain injury on day 14 after treatment.
To further observe the retention, survival, and differentiation of NSCs after implantation, immunofluorescence staining for nestin and GFAP was performed on the rat brain samples.The MTX (Figure 8A) and MTX + MEL(Figure 8D) groups had no CM-Dil-positive cells in the lesions.Tissue samples of lesions in NSC (Figure 8B), MTX + NSC (Figure 8C), and MTX + NSC + MEL(Figure 8E) groups expressed nestin and GFAP on day 14.It is clear that the MTX + NSC + MEL group exhibited more CM-Dil-positive cells compared with the NSC and MTX + NSC groups (Figure 8F), which indicates that the combination of Matrigel scaffold and melatonin effectively protected the retention and survival of NSCs within the brain lesions of rats with TBI.Furthermore, the increased number of GFAP-positive cells in the NSC, MTX+ NSC, and MTX + NSC + MEL groups suggests that NSCs mainly differentiate into glial cells.
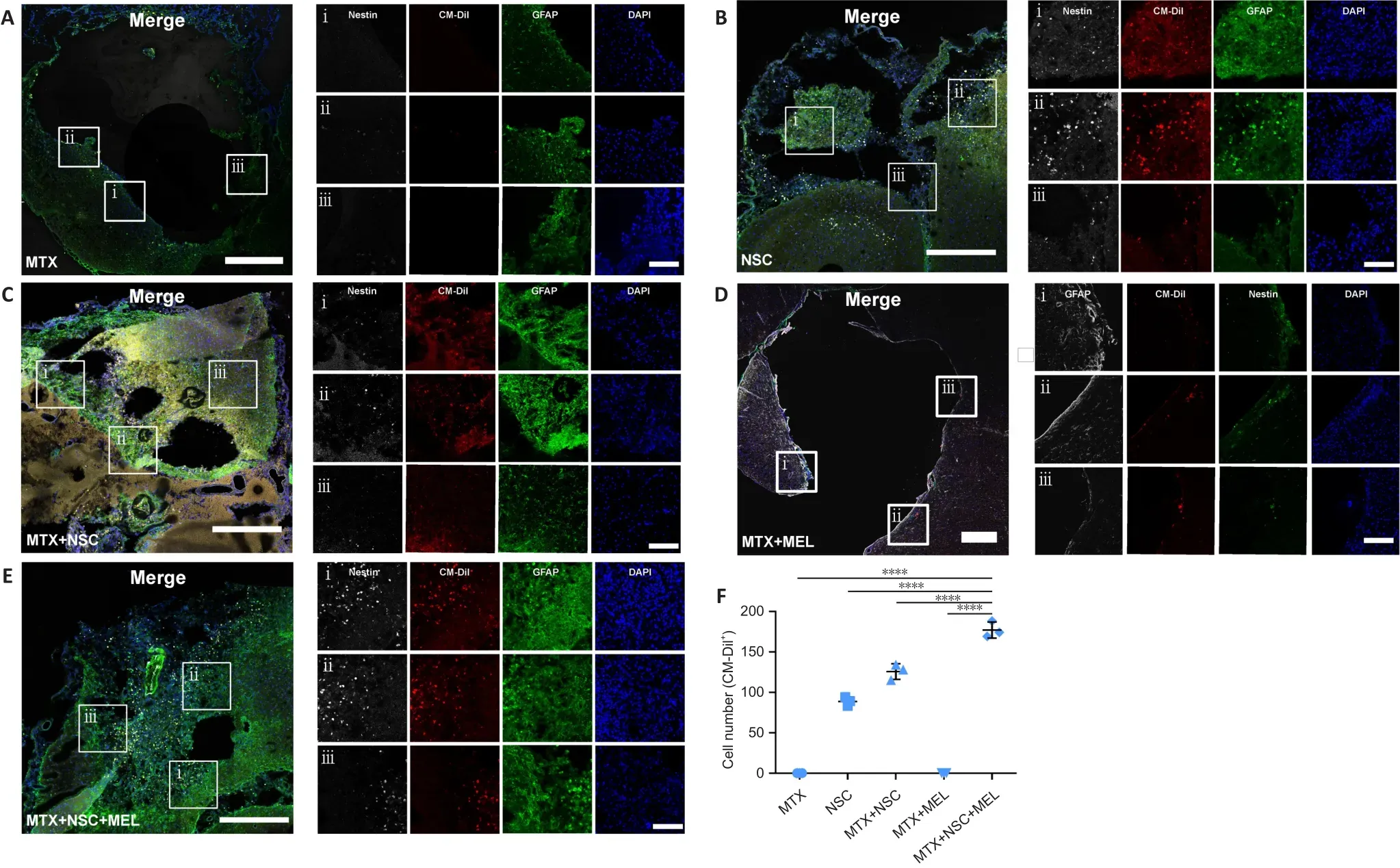
Figure 8 | Effect of the three-dimensional matrix system containing melatonin and neural stem cells on the retention, survival, and differentiation of neural stem cells in the brains of rats with traumatic brain injury on day 14 after treatment.
