Cerebellar pathology in motor neuron disease:neuroplasticity and neurodegeneration
Rangariroyashe H.Chipika , Grainne Mulkerrin , Pierre-François Pradat,Aizuri Murad Fabrice Ango, Cédric Raoul, Peter Bede ,
Abstract Amyotrophic lateral sclerosis is a relentlessly progressive multi-system condition.The clinical picture is dominated by upper and lower motor neuron degeneration, but extra-motor pathology is increasingly recognized, including cerebellar pathology.Post-mortem and neuroimaging studies primarily focus on the characterization of supratentorial disease, despite emerging evidence of cerebellar degeneration in amyotrophic lateral sclerosis.Cardinal clinical features of amyotrophic lateral sclerosis, such as dysarthria, dysphagia, cognitive and behavioral deficits, saccade abnormalities, gait impairment,respiratory weakness and pseudobulbar affect are likely to be exacerbated by co-existing cerebellar pathology.This review summarizes in vivo and post mortem evidence for cerebellar degeneration in amyotrophic lateral sclerosis.Structural imaging studies consistently capture cerebellar grey matter volume reductions, diffusivity studies readily detect both intra-cerebellar and cerebellar peduncle white matter alterations and functional imaging studies commonly report increased functional connectivity with supratentorial regions.Increased functional connectivity is commonly interpreted as evidence of neuroplasticity representing compensatory processes despite the lack of post-mortem validation.There is a scarcity of post-mortem studies focusing on cerebellar alterations, but these detect pTDP-43 in cerebellar nuclei.Cerebellar pathology is an overlooked facet of neurodegeneration in amyotrophic lateral sclerosis despite its contribution to a multitude of clinical symptoms,widespread connectivity to spinal and supratentorial regions and putative role in compensating for the degeneration of primary motor regions.
Key Words: amyotrophic lateral sclerosis; ataxia; cerebellum; magnetic resonance imaging; motor neuron disease; neuroimaging; neuroplasticity; pathology; primary lateral sclerosis; pseudobulbar affect
Introduction
Amyotrophic lateral sclerosis (ALS) is a relentlessly progressive neurodegenerative condition.While it is primarily characterized by the degeneration of upper and lower motor neurons, ALS is now recognized as a multi-system disorder.Cerebellar pathology in ALS has been demonstrated by a multitude of neuropathology and neuroimaging studies (Prell and Grosskreutz, 2013), but its role in the disease process is poorly characterized.Clinical reports of overt cerebellar signs are sparse, frank dysmetria,dysdiadochokinesia and ataxia are rarely reported in ALS (Machida et al.,1999; Schimke et al., 2002; Yasser et al., 2010; De Marco et al., 2015).The clinical assessment of the cerebellum in ALS is notoriously challenging due to concomitant upper and lower motor neuron degeneration.Eye-movement abnormalities, such as nystagmus (Kushner et al., 1984), pursuit (Jacobs et al.,1981; Gizzi et al., 1992; Donaghy et al., 2010) and saccadic impairment (Gizzi et al., 1992; Averbuch-Heller et al., 1998; Donaghy et al., 2010) have been consistently described in ALS.Bulbar dysfunction is a pathognomonic feature of ALS (Yunusova et al., 2019) which is often exclusively linked to corticobulbar tract and brainstem nuclei degeneration despite the likely contribution of cerebellar pathology to bulbar symptoms (Sasegbon and Hamdy, 2021).Primary lateral sclerosis (PLS) is a progressive, low-incidence upper motor neuron disorder which carries a better prognosis than ALS.A common symptom of both ALS and PLS is pseudobulbar affect, which is traditionally linked to corticobulbar disconnection, but cerebellar pathology is increasingly recognized as an important aetiological factor (Parvizi et al., 2007; Floeter et al., 2014; Christidi et al., 2018c).
