Interplay of SOX transcription factors and microRNAs in the brain under physiological and pathological conditions
Milena Stevanovic , Danijela Stanisavljevic Ninkovic Marija MojsinDanijela Drakulic Marija Schwirtlich
Abstract Precise tuning of gene expression, accomplished by regulatory networks of transcription factors,epigenetic modifiers, and microRNAs, is crucial for the proper neural development and function of the brain cells.The SOX transcription factors are involved in regulating diverse cellular processes during embryonic and adult neurogenesis, such as maintaining the cell stemness, cell proliferation, cell fate decisions, and terminal differentiation into neurons and glial cells.MicroRNAs represent a class of small non-coding RNAs that play important roles in the regulation of gene expression.Together with other gene regulatory factors, microRNAs regulate different processes during neurogenesis and orchestrate the spatial and temporal expression important for neurodevelopment.The emerging data point to a complex regulatory network between SOX transcription factors and microRNAs that govern distinct cellular activities in the developing and adult brain.Deregulated SOX/microRNA interplay in signaling pathways that influence the homeostasis and plasticity in the brain has been revealed in various brain pathologies, including neurodegenerative disorders, traumatic brain injury,and cancer.Therapeutic strategies that target SOX/microRNA interplay have emerged in recent years as a promising tool to target neural tissue regeneration and enhance neurorestoration.Numerous studies have confirmed complex interactions between microRNAs and SOX-specific mRNAs regulating key features of glioblastoma.Keeping in mind the crucial roles of SOX genes and microRNAs in neural development, we focus this review on SOX/microRNAs interplay in the brain during development and adulthood in physiological and pathological conditions.Special focus was made on their interplay in brain pathologies to summarize current knowledge and highlight potential future development of molecular therapies.
Key Words: dysregulation of miRNA expression; glioblastoma; gliogenesis; glioma stem cells; ischemic stroke; neural stem cells; neural tissue regeneration; neurodegenerative diseases; neurodevelopment;neurogenesis; SOX/miRNA interplay; traumatic brain injury
Introduction
Brain development and homeostasis consist of a series of coordinated events that rely on precise control of gene expression.Neural stem cells(NSCs) represent a self-renewing stem cell population that is essential for the proper development of the central nervous system (CNS) as well as adult neurogenesis (De Filippis and Binda, 2012; Obernier and Alvarez-Buylla, 2019).During development, primary NSCs directly differentiate into early neurons.With the transition from single to multi-layered nervoustissue, a novel population of NSCs is generated that gives rise to the neural progenitor cells (NPCs) contributing to the majority of neurons in the brain.At the later stages of development, NSCs also generate glial precursors,astrocyte progenitor, and oligodendrocyte progenitor cells (OPCs) that further differentiate into astrocytes and oligodendrocytes, respectively (Kriegstein and Alvarez-Buylla, 2009).In the adult brain, the majority of NSCs, found in the two neurogenic niches, the subgranular zone of the hippocampal dentate gyrus and subventricular zone of the lateral ventricle, are involved in adult neurogenesis.The generation of both new neurons and glial cells in the adult brain contributes to neural plasticity and, to some extent, to neural repair(Frisen, 2016).In addition, growing evidence indicates that impaired adult neurogenesis is associated with some neurodegenerative diseases (NDs),including Parkinson’s (PD), Alzheimer’s (AD), and Huntington’s diseases (HD)(Horgusluoglu et al., 2017).
Several lines of evidence have supported the hypothesis that brain tumors arise from aberrant NSCs proliferation (Oliver and Wechsler-Reya, 2004).Many brain tumors contain stem cells that share many similarities to NSCs(Nakano and Kornblum, 2006).For instance, glioblastoma (GBM), one of the most common and the most aggressive malignant brain tumors in adults,contains neural carcinoma stem cells, known as glioma stem cells (GSCs),which are responsible for tumor initiation, progression, resistance to chemoand radiotherapy and tumor relapse (Bryukhovetskiy et al., 2020; Vieira de Castro et al., 2020).NSCs are considered one of the major candidates for the GBM cell of origin (Fan et al., 2019).
Numerous transcription regulators, including SOX proteins, play important roles during brain development and homeostasis, starting from maintenance of stemness, cell fate decision, coordination of initial phases of differentiation until the generation of mature neurons, astrocytes, and myelinating oligodendrocytes (Stevanovic et al., 2021).The SOX regulatory proteins display properties of both classical transcription factors (TFs) and architectural components of chromatin (Pevny and Lovell-Badge, 1997).Based on similarity between the proteins they encode, their structure and expression profiles,SOX/Soxgenes (in human and mammals, respectively) have been divided into eight groups, A to H (Table 1), with group B being further split into subgroups B1 and B2 (Bowles et al., 2000).