Discussion
TBI is a complex disease that causes structural damage and functional deficit.Knowledge of the pathological mechanisms of TBI has improved in recent years (Pavlovic et al., 2019).However, complex pathological changes still present challenges for the treatment of TBI (Galgano et al., 2017).Thus far,neuroprotective drugs have proven ineffective in clinical trials for TBI (Green and Shuaib, 2006; Kaur et al., 2013; Neuhaus et al., 2017).The discovery of NSCs has led to recent progress in treatment opportunities for TBI(Ahmed et al., 2016; Zibara et al., 2019).Nevertheless, challenges remain,including successful survival, proliferation, and differentiation of NSCs;in vivoreconstruction of three-dimensional structures; and clinical safety.Many recent studies have shown that MEL plays an important role in the survival(Kong et al., 2008), proliferation (Li et al., 2019), and differentiation of NSCs(Shu et al., 2016).MEL also enhances the stress resistance of NSCs under adverse conditions (Chern et al., 2012; Deng et al., 2015; Chen et al., 2020).MEL possesses antioxidant properties; it scavenges excess free radicals and increases the synthesis of antioxidant enzymes, including superoxide dismutase, glutathione peroxidase, and glutathione reductase (Poeggeler et al., 1994).In addition, MEL has other biological properties, including antiinflammatory (Yu et al., 2017; Chen et al., 2018), antiapoptotic (Xu et al.,2018), and mitochondrial-modulating activities (Mendivil-Perez et al., 2017;Suofu et al., 2017).In this study, MEL significantly promoted the survival rates of NSCs under conditions of OGD.
The microenvironment helps determine the fate of NSCs; some detrimental effects of TBI can be explained by increased neuroinflammation (Smith et al., 2013; Faden and Loane, 2015).Glial cells assemble to build glial scars to defend against neuroinflammation; however, glial cells can impede tissue repair and neural-network rebuilding, which contributes to poor outcomes after TBI (David and Kroner, 2011; Kumar and Loane, 2012).Secondary pathological reactions after trauma are detrimental to cell survival and can limit the benefits of therapeutics.Research efforts have focused on ways to improve the effects of therapeutics.Some biomimetic biomaterials enhance the retention, survival, and proliferation of stem cells (Barros et al.,2019; Wilems et al., 2019) and thereby accelerate the remodeling of brain structures and the recovery of neurological functions in rat models of TBI(Betancur et al., 2017; Sahab Negah et al., 2019).We used this knowledge as a starting point for our experiments.A new three-dimensional system based on MTX, NSCs, and MEL was developed and transplanted into the lesion cavity of the rat model of TBI.
In our study, the lesion cavity was precisely established by surgery in the M1 and M2 motor cortices.Our precise model provided a way to estimate the effect of damage to specific regions.Fourteen days after transplantation,T2WIs revealed that MTX was filling in the lesion cavities.Histological results revealed that MTX created a sustaining network structure inside the lesion cavity suitable for the support of cells.More residual MTX and surviving cellswere observed in the presence of MEL, NSCs, and MTX combined, compared with the treatment with NSCs or MTX alone.No cell retention was observed in the lesion cavity of rats treated with NSCs alone.Our results show that MTX can be used as a supporting structure to provide a three-dimensional scaffold for NSC retention, survival, proliferation, and differentiation.We also found a significant decline in mNSS in rats treated with the combination of MTX, NSCs,and MEL on the 7thand 14thdays, compared with the other groups.
Our study has several limitations.We confirmed that the NSCs differentiated into neuronsin vitroand into astrocytesin vivo.Unfortunately, our data were insufficient to confirm neuronal differentiation of NSCsin vivo, and this effect needs to be studied further.We should test NSCs using more precise observation time-frames, and more in-depth studies are needed to clarify the effect of our MTX-based three-dimensional system on the repair of damaged neural structures.In addition, we still need to understand more about the protective mechanisms of MEL that makes cells react to brain damage after TBI.It would be interesting to test why different concentrations of melatonin give different resultsin vitro, and the concentration of melatonin required for successful clinical treatment should be determined.
In summary, our study shows that the combination of a three-dimensional system based on MTX, MEL, and stem cell transplantation has potential to improve tissue structure and neural-network rebuilding in rats with TBI.MTX provides the spatial physical-support structure to support stem cells, whereas MEL exerts its neuroprotective functions by promoting the survival of NSCs.Cell-based therapies combined with biomaterials could be the future strategy of choice for tissue repair, and we look forward to further development of these therapies for the treatment of TBI.
Author contributions:Study conception and design, and manuscript revision:RH, HF; experiment implementation: XYF, XYZ, FCZ, HFG; data analysis: RH,DWZ, CZ, YBJ; image preparation and manuscript draft: XYF, DWZ.All authors read and approved the final manuscript.
Conflicts of interest:All authors declare no conflicts of interest.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long asappropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Peng Li, VCU Massey Cancer Center, USA.
Additional files:
Additional Table 1:Modified neurological severity score.
Additional file 1:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Interplay of SOX transcription factors and microRNAs in the brain under physiological and pathological conditions
- Cerebellar pathology in motor neuron disease:neuroplasticity and neurodegeneration
- Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease
- An atypical ubiquitin ligase at the heart of neural development and programmed axon degeneration
- The endogenous progenitor response following traumatic brain injury: a target for cell therapy paradigms
- The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer’s disease