Functional Anatomy
The cerebellum comprises two hemispheres with deep fissures and folia connected by the vermis.It is located in the posterior cranial fossa, inferior to the occipital and temporal lobes and posterior to the pons.Each hemisphere has three lobes, the flocculonodular lobe, anterior lobe, and posterior lobe divided by two fissures - the posterolateral fissure and the primary fissure.(Figure 1) The white matter surrounds four cerebellar nuclei, the fastigial,emboliform, globose and dentate nuclei.Functionally, the cerebellum is classically divided into three zones: (1) the vestibulocerebellum which is the flocculonodular lobe.It is primarily involved in balance and ocular reflexes.Inputs arise from vestibular nuclei and semicircular canals as well as visual inputs from superior colliculi and visual cortex and output synaps onto the vestibular nuclei; (2) the spinocerebellum which is made up of the vermis and the paravermis or intermediate zone on either side of the vermis.It is involved in the adjustment of body movements, error correction, and proprioception.It receives proprioceptive inputs from the dorsal columns of the spinal cord and trigeminal nerve and also visual and auditory inputs and has projections onto the deep cerebellar nuclei and brainstem; (3) the cerebrocerebellum which comprises the lateral hemispheres, lateral to the intermediate zone.It is involved in planning movements, motor learning as well as cognitive functions.It receives inputs from the cerebral cortex and pons and sends outputs to the thalamus and red nuclei (Fitzgerald et al., 2012).Phylogenetically, the cerebellum is divided as follows: (1) the archicerebellum which is the vestibulocerebellum, and is evolutionarily the oldest structure;(2) the paleocerebellum which is the spinocerebellum; (3) the neocerebellum which is the cerebrocerebellum, and is evolutionarily the newest structure(Fitzgerald et al., 2012).There are three major fiber bundles connecting the cerebellum to the rest of the brain known as the cerebellar peduncles.The superior cerebellar peduncle or brachium conjunctivum is the primary output pathway of the cerebellum.It is mostly made up of efferent fibers originating from the dentate nucleus projecting onto the midbrain, thalamus and medulla.The dentato-rubro-thalamo-cortical and cerebello-thalamo-cortical pathways pass through this peduncle.This peduncle is primarily involved in motor planning.The middle cerebellar peduncle or brachium pontis is primarily made up of afferent fibers originating from the pontine nuclei as part of the cortico-ponto-cerebellar tract.The inferior cerebellar peduncle(restiform and juxtarestiform bodies) is made up of both afferent and efferent fibres involved in proprioception and vestibular functions.The dorsalspinocerebellar tract passes through the inferior peduncle.This peduncle is involved in the maintenance of posture and balance (Fitzgerald et al., 2012).(Figure 1) Neurulation is the process by which the central portion of the ectoderm forms the neural plate which is the origin of the entire nervous system.At week 3 of gestation, the neural plate folds to form the neural tube,which in week 4 undergoes flexion at the region of the mesencephalon, which is the future midbrain.Above the mesencephalon is the prosencephalon,which is the future forebrain and beneath it is the rhombencephalon, which is the future hindbrain.The caudal rhombencephalon develops into the medulla oblongata and the rostral part becomes the pons and cerebellum (Fitzgerald et al., 2012).
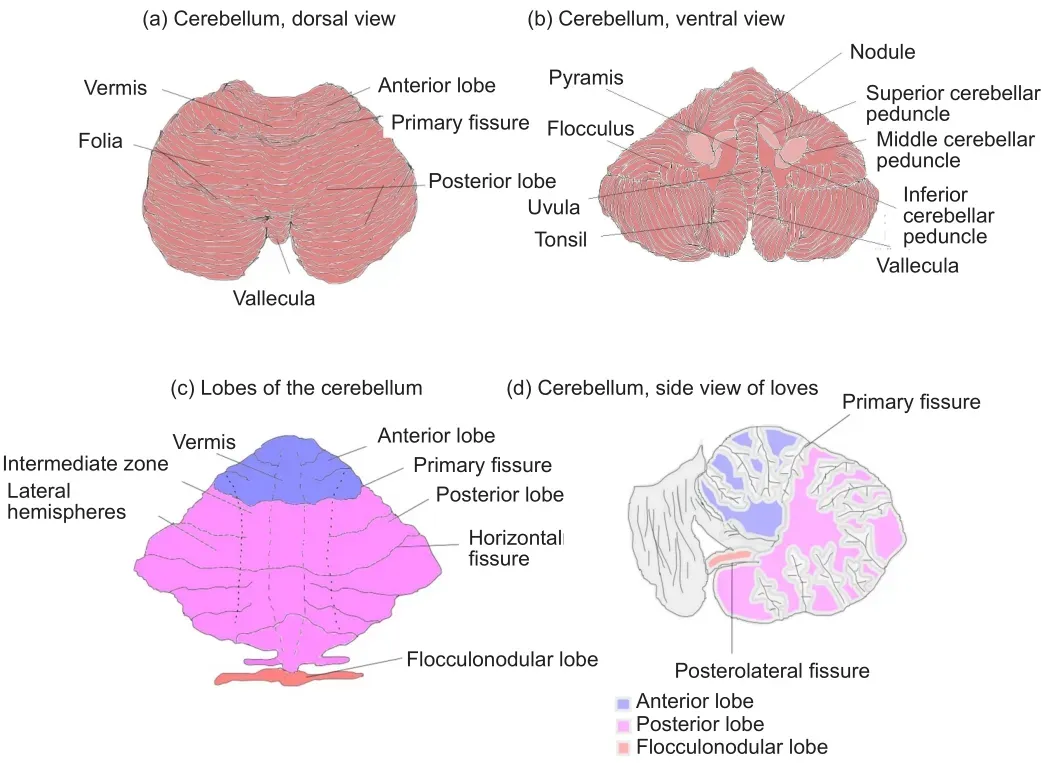
Figure 1|Gross anatomy of the cerebellum.