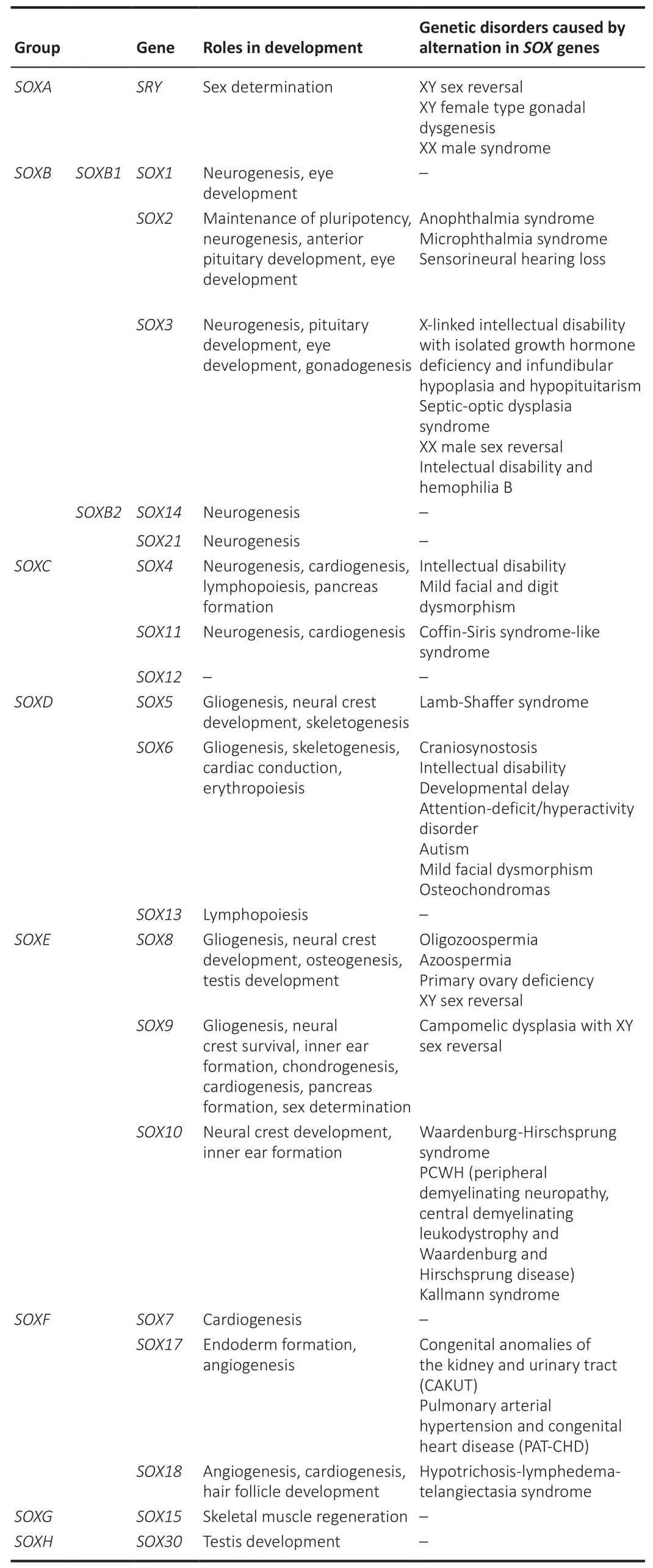
Table 1 |Classification of the human SOX genes and their roles in development and association with genetic disorders
MicroRNAs (miRNAs) are small non-coding single-stranded RNA molecules that regulate the expression of genes at the post-transcriptional level(Dexheimer and Cochella, 2020).miRNAs act together with other gene regulatory factors to orchestrate the spatial and temporal expression important for neurodevelopment.Literature data revealed that miRNAs regulate different processes during neurogenesis, including self-renewal,cell-type specification/differentiation, and synaptic plasticity (Stappert et al.,2015).Importantly, miRNAs play roles in the conversion of NSCs into neural cancer stem cells (Diana et al., 2019).
Both,SOXgenes and miRNAs are crucial regulatory components in neurogenesis and brain plasticity affecting similar processes (Stappert et al.,2015; Zhang et al., 2017; Stappert et al., 2018; Prodromidou and Matsas,2019; Stevanovic et al., 2021).About 70% of all miRNAs are highly expressed in the CNS (Cao et al., 2016) and extensive changes in the expression of bothSOXgenes and miRNAs are revealed during brain development (Bylund et al., 2003; Miska et al., 2004; Bergsland et al., 2011; Hoshiba et al., 2016; Cho et al., 2019).Some of theSOXgenes and miRNAs have age-specific (Smith-Vikos and Slack, 2012; Kuipers et al., 2015; Carrasco-Garcia et al., 2019; Cho et al., 2019; Goodall et al., 2019; Kinser and Pincus, 2020) and gender-specific(Guo et al., 2017b; Zaletel et al., 2018; Piscopo et al., 2021) expression in the brain.miRNAs andSOXgenes are already recognized as novel diagnostic and prognostic biomarkers as well as possible therapeutic targets for various pathologies (Adlakha and Saini, 2014; Hu et al., 2019; Condrat et al., 2020;Grimm et al., 2020).Accordingly, a better understanding of the general principles of the interplay betweenSOXgenes and miRNAs in the brain under physiological and pathological conditions could contribute to translating basic studies into novel clinical approaches, particularly in the fight against brain disorders.
Search Strategy and Selection Criteria
The studies cited in the current review, published from 1993 to 2021, were retrieved by an electronically search on Google, Web of Science, and PubMed databases using the following keywords/terms: SOX, miRNA, self-renewal,differentiation, neurodevelopment, neurodegeneration, ischemia, trauma brain injury, brain disorders, cancer, and glioblastoma.Furthermore, we also used various combinations of the above search terms to reach the literature data more specifically.
The Roles of SOX Genes in Development and Diseases
SOXgenes are widely expressed in different cells and tissues having important roles during various developmental processes including sex determination,gonadogenesis, neurogenesis, gliogenesis, eye development, ear formation,neural crest development, cardiogenesis, chondrogenesis, skeletogenesis,pituitary development, angiogenesis, and lymphopoiesis (Table 1).Literature data also revealed that mutations, dysfunction, and altered expression ofSOXgenes are linked to a wide spectrum of genetic disorders (Table 1) and different types of cancers (Table 2).In malignancies,SOXgenes may function as oncogenes, tumor suppressors or both, depending on the cellular context and interacting partners (Grimm et al., 2020).It is interesting to point out that increased levels of some SOX TFs result in tumorigenesis in one organ,while their decreased expression results in tumorigenesis in another organ(Grimm et al., 2020).In addition, down-regulation ofSOX9gene expression is associated with inhibition of proliferation of glioma cells and increased proliferation of melanoma cells (Olbromski et al., 2020).
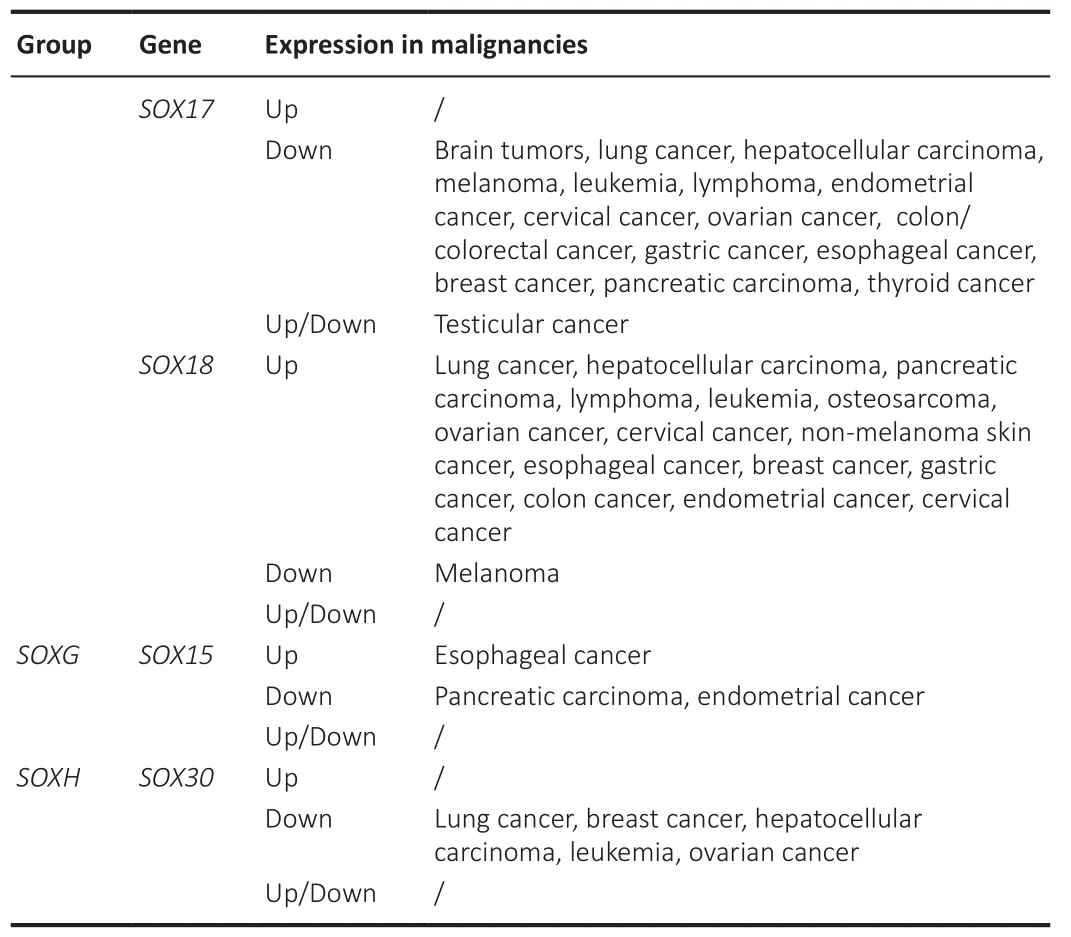
Table 2 |Continued

Table 2 |Deregulation of SOX genes expression in malignancies
It has been shown that numerousSOXgenes are expressed in brain tumors and exert different important roles in this type of cancer (Ferletta, 2011;Grimm et al., 2020).The roles ofSOXgenes in GBM, the most common,most aggressive, and deadliest brain tumor, have been extensively studied,and it has been revealed that numerous SOX TFs influence the initiation and progression of this type of tumor acting as oncogenes, tumor suppressors,or both, depending on the cellular context (Castillo and Sanchez-Cespedes,2012; Thu et al., 2014; Bryukhovetskiy et al., 2020; Vieira de Castro et al.,2020).
Despite enormous data indicating the key roles ofSOXgenes in the regulation of NSCs proliferation and differentiation during embryonic and adult neurogenesis, their expression and function in neurodegenerative processes are largely unknown with a very limited number of publications focusing on this issue.Data from a recent study have demonstrated a reduction in the number of SOX2 positive NSCs in the hippocampus of AD patients, which correlated with the severity of the disease or the patient’s cognitive capacity(Briley et al., 2016).Another study has demonstrated a significant decrease in the number of cells expressing SOX1, SOX2, and SOX21 within the subgranular zone in the transgenic mouse model of AD compared to their non-transgenic counterparts (Zaletel et al., 2018).
The Roles of MicroRNAs in Development and Diseases
The expression profiles of miRNAs are specific for the particular type of tissue and stage of cell differentiation playing important roles in development,including neurogenesis and synaptic plasticity, immune system development and response, regulation of various metabolic pathways (cholesterol and fat metabolism), adipogenesis, establishment of hematopoietic lineages and regulation of cardiac development and pathophysiology (Gomase and Parundekar, 2009).Besides theSOXgenes, the emerging data also point to the association between the dysregulation of miRNAs and various pathologies.Aberrant expression profiles of miRNAs have been detected in various diseases, including NDs, spinal cord injury, Duchene muscular dystrophy, cardiovascular diseases, diabetic nephropathy, sepsis, premature ovarian failure, and cancers (Fu et al., 2019; Davey et al., 2021; Ghafouri-Fard et al., 2021; Lin and Hu, 2021; Xu et al., 2021).
miR-200 family members, miR-147 and miR-124, are linked with the NDs (Fu et al., 2019; Lin and Hu, 2021; Xu et al., 2021), while miR-204 is deregulated in cardiovascular and renal diseases (Liu et al., 2021).In cancers, likeSOXgenes, miRNAs may function as oncogenes, tumor suppressors, or both,depending on the cellular context (Gajda et al., 2021).Important roles of miRNAs, for example, miR-138, miR-204, miR-145, miR-335, miR-338 andmiR-21, have been revealed in different cancers (Li et al., 2016; Yeh et al.,2019; Xu et al., 2020; Moghbeli, 2021; Nguyen et al., 2021; Ye et al., 2021).Accordingly, a comprehensive understanding of the roles of miRNAs is crucial for determining whether miRNAs-related pathways could be recognized as novel targets for these diseases.