The cerebellar function has been traditionally associated with motor control and motor learning, however, physiological role in mediating cognitive and behavioral functions such as executive functions, language, spatial cognition,and mood regulation is also increasingly recognized (Buckner, 2013).The‘Dysmetria of thought’ theory was coined to denote cognitive and emotional disorders arising from cerebellar pathology (Schmahmann, 1998).Cerebellar lobules are not only activated during motor and sensorimotor task, but during executive, working memory, language, and emotional processing paradigms (Stoodley and Schmahmann, 2009).Cerebellar pathology has been consistently implicated in impaired social cognition (Van Overwalle et al.,2015), pathological crying and laughing (Bede and Finegan, 2018; Finegan et al., 2019a), and language deficits (Runnqvist et al., 2016).Neuropsychological domains that show focal cerebellar activity on cerebellar imaging studies include attention (Allen and Courchesne, 2003), working memory (Desmond et al., 1997), and envisioning future events (Szpunar et al., 2007).Attention and visuospatial skills are noted to be particularly impaired in subjects with cerebellar insults (Malm et al., 1998).Emotional dysregulation including irritability, disinhibition, and impulsivity has been linked to lesions of the vermis (Levisohn et al., 2000).While lesions in the anterior lobe have been primarily linked to motor deficits, pathology of the posterior lobe is associated with cerebellar cognitive affective syndrome, encompassing problems with language, speech, executive and visuospatial functions and emotional affect(Tedesco et al., 2011; Argyropoulos et al., 2020).Evidence is slowly mounting that specific lobules have specific roles in mediating cognitive processes(Stoodley and Schmahmann, 2009; Argyropoulos et al., 2020).Based on the expanding spectrum of physiological roles of the cerebellum and the high number of recently published imaging studies in ALS, the main aim of this review is to summarise cerebellar neuroimaging and post-mortem findings in ALS and other motor neuron disease phenotypes.An additional objective of this manuscript is to examine the evidence for cerebellar neuroplasticity in ALS and link radiological observations to post mortem findings.
Search Strategy and Selection Criteria
A systematic literature search was performed on PubMed using the core search terms “amyotrophic lateral sclerosis”, “primary lateral sclerosis” and“motor neuron disease” combined individually with each of the following keywords as search word pairs: “cerebellum”, “cerebellar”, “magnetic resonance imaging”, “positron emission tomography”, and “post mortem”.Only original research papers were systematically reviewed.Conference abstracts, review papers, opinion pieces, and editorials were excluded.No exclusion criteria were set based on the year of publication.Based on the above criteria, a total of 69 original research papers were identified, selected,and reviewed.
Results
Imaging
Only two cerebellar studies included pre-symptomatic patients (Menke et al., 2016; Papma et al., 2017).About half of the identified studies collected genetic information, but only 11 studies stratified their ALS cohort by genotype (Tanaka et al., 1993; Canosa et al., 2016; Agosta et al., 2017;Schonecker et al., 2018; Calvo et al., 2019; Abidi et al., 2020, 2021; Hu et al., 2020).The vast majority of identified studies were cross-sectional with only 4 longitudinal imaging studies describing cerebellar changes (Keil et al., 2012; Menke et al., 2018; Calvo et al., 2019).Sample size limitations are evident, with only a minority of studies including over 100 participants.Four studies investigated over 200 participants and most of these were PET studies(Pagani et al., 2014, 2016; Canosa et al., 2020).The majority of studies were case-control studies, with only a few exceptions.While most studies reported complementary clinical or neuropsychological assessments, only a handful of studies performed clinic-radiological correlations (Verstraete et al., 2015; Consonni et al., 2019; Sala et al., 2019; Abidi et al., 2020; Canosa et al., 2021b).A minority of studies acquired data on 1.5 Tesla (T) scanners,3T scanners were most commonly used and only one study collected data on a 7T platform (Barry et al., 2021).Over half the studies assessed multiple imaging parameters.
Grey matter alterations
While the cerebellar imaging data are characteristically challenging to quantitatively interpret, novel pipelines have been developed to evaluate infratentorial structures.Cerebellar changes are often observed on wholebrain analyses (Prell and Grosskreutz, 2013).Structural imaging readily captures cerebellar grey matter atrophy in ALS and ALS-Plus patients (Kim et al., 2017; Christidi et al., 2018d, e) and progressive cerebellar degeneration has been detected longitudinally.Cerebellar degeneration has been described inC9orf72positive ALS patients (Agosta et al., 2017), as well as in presymptomatic GGGGCC hexanucleotide carriers above 40 years of age (Papma et al., 2017).A study specifically investigating genotype-associated cerebellar profiles in ALS patients revealed symmetrical patterns of cerebellar atrophy inC9orf72ALS preferentially affecting lobules I-IV, V, VIIIA/B, IX, and the vermis (Bede et al., 2021a).InC9orf72negative sporadic ALS patients, more focal changes were observed in lobules I-IV and V on morphometric analyses(Bede et al., 2021a).Structural cerebellar changes in the anterior lobule have been correlated to disease severity, and changes in the posterior lobule have been linked to cognitive impairment (Consonni et al., 2019).Interestingly, one study reported increased grey matter volume in the cerebellum in ALS (Qiu et al., 2019), and another detected no cerebellar atrophy (Feron et al., 2018;Schonecker et al., 2018; Additional Table 1).