SOX and MicroRNAs Interplay during Neural Development
The majority of data about the interplay betweenSOXBgroup members and miRNAs in brain development came from the studies ofSOX1andSox2genes.For instance, it was revealed thatSOX1was a direct target of miR-184 in human NPCs.Over-expression of this miRNA reduced the expression ofSOX1and other neuron and astrocyte-specific genes and promoted the differentiation of oligodendrocytes (Afrang et al., 2019).The interplay ofSox2and miRNAs presents an important regulatory network controlling the balance between cell proliferation and differentiation in the brain.It has been demonstrated that a negative feedback loop betweenSox2and miR-200 is important for neural differentiation of NSCs and NPCs in the murine midbrain/hindbrain region (Peng et al., 2012).The authors show that when miR-200 suppresses the expression ofSox2in both NSCs and NPCs, these cells exit the cell cycle and enter toward neuronal differentiation (Peng et al.,2012).Further, increased expression of miR-145 is essential for proper neural differentiation of NSCs through direct regulation ofSox2andSox2-Lin28/let-7 signaling pathway (Morgado et al., 2016).Interestingly, the interplay between SOX2 and miR-145 was also shown in oligodendroglia.The authors demonstrated that SOX2 represses the expression of miR-145 and speculated that SOX2 might be involved in the regulation of terminal differentiation of oligodendrocytes through inhibition of this miRNA (Hoffmann et al., 2014).Another axis important for neural differentiation of NSCs is miR-21/Sox2interplay, where miR-21 directly regulatesSox2expression, while both factors show mutually exclusive expression patterns in the mouse brain (Sathyan et al., 2015).All presented results indicate thatSox2gene is regulated by several miRNAs in NSCs, where the interplay between them directs neural cell fate determination as well as region-specific differentiation of mature neurons or glial cells (Figure 1).
There are a number of studies that are focused on how miRNAs regulateSOXCgenes expression in various cancer types, while to the best of our knowledge,there is only one study focused on the interplay betweenSOXCgenes and miRNAs during neural development.The results of this study demonstrated thatSox4gene is expressed in OPCs.Down-regulation of its expression by miR-204 leads to oligodendrocytes differentiation and onset of myelination(Figure 1) (Wittstatt et al., 2020).
SOX5andSOX6genes that belong to theSOXDgroup are also regulated by different miRNAs during neural development.SOX5is directly regulated by miR-96 in 3D cultures of human NSCs, and both these factors show exclusive expression patterns (Stevanato and Sinden, 2014).It was also suggested that miR-96 is involved in controlling cell-cycle progression and axon length modulation through direct regulation ofSOX5gene expression (Stevanato and Sinden, 2014).In OPCs,Sox6gene is a direct target of two miRNAs, miR-219 and miR-338 (Dugas et al., 2010; Zhao et al., 2010).These miRNAs further initiate oligodendrocyte differentiation and myelination through inhibition of not only theSox6gene expression (Figure 1), but also the expression of other TFs involved in the promotion of oligodendrocyte progenitor state, likeplatelet-derived growth factor receptor alpha, forkhead box J3, and zinc finger protein 238 (Dugas et al., 2010; Zhao et al., 2010).Due to the importance of these miRNAs in promoting myelination, it was suggested that miR-219 and miR-338 could be considered as promising targets for the treatment and enhancement of axonal remyelination after nerve injuries in CNS (Nguyen et al., 2020).

Figure 1|The interplay between SOX TFs and miRNAs in the regulation of selfrenewal or differentiation of NSCs.
The members ofSOXEgroup,SOX9, andSOX10genes, in particular, are involved in the differentiation of oligodendrocytes (Figure 1) (Stolt et al., 2002;Weider et al., 2013; Klum et al., 2018).Therefore, the majority of studies were focused on miRNAs andSOXEinterplay during differentiation of OPCs toward mature/myelinating oligodendrocytes (Gokey et al., 2012; Reiprich et al., 2017; Wittstatt et al., 2020).It is important to point out that, by regulating miRNAs expression, SOX9 and SOX10 regulate the expression ofSox4andSox9, respectively.SOX9 regulates the expression of miR-204 in OPCs that further directly regulatesSox4expression (Figure 1) (Wittstatt et al., 2020).Further, miR-338 and miR-335 inhibit the expression ofSox9gene in OPCs and promote differentiation of oligodendrocytes (Figure 1) (Reiprich et al., 2017).On the other side, SOX10 regulates the expression of miR-338 and miR-335 that leads to suppression of Hes Family BHLH Transcription Factor 5 (Hes5)and Hes Family BHLH Transcription Factor 6 (Hes6) gene expression leading to terminal differentiation of oligodendrocytes (Gokey et al., 2012).By regulating the expression of these two miRNAs, SOX10 is indirectly involved in the regulation ofSox9gene expression in OPCs (Figure 1) (Reiprich et al., 2017).The proper expression of SOX9 is essential for NSCs maintenance during both embryonic and adult neurogenesis (Cheng et al., 2009; Scott et al., 2010).Cheng and colleagues demonstrated thatSox9is a target of miR-124 in the subventricular zone.Silencing the expression of miR-124 led to increased expression ofSox9and decreased neurogenesis (Cheng et al., 2009).Based on the presented results, it might be concluded that the interplay between miRNAs and SOXE TF is important for cell fate determination, pointing to them as fine tuners essential for proper differentiation of oligodendrocytes in particular (Figure 1).
It is more than evident that the interplay between SOX TFs and miRNAs is crucial for different aspects of neural development.As previously suggested,various SOX TFs and miRNAs show a functional link in orchestrating cell fate determination and differentiation (Stevanovic et al., 2021).Here we are focused on highlighting the interplay between SOX TFs and miRNAs during neural development, particularly in NSCs, NPCs, and OPCs (Figure 1).The interplay between SOX TFs and miRNAs is important for the regulation of the self-renewal and proliferative capacity of NSCs and progenitors, thus influencing the cell fate of these cells (Figure 1).The fact that the interplay between miRNAs and SOX TFs (Figure 1) is included in generating various types of neural cells from a limited pool of NSCs during adult neurogenesis is striking.Most of the studies regarding how miRNAs regulateSOXexpression are conducted in animal models.The enormous progress in pluripotent stem cells research enabled the comprehensive study of miRNAs and SOX interplay in the human model systems.Keeping in mind that both SOX TFs and miRNAs are important for neural development, the interplay between them in NSCs and NPCs can be exploited to better understand nervous system development, facilitating the progress in developing novel and more effective strategies for the treatment of brain pathologies.
The Interplay between SOX TFs and MicroRNAs in Brain Pathologies
Since the altered expression of miRNAs and SOX TFs is detected in different brain disorders, we present the overview of the current literature data about their interplay in different brain pathologies.We are focused on experimentally validated interactions between SOX and miRNAs and their potential interplay in various brain pathologies.
The Interplay between SOX TFs and MicroRNAs in Neurodegenerative Diseases
Neurodegenerative diseases, as a large group of neurological disorders characterized by progressive loss of neuronal and glial cells, have enormous and growing social and economic implications (Maciotta et al., 2013).These incurable, debilitating, and age-dependent disorders are becoming increasingly prevalent, which is associated with an increase in the elderly population in recent years (Gitler et al., 2017).
Here we present data indicating that some SOX TFs represent potential miRNAs targets in NDs (Figure 2).We also pointed out that modulation ofSOXgenes expression by miRNAs might be considered as a future strategy for the clinical treatment of NDs.
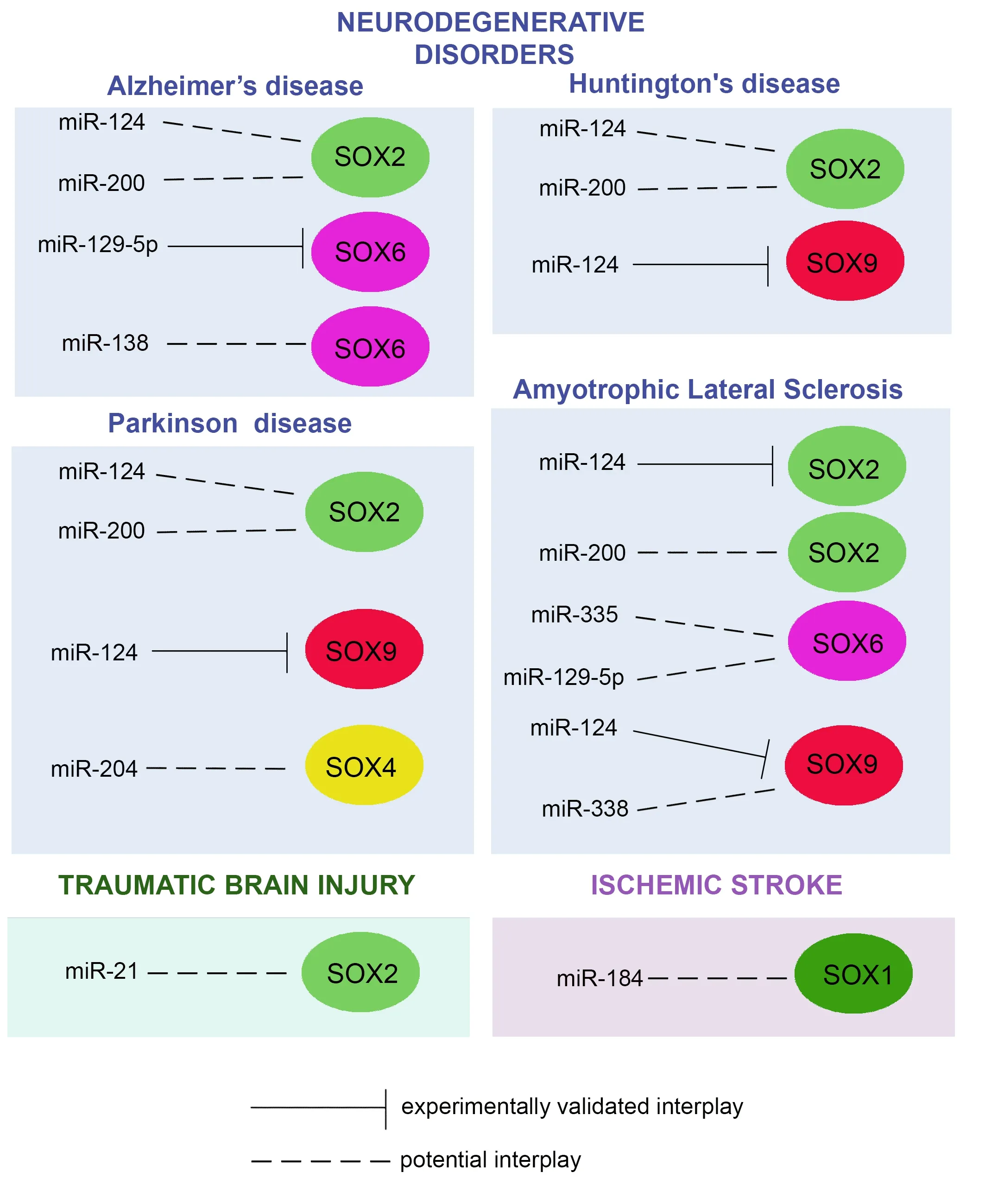
Figure 2|The interplay between miRNAs and SOX genes in brain pathologies.