White matter changes
Cerebellar white matter alterations have also been specifically studied (Keil et al., 2012; Hartung et al., 2014; Trojsi et al., 2015) and genotype-associated patterns have recently been proposed (Bede et al., 2021a).Decreased fractional anisotropy and increased axial diffusivity and radial diffusivity in lobules I-IV, V have been observed inC9orf72ALS, while in sporadic ALS,decreased fractional anisotropy in lobules I-IV, V, IX and crura I and II and increased radial diffusivity in lobules I-IV, V, and VI was detected (Bede et al., 2021a).Cerebro-cerebellar tractography in ALS reveals preferential white matter degeneration in the cerebellar peduncles and ponto-cerebellar tracts(Minnerop et al., 2009; Prudlo et al., 2012; Christidi et al., 2018c; Baek et al.,2020; Bharti et al., 2020; De Marchi et al., 2020; Trojsi et al., 2020; Bede et al., 2021a).Significant alterations were also observed in the dentato-rubrothalamo-cortical tract proximal to the motor cortex in both ALS and PLS (Tu et al., 2019).Progressive diffusion tensor imaging changes were noted in the cerebellum in a longitudinal study conducted over 2 years (Menke et al.,2018; Additional Table 1).
Functional and metabolic studies
Functional magnetic resonance imaging findings are relatively inconsistent(Proudfoot et al., 2018).Both decreased (Fekete et al., 2013; Trojsi et al., 2020; Barry et al., 2021) and increased cerebro-cerebellar connectivity have been reported (Agosta et al., 2011; Zhou et al., 2013; Menke et al.,2016; Abidi et al., 2021), the latter postulated to represent a compensatory process (Abidi et al., 2021).An increase in functional connectivity has been observed between the cerebellum and a network comprising the precuneus,cingulate, and middle frontal lobe in both presymptomatic and symptomatic ALS (Menke et al., 2016).Increased functional connectivity between bilateral superior parietal lobule and right anterior inferior cerebellum is thought to correlate with disease severity (Zhou et al., 2013), but direct clinicoradiological correlations are widely seen as contentious (Verstraete et al.,2015).In the paradigm-based studies increased cerebellar activation is often observed during various motor tasks (Han and Ma, 2006; Konrad et al., 2006),and particularly in patients with upper motor neuron (UMN) predominant symptoms (Abidi et al., 2020, 2021).In ALS with cognitive impairment,alterations of regional homogeneity in the right inferior cerebellar area have been noted (Hu et al., 2020).Increased cerebellar ‘degree centrality’ was observed in the posterior lobes and bilateral cerebellum crura (Zhou et al.,2016).The involvement of the cerebellar nuclei has also been demonstrated.While no volume reductions were noted in the dentate nucleus, the restingstate functional connectivity of the dentate nucleus is thought to correlate with WM changes at the superior cerebellar peduncle (Bharti et al., 2020;Additional Table 2).Significantly reduced mean regional cerebral blood flow has been also observed in the cerebellar hemispheres (Tanaka et al., 1993).The cerebellum is rarely evaluated by spectroscopy, but one study found that the cerebellar N-acetylaspartate concentrations are unaltered in patients with motor neuron disease (Gredal et al., 1997; Additional Table 2).While some PET studies detect no changes in the cerebellum (Ludolph et al., 1992), somedescribe hypometabolism (Calvo et al., 2019), the majority of PET studies identify cerebellar hypermetabolism in ALS (Cistaro et al., 2012; Canosa et al., 2016; Matías-Guiu et al., 2016; Buhour et al., 2017), both in spinal-onset disease (Pagani et al., 2014; Sala et al., 2019), bulbar-onset (Sala et al., 2019),as well as in ALS-FTD (Canosa et al., 2020).The reports are conflicting, with another study reporting (Additional Table 3).
Post-mortem findings
While cerebellar changes are seldom evaluated specifically, cerebellar pathology is readily observed post-mortem (Ishihara et al., 2006; Kobayashi et al., 2011).UBQLN-positive cytoplasmic inclusions in the cerebellar granular layer (Brettschneider et al., 2012), neurofibrillary tangles (Yokota et al., 2006),and neuronal and glial TDP-43 pathology have been consistently described(Geser et al., 2008).Cerebellar degeneration has been linked to bulbar ALS(Shellikeri et al., 2017), but other studies do not identify significant cerebellar alterations (Przedborski et al., 1996; Petri et al., 2006; Additional Table 4).p62 positive, p-TDP-43 negative neuronal intranuclear inclusions (Rohrer et al., 2011) have been observed inC9orf72and the severity of cerebellar changes are thought to depend on repeat length expansion (van Blitterswijk et al., 2013).In PLS, cerebellar involvement is clearly established.Some studies observed ballooning of dendrites in molecular and Purkinje cell layers of the cerebellum (Sugihara et al., 1999), and dentate nucleus degeneration(Kosaka et al., 2012), while others found no significant changes (Engel and Grunnet, 2000).
Animal models
With few exceptions (Aguirre et al., 2005), animal models of ALS also exhibit cerebellar pathology.InSOD1(G93A)transgenic mice, PARP-immunoreactive astrocytes (Chung et al., 2004) and pERK-immunoreactive astrocytes(Chung et al., 2005) were observed in cerebellar nuclei.Reduced tau-mRNA expression was observed at the symptomatic stage of ALS (Barańczyk-Kuźma et al., 2007).Increased cerebellar TRPV4 expression (Lee et al., 2012)and enhancedLOXgene expression (Li et al., 2004) have also been shown.Cerebellar Purkinje cell degeneration and neuroinflammation were noted inMATR3 S85Cknock-in mice (Kao et al., 2020),C9orf72mice (Chew et al.,2015) as well as inSOD1mice (Li et al., 2004).
Discussion
Neuroimaging and post-mortem infratentorial pathology in ALS
It is increasingly recognized that symptom manifestation in ALS is preceded by a long pre-symptomatic phase (Eisen et al., 2014b; Schuster et al.,2015), and to clarify the role of the cerebellum in the pathogenesis and verify proposed compensatory processes, more pre-symptomatic imaging studies are needed specifically evaluating infratentorial changes (Menke et al., 2016; Papma et al., 2017).Cerebellar findings in ALS are strikingly inconsistent; many studies report widespread cerebellar degeneration (Tu et al., 2019; Bede et al., 2021a), others detect no changes (Przedborski et al., 1996; Gredal et al., 1997; Petri et al., 2006; Schonecker et al., 2018).Both increased (Qiu et al., 2019) and decreased grey matter volumes have been reported, both increased (Agosta et al., 2011; Zhou et al., 2013; Menke et al., 2016; Abidi et al., 2021) and reduced functional cerebro-cerebellar connectivity (Trojsi et al., 2020), cerebellar hypermetabolism (Canosa et al.,2016; Matías-Guiu et al., 2016; Buhour et al., 2017) and hypometabolism(Calvo et al., 2019) have been observed.Very few imaging studies assessed disease burden in specific cerebellar lobules (Consonni et al., 2019; Barry et al., 2021; Bede et al., 2021a), cerebellar nuclei (Bharti et al., 2020) or the cerebellar peduncles (Minnerop et al., 2009; Floeter et al., 2014; Baek et al.,2020; Bharti et al., 2020; De Marchi et al., 2020).Because of the considerable clinical heterogeneity of ALS, stratification by genotype and phenotypespecific changes is essential.Delineating disease heterogeneity and objective stratification in ALS are particularly critical for drug development (Chipika et al., 2019).Only a minority of cerebellar imaging studies investigated different subtypes of ALS contrasting LMN versus UMN predominant (Abidi et al., 2020, 2021), ALS with and without cognitive impairment (Tanaka et al., 1993; Canosa et al., 2016; Schonecker et al., 2018; Hu et al., 2020), ALS-motor and ALS-plus, andC9orf72positive andC9orf72negative ALS (Agosta et al., 2017; Schonecker et al., 2018).Only a handful of studies included PLS(Floeter et al., 2014; Menke et al., 2018; Tu et al., 2019), PMA (Gredal et al.,1997), FTD, and ALS-FTD (Bae et al., 2016; Schonecker et al., 2018) cohorts.The strikingly different stratification strategies illustrate just one of the many methodological differences across cerebellar imaging studies that may explain the inconsistencies in the literature.It is also noteworthy that the vast majority of ALS imaging studies are cross-sectional encompassing patients at various stages of their disease trajectories adding to sample heterogeneity(Chipika et al., 2019).Sample heterogeneity (genetic, phenotypic, symptom duration) coupled with sample size limitations are likely to be key contributors to the contradictory findings.It is also widely recognized that imaging studies in ALS have an inherent inclusion bias to patients with less severe disability, limited bulbar impairment, less severe sialorrhoea, and relatively good respiratory function.Patients with frank orthopnoea, patients reliant on continuous non-invasive ventilation, patients with very poor mobility are much less likely to participate in imaging studies, therefore our insights gained from lengthy imaging protocols may not be fully representative of disease burden across the entire clinical spectrum of ALS.