Insight into the interplay betweenSOXgenes and miRNAs and their effects on the transition from NSCs to differentiated neural cells during development can improve NDs treatments.By directly targetingSOXgenes, miRNAs can influence NSCs fate decisions during CNS development.The interplay of SOX2 and miR-200 family members regulates the proper generation and survival of ventral neuronal populations, including dopaminergic neurons (Peng et al., 2012).Both miR-124 and miR-200, which regulateSox2gene expression in NSCs, are associated with the pathogenesis of AD (Fu et al., 2019; Han et al., 2019) (Figure 2).These miRNAs are involved in the regulation of amyloid-β peptide secretion, which is considered the major cause of AD (Fu et al., 2019; Han et al., 2019).The functional link between SOX2 and betaamyloid precursor protein, a precursor of amyloid-β, has also been revealed(Zhao et al., 2015).Accordingly, it has been proposed that SOX2 might play an important role in AD (Zhao et al., 2015).We hypothesize that the interplay between miR-124, miR-200, andSOX2might be considered a new therapeutic target for AD treatment (Figure 2).The interplay betweenSox6and miR-129-5p is also shown in AD, whereSox6and miR-129-5p are involved in the regulation of nerve injury and inflammatory response in the transgenic rat model of AD (Zheng et al., 2017).Another miRNA involved in the regulation ofSox6expression, miR-138, is involved in the promotion of amyloid-β production through different pathways (Boscher et al., 2020).However, theinterplay betweenSox6and miR-138 in AD is yet to be confirmed in future studies (Figure 2).
One of the earliest stages of AD pathology includes loss of myelin sheaths as a result of impaired repair of OPCs, implying oligodendrocytes as novel therapeutic targets for the prevention and treatment of AD (Cai and Xiao,2016).Here we point out that SOX TFs and miRNAs serve as fine tuners essential for proper differentiation of oligodendrocytes.The interplay between SOX and miRNAs involved in oligodendrocyte differentiation should be considered for better understanding the treatment of, not only AD, but also of the demyelinating diseases.
Since NDs are mainly characterized by progressive loss of neural cells, SOX and miRNAs interplay becomes essential for a better understanding of the mechanisms underlying the loss of dopaminergic neurons in PD or motor neurons in amyotrophic lateral sclerosis (ALS).miR-124, which regulatesSox2andSox9genes expression in NSCs, is associated with the pathogenesis of PD (Figure 2), and it was suggested to be involved in the suppression of the neuro-inflammation process during the development of this disease(Han et al., 2019).Based on the detected reduction in plasma levels, it was proposed that miR-124 could serve as a potential diagnostic biomarker in PD(Angelopoulou et al., 2019).Intracerebral administration of nanoparticles coated with miR-124 increased the number of migrating neuroblasts, induced migration of neurons into the lesioned striatum, and improved motor symptoms in 6-hydroxydopamine mouse model of PD (Saraiva et al., 2016).Besides the role of miR-124, there is increasing interest in the therapeutic potential ofSOX9as its target in PD.Keeping in mind the crucial role of SOX9 in neuronal-glial switch during neural development and the fact that NDs can be characterized by either loss of neurons or astrocytes, it is important to decipher how SOX9/miR-124 interplay could be exploited for improvement of the future outcome of all NDs.
Further, miR-200 family members that regulateSox2expression in NSCs are recognized as an effective indicator of the progression of PD (Fu et al., 2019).SinceSOX2is important for proper neuronal differentiation, future studies are needed to identify how the interplay betweenSOX2and miR-124 and miR-200 contributes to PD pathology (Figure 2).Also, miR-204 is involved in the regulation of the apoptotic signaling pathway, which leads to a loss of dopaminergic neurons, a hallmark of PD (Chiu et al., 2019), whileSOX4, a direct target of this miRNA, is down-regulated in the brain of patients affected with this disease (Sakib, 2018).Future studies are needed to identify how the interplay between SOX4 and miR-204 is involved in the pathogenesis of PD(Figure 2).
In addition to AD and PD, some studies associated deregulated miR-124 expression with HD pathology (Han et al., 2019).Particularly, miR-124 injected into the brain promoted neuronal differentiation and neuron survival in the striatum and slowed down the progress of this disease (Liu et al., 2015).The authors revealed that it was accomplished through modulation of the expression of proteins that are imbalanced in HD, including SOX9, Peroxisome proliferator-activated receptor-γ coactivator, and brain-derived neurotrophic factor (Liu et al., 2015).A recent study demonstrated that another miRNA involved in the regulation ofSOXgene expression, miR-200, can serve as an early marker of HD.It is proposed that miR-200 family members in HD might induce neuronal degeneration through the inhibition of the expression of their target genes (Fu et al., 2019).Considering the role of SOX2 in NSCs maintenance and differentiation during adult neurogenesis (Ferri et al., 2004;Favaro et al., 2009; Amador-Arjona et al., 2015), future studies are needed to identify how the interplay betweenSOX2and miR-124 and miR-200 contributes to HD pathology (Figure 2).