There is often an expectation in imaging studies to explore clinico-radiological correlations even though cerebellar signs, gait abnormalities, eye-movement abnormalities, and cognitive deficits are confounded by coexisting UMN, LMN and frontotemporal degeneration in ALS (Verstraete et al., 2015; Christidi et al., 2018a, 2019).Nevertheless, cerebellar changes have been linked to disease severity (Consonni et al., 2019), motor symptoms (Sala et al., 2019),cognitive impairment (Consonni et al., 2019), and apathy (Canosa et al.,2021b).One study using computational gait analysis reveals the contribution of extrapyramidal deficits rather than cerebellar pathology to gait impairment highlighting the need for objective measures of clinical findings (Feron et al., 2018).The majority of cerebellar imaging studies evaluate multiple parameters which is helpful to determine which biomarkers are most sensitive to detect and track progressive cerebellar changes.Despite the overwhelming focus on spinal and supratentorial changes, cerebellar pathology has also been evaluated post-mortem (Ishihara et al., 2006).UBQLN-positive cytoplasmic inclusions (Brettschneider et al., 2012), neurofibrillary tangles(Yokota et al., 2006), and TDP-43 pathology (Geser et al., 2008) have been described in the cerebellum.The predilection to specific cerebellar lobules,vermis or deep cerebellar nuclei however has not been systematically characterized post mortem in specific ALS phenotypes and genotypes.There is a notion that neuronal networks underpinning phylogenetically “recent”skills, such as fine motor skills, vocalization, ambulation, etc.are particularly vulnerable to ALS (Eisen et al., 2014a; Eisen and Bede, 2021), which may explain the preferential atrophy of certain cerebellar regions, but these associations remain to be elucidated.It is also unfortunate that large imaging studies of well-characterized patients do not typically offer complementary post-mortem analyses to interpret their ante-mortem imaging findings.
A multitude of functional magnetic resonance imaging studies showed increased cerebellar activation when performing motor tasks (Konrad et al.,2006; Prell and Grosskreutz, 2013; Proudfoot et al., 2018; Bede et al., 2021a),and resting-state functional magnetic resonance imaging studies often reveal increased cerebro-cerebellar connectivity (Agosta et al., 2011; Zhou et al., 2013; Menke et al., 2016; Abidi et al., 2021).These two observations are often interpreted as a compensatory, adaptive change to motor cortex degeneration despite the lack of supporting structural findings and compelling post mortem evidence.Neuroplasticity is an intrinsic property of the nervous system, allowing some degree of reorganization at a molecular,cellular, or anatomical level (Pascual-Leone et al., 2005) which enables the nervous system to adapt to insult and compensate for injury (Villamar et al.,2012).Effective neuroplasticity has been demonstrated in a multitude of neurological conditions such as traumatic brain injury (Villamar et al., 2012),stroke (Dimyan and Cohen, 2011), multiple sclerosis (Straudi and Basaglia,2017), Alzheimer’s disease (Herholz et al., 2013) and spinal cord injury(Hutson and Di Giovanni, 2019), and similar adaptive mechanisms have also been proposed in ALS (Mohammadi et al., 2011).Based largely on abnormal activation patterns during motor tasks, it has been proposed that additional brain regions compensate for primary motor cortex degeneration such as the ipsilateral motor cortex, supplementary motor and premotor areas(Konrad et al., 2002), as well as in the basal ganglia (Tessitore et al., 2006) and cerebellum (Schoenfeld et al., 2005; Han and Ma, 2006; Prell and Grosskreutz,2013; Proudfoot et al., 2018).The cerebellum in particular is thought to exhibit remarkable neuroplasticity, withstand considerable cerebellar insults(Konczak et al., 2010) and adapt to extra-cerebellar pathologies (Mitoma et al., 2020).The activation shift from primary motor areas to the cerebellum during movement execution is often interpreted as evidence of an adaptive or compensatory process, but very few structural studies (Qiu et al., 2019)and no post mortem studies have actually captured frank hypertrophy.An alternative explanation for increased functional connectivity center on the loss of cortical inhibition (Prell and Grosskreutz, 2013; Proudfoot et al., 2018).
Cerebellar findings in related neurodegenerative conditions
The link between ALS and FTD is increasingly accepted based on shared clinical, genetic, and imaging features (Omer et al., 2017; Christidi et al., 2018a, b).Frontotemporal dysfunction is a key dimension of clinical heterogeneity in ALS and the concept of an ALS-FTD spectrum or continuum is now widely accepted.Cerebellar changes have been consistently described in GGGGCC hexanucleotide repeat carriers inC9orf72(Mahoney et al.,2012; Rohrer et al., 2015; Bede et al., 2021a; McKenna et al., 2021).While comorbid FTD was once primarily associated with hexanucleotide repeats,recent data have shown that frontotemporal degeneration is not exclusively caused byC9orf72repeat expansions in ALS (Westeneng et al., 2016).Cerebellar changes have also been reported in FTD cohorts without ALS and GGGGCC repeat expansions (Bocchetta et al., 2016) For example, preferential vermis atrophy was described inMAPTcarriers (Bocchetta et al., 2016).Other cerebellar studies of FTD stratified their patients by the clinical phenotype(Gellersen et al., 2017; Chen et al., 2018, 2020; McKenna et al., 2021) and associations were identified between cerebellar pathology and performance in specific cognitive domains such as attention, working memory, visuospatial skills, and language (Chen et al., 2018, 2020).Lobule VI, VIIb, VIIIb atrophy was noted in bvFTD and crus I and lobule VI volume loss was seen in semantic variant primary progressive aphasia (svPPA) (Chen et al., 2019).Cerebellar grey matter atrophy was captured pre-symptomaticC9orf72carriers (Cash et al., 2018) and ALS-FTD (Tan et al., 2014).PLS is a low incidence disorder of the upper motor neurons with relatively limited imaging literature (Pioro et al., 2020) and overlapping clinical, radiological, and genetic features with ALS(Bede et al., 2019; Finegan et al., 2019b, c).Very few imaging studies have specifically commented on cerebellar changes in PLS.Intra-cerebellar white matter alterations (Finegan et al., 2021), dentato-rubro-thalamo-cortical and spino-cerebellar tract diffusivity changes (Tu et al., 2019) and increased functional connectivity were reported between the cerebellum and cortical motor, frontal and temporal areas (Meoded et al., 2015).PLS patients with cognitive deficits are thought to exhibit fractional anisotropy reductions andincreased radial diffusivity in cerebellar white matter (Canu et al., 2013).In spinal muscular atrophy preferential lobule VIIIB, IX and X atrophy has been observed without notable correlations with clinical metrics (de Borba et al., 2020).Hereditary spastic paraplegia, which can be mistaken for UMN-predominant ALS or PLS, is also associated with considerable cerebellar grey and white matter degeneration (Orlacchio et al., 2004; Seidel et al., 2009;Lindig et al., 2015; Thal et al., 2015; Olivieri et al., 2019; Servelhere et al.,2021).Cerebellar changes are also commonly observed in spinal and bulbar muscular atrophy or Kennedy’s disease which is an X-linked recessive, slowly progressive, LMN-predominant motor neuron disease (Pieper et al., 2013;Pradat et al., 2020).Interestingly, cerebellar integrity metrics are typically superior in poliomyelitis survivors compared to demographically-matched healthy controls (Li Hi Shing et al., 2021b), which is often regarded as evidence of neuroplasticity and compensation for longstanding spinal anterior horn insult (Li Hi Shing et al., 2021a, c).
Clinical relevance
Though woefully understudied in ALS, cerebellar pathology is thought to contribute to pseudobulbar affect (Floeter et al., 2014; Finegan et al., 2019a),cognitive deficits (Consonni et al., 2019; Bede et al., 2021a), behavioral dysfunction (Bae et al., 2016; Canosa et al., 2021b), bulbar symptoms (Sala et al., 2019; Yunusova et al., 2019), respiratory problems (Xu and Frazier, 2002),gait impairment (Schimke et al., 2002; Yasser et al., 2010) and eye movement abnormalities (Jensen et al., 2019).The role of the cerebellum in mediating cognitive, and behavioral processes is increasingly well established (Van Overwalle et al., 2015; Guo et al., 2016; Schmahmann, 2019; Argyropoulos et al., 2021).Multi-time point longitudinal studies have shown that the corticospinal tracts and the corpus callosum are involved very early in the disease process (Schuster et al., 2016b; Bertrand et al., 2018; Querin et al.,2019a; Wen et al., 2019), whereas cerebellar changes exhibit a progressive change in the symptomatic phase of the disease (Menke et al., 2018).Regions that are affected early in the disease process may help diagnostic or phenotypic classification (Schuster et al., 2016a; Querin et al., 2018;Grollemund et al., 2019), but regions which exhibit progressive change in the later disease phases are better suited as tracking biomarkers (Schuster et al., 2015; Chipika et al., 2019).It is noteworthy that commonly used image analysis suites which evaluate cortical thickness profiles, such as FreeSurfer,only provide supratentorial segmentation, therefore many longitudinal ALS studies using this software do not evaluate cerebellar changes at all(Tahedl et al., 2021a).The prevailing clinical understanding of ALS-associated disability is overwhelmingly centered in UMN/LMN degeneration and the wider recognition of cerebellar pathology would also inform and refine multidisciplinary rehabilitation strategies, fall prevention, occupational therapy, speech and language therapy, management of pseudobulbar affect and synchronization with non-invasive ventilation.ALS is now universally regarded as part of the ALS-FTD spectrum, but mounting clinical and genetic evidence suggests that an ataxia-ALS continuum also exists.Ataxia-ALS overlap syndromes have been described in association with spinocerebellar ataxia type 1 and type 2 (Van Damme et al., 2011; Tazelaar et al., 2020).While 33 or more CAG repeats in theATXN1andATXN2genes are linked to spinocerebellar ataxia, an intermediate number of repeats in these genes is a risk factor for ALS (Neuenschwander et al., 2014; Tazelaar et al., 2020).ALS patients with intermediateATXN1andATXN2mutations often have a positive family history of spinocerebellar ataxia (Morello et al., 2020; Tazelaar et al.,2020) and spinocerebellar ataxia type 1 and type 2 patients can present with or eventually develop an ALS-like phenotype (Nanetti et al., 2009; Morello et al., 2020).Despite the emerging recognition of an ataxia-ALS continuum,ALS patients are not routinely screened for trinucleotide repeat expansions inATXN1andATXN2and a targeted cerebellar examination is seldom performed in ALS clinics (Bede et al., 2021a).Subtle cerebellar manifestations in ALS are notoriously difficult to ascertain due to confounding UMN and LMN signs which dominate the clinical picture.Spinocerebellar ataxias also share some clinical features with ALS such as dysphagia, and even muscle wasting and dementia, particularly in advanced stages (Sun et al., 2016; Antenora et al., 2017).A key lesson of the emergingATXN1andATXN2literature is that patients presenting to ataxia clinics should be carefully evaluated for UMN and LMN signs, and patients attending ALS clinics should be assessed for cerebellar signs.