miR-124 suppresses the expression ofSox2in the transgenic mouse model of ALS, thus inducing glial differentiation of NSCs (Zhou et al., 2018a).miR-200 is also associated with the pathogenesis of ALS.It was shown that the expression levels of miR-200 family members are different in the early and later stages of ALS in the transgenic mouse model, indicating them as potential biomarkers for the progression of ALS (Fu et al., 2019).Interestingly, it was recently shown that SOX2 is involved in regulating motor neuron development in zebrafish by regulating neuron differentiation and morphology of neuron axons (Gong et al., 2020).Since ALS is known as motor neuron disease (Rowland and Shneider, 2001), it would be interesting to investigate if there is an interplay betweenSOX2and miR-200 in ALS (Figure 2).miR-335, which regulates the expression ofSox6gene, is involved in motor neuron loss in ALS (De Luna et al., 2020).In addition, miR-129-5p, which regulates the expression ofSox6gene, is also recognized as a key factor and therapeutic target in ALS (Loffreda et al., 2020).The potential relevance of the interplay ofSOX6and miR-335 and miR-129-5p in this disease is yet to be determined (Figure 2).In NSCs of a transgenic mouse model of ALS,Sox9is direct target of miR-124, where the inhibition ofSox9gene induces astrocytes differentiation (Zhou et al., 2018a).In addition,Sox9gene was highly upregulated in the spinal cord at the symptomatic stage in mouse models of ALS(Sun et al., 2017).The expression of miR-338, involved in the regulation ofSox9expression in NSCs, was shown to be over-expressed in the spinal cord of patients with ALS and it was suggested that this miRNA could be a potential biomarker for this disease (De Felice et al., 2014).Future studies are needed to confirm the interplay between miR-338 andSOX9in ALS (Figure 2).
Presented results suggest that SOX/miRNA interplay could be considered as a potential therapeutic tool for treating NDs.
The Interplay between SOX TFs and MicroRNAs in Traumatic Brain Injury and Ischemic Stroke
Traumatic brain injury (TBI) is an alternation in brain anatomy or/and function caused by an external force.Cumulative damage comprises the immediate impact on the tissue followed by biochemical responses to injury that result in neuronal repair or apoptotic cell death (Galgano et al., 2017).Currently,no reliable biomarkers could be applied to assess the severity of damage or predict recovery.However, emerging evidence on altered miRNAs expression in different animal models of TBI suggested their potential roles in the diagnosis and treatment of this severe pathology (Di Pietro et al., 2018;Atif and Hicks, 2019; Pinchi et al., 2020).For example, increased expression of miR-21, the most studied miRNA in TBI, was found to improve the neurological outcome through inhibiting apoptosis and targeting angiogenesis(Ge et al., 2015).Furthermore, a significant increase in miR-21 in neurons and extracellular vesicles, detected after TBI, suggested its additional role in cell-cell communication and neuroinflammation (Harrison et al., 2016).On the other hand, a recent study demonstrated that conditional deletion ofSOX2in reactive astrocytes improved the recovery in mice after TBI (Chen et al., 2019).SOX2is a functional target of miR-21 in mouse NSCs (Sathyan et al., 2015); however, their interplay in neural restoration following TBI needs further investigation.Since SOX2 can bind to the regulatory regions of many genes that control proliferation, differentiation, and cytokine signaling(Garros-Regulez et al., 2016; Mercurio et al., 2019; Stevanovic et al., 2021),a possible modulation of its expression by miR-21 (Figure 2) should be evaluated for future therapeutic strategies.
Impaired miRNAs profiles which were detected following cerebral ischemic stroke provided evidence that modulation of their expression could be considered as a diagnostic and prognostic tool providing a basis for potential therapeutic strategy (Khoshnam et al., 2017).A recent study in rodents demonstrated that the level of miR-184 is significantly reduced following ischemic stroke and that the over-expression of this miRNA alleviates brain damage (Yang et al., 2021).A potential interplay between miR-184 and SOX1,which is critical for oligodendroglia differentiation during development (Afrang et al., 2019), suggests the importance of this interplay in neuroregenerative processes that could be applied in future therapeutic strategies.
Astrocytes, the most abundant cell type in the brain, play a dual role in neuronal injury - protecting neurons and increasing the injured area by forming edema (Stary and Giffard, 2015; Zhou et al., 2020).Numerous data suggested astrocytes as an attractive cellular candidate for stroke therapy(Stary and Giffard, 2015).A recent study demonstrated a protective role of miR-145 in these cells following ischemia-induced injury (Zheng et al., 2017).A high level of SOX2 expression is detected in developing and reactive astrocytes (Bani-Yaghoub et al., 2006; Gotz et al., 2015) indicating an important role of this TF in cell homeostasis.Furthermore, results from a recent study demonstrated the roles of this TF in functional recovery upon ischemic stroke by axonal regeneration (Zhao et al., 2018).Taken together, the interplay between miR-145 and SOX2, which was demonstrated previously in NSCs (Hoffmann et al., 2014), and its possible effect on astrocyte and neuron recovery following stroke should be further investigated.
The possibility of expanding the pool of self-renewing NSCs or directing their cell fate towards certain neural phenotypes is a hallmark of regenerative medicine.Based on numerous data on the role ofSOXgenes in diverse cell processes during development as well as the effect of different miRNAs on their expression, we can speculate that SOX/miRNA interplay should be considered as a target in the future strategies for prevention and therapy of various impairments of brain structure and function.
The Interplay between SOX TFs and MicroRNAs in Glioblastoma
GBM represents a prototypic brain tumor for studying neural cancer stem cells (Diana et al., 2019).Literature data indicate that miRNAs serve as glioma biomarkers and can be used for targeted therapy of GBM (Mondal and Kulshreshtha, 2021).Moreover, it has been shown that miRNAs have an important function during the conversion of neural stem cells into neural cancer stem cells (Diana et al., 2019).
Numerous studies have confirmed complex interactions between miRNAs andSOX-specific mRNAs in GBM.Multiple miRNAs inhibit the expression of theirSOXtargets; thus, miRNAs regulate the key features of GBM by acting as oncogenes or tumor suppressors.Figure 3 shows the miRNA-SOXaxes for miRNAs associated with GBM and their effects on the main GBM characteristics.Detailed information about specific miRNAs, theirSOXtargets and the GBM cell properties affected by down-regulation ofSOXexpression is presented in Table 3.