Future directions
The contribution of cerebellar pathology to the clinical manifestations of ALS are poorly characterized and robust dedicated cerebellar studies are needed to dissect the role of infratentorial and supratentorial changes to motor disability, cognitive deficits, behavioral disturbances, pseudobulbar affect, dysphagia, and respiratory regulation.While putative compensatory processes were inferred from functional imaging studies, these have not been demonstrated by compelling structural and post mortem data to date.Emerging genetic data reveal an ataxia-ALS spectrum, the anatomical correlates of which have not been duly explored by dedicated imaging and post mortem studies.Cerebello-spinal connectivity is particularly poorly explored in ALS, despite emerging quantitative spinal imaging techniques (El Mendili et al., 2019; Querin et al., 2019b).From an academic perspective, the role of the cerebellum in the pathogenesis of ALS and disease propagation is not established, despite its ample projections to key ALS-associated foci; the spinal cord and motor cortex.At present, cerebellar disease burden remains an overlooked facet of ALS despite its wide-ranging clinical and academic relevance.
The field of motor neuron disease imaging has seen unprecedented technological innovations in recent years, including the emergence of ultrahigh-field platforms, a gradual transition to cloud-based applications, the increasing implementation of machine-learning frameworks, emphasis on longitudinal modeling, and a shift to non-Gaussian diffusion imaging(Barritt et al., 2018; Broad et al., 2019; Bede et al., 2021b; Tahedl et al., 2021b).Methods for evaluating the cerebellum have also improved considerably; robust cerebellar analysis pipelines have been published,high-resolution cerebellar atlases have been made available and novel cerebellar normalization templates have been developed (Diedrichsen, 2006;Diedrichsen et al., 2009; Diedrichsen and Zotow, 2015; Romero et al., 2017).Thanks to momentous methodological advances in neuroimaging, animal model technologies and immunohistochemistry, the armamentarium of clinical, radiological and biological tools are finally at our disposal to conduct authoritative integrative cerebellar studies.
Conclusions
While the cerebellum is recognized to be involved in the disease process in ALS, the specific contribution of cerebellar pathology to cardinal clinical manifestations is poorly characterized.There is currently a disproportionate emphasis on supratentorial changes both in imaging and post mortem studies.Recent advances in genetics, imaging technology, and data analysis pipelines offer unprecedented opportunities to clarify the role of the cerebellum in mediating disease propagation in ALS and its contribution to clinical symptoms.
Author contributions:All authors contributed equally to the conceptualization and drafting of the manuscript.CR, FA, PFP and PB are part of the CEREBRALS(Cerebellum in ALS) research consortium.All authors approved the final version of the manuscript.
Conflicts of interest:The authors have no conflicts of interest to declared.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:A selection of grey and white matter studies investigating cerebellar pathology in motor neuron disease.
Additional Table 2:A selection of functional magnetic resonance studies evaluating cerebellar pathology in amyotrophic lateral sclerosis: functional magnetic resonance imaging & spectroscopy.
Additional Table 3:A selection of positron emission tomography studies commenting on cerebellar pathology in amyotrophic lateral sclerosis.
Additional Table 4:A selection of post mortem studies describing cerebellar pathology in amyotrophic lateral sclerosis.
- 中国神经再生研究(英文版)的其它文章
- Interplay of SOX transcription factors and microRNAs in the brain under physiological and pathological conditions
- Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease
- An atypical ubiquitin ligase at the heart of neural development and programmed axon degeneration
- The endogenous progenitor response following traumatic brain injury: a target for cell therapy paradigms
- The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer’s disease
- Telomerase and neurons: an unusual relationship