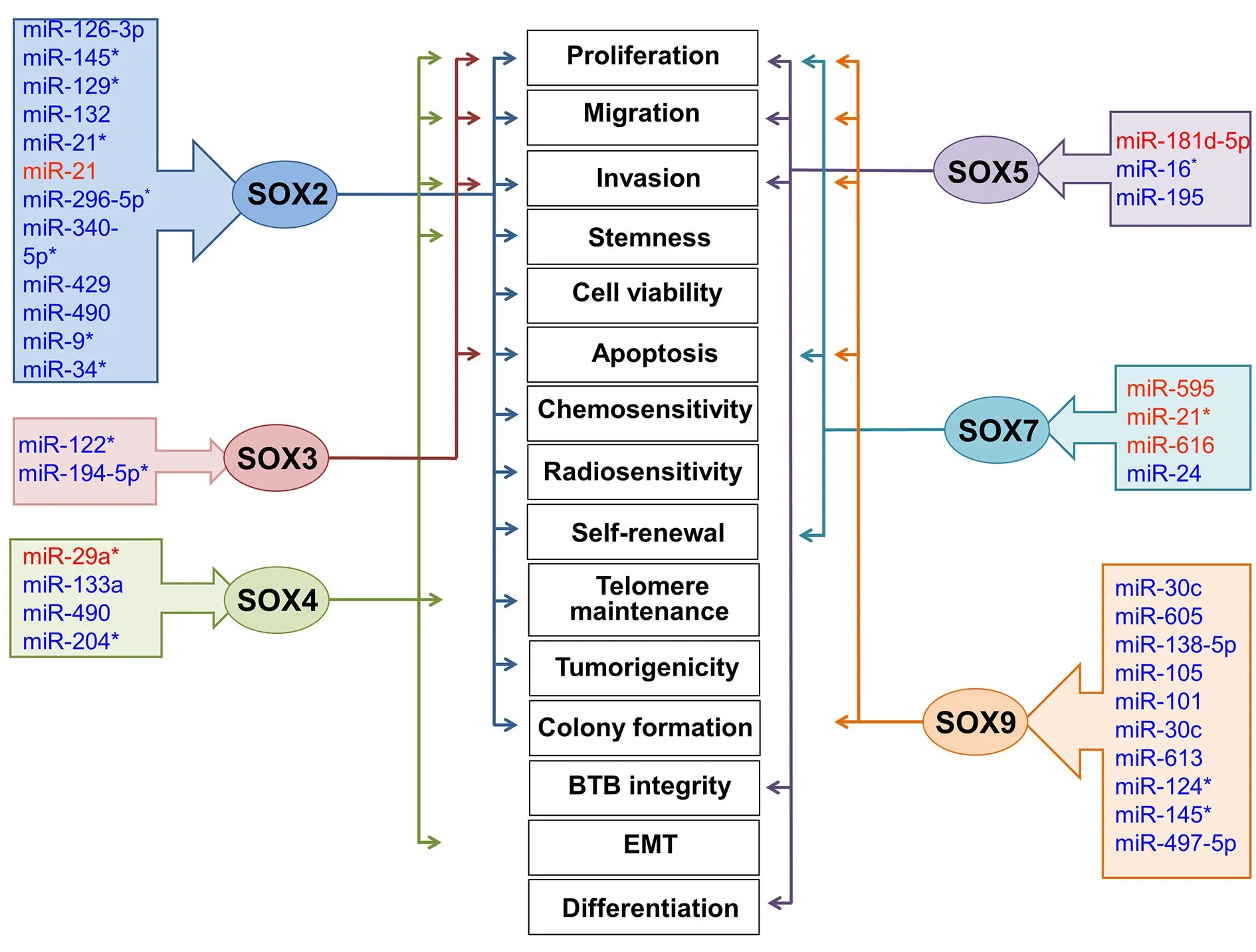
Figure 3 | The effects of specific miRNAs on the key characteristics of GBM operating through modulations of SOX protein expression.
The Interplay between SOX TFs and MicroRNAs in Glioma Stem Cells
The roles of miRNAs in GSCs have been extensively investigated since they regulate tumor-related miRNAs, thus controlling the stem-like properties of GSCs (Virant-Klun et al., 2016), differentiation, chemo- and radioresistance(Besse et al., 2013) playing both oncogenic and tumor-suppressive roles in GBM (Esquela-Kerscher and Slack, 2006).
Among miRNAs listed in Table 3, several of them also play important roles in the maintenance of GSCs (Jeon et al., 2011; Yang et al., 2012; Rani et al.,2013; Ying et al., 2013; Sathyan et al., 2015; Lopez-Bertoni et al., 2016; Xu et al., 2016; Su et al., 2017; Tian et al., 2017; Xiong et al., 2018; Kim et al.,2019; Qian et al., 2019; Sabelstrom et al., 2019; Zhao et al., 2019; Guan et al.,2020; Jiang et al., 2020).By down-regulation ofSOXtargets, these miRNAs control cell processes essential for glioma progression, such as proliferation,migration, invasion, apoptosis, stemness, differentiation, and chemosensitivity(Jeon et al., 2011; Yang et al., 2012; Rani et al., 2013; Ying et al., 2013; Sathyan et al., 2015; Lopez-Bertoni et al., 2016; Xu et al., 2016; Su et al., 2017; Tian et al., 2017; Xiong et al., 2018; Kim et al., 2019; Qian et al., 2019; Sabelstrom et al., 2019; Zhao et al., 2019; Guan et al., 2020; Jiang et al., 2020).Schematic representation of miRNAs regulatingSOXsin GSCs is given in Figure 4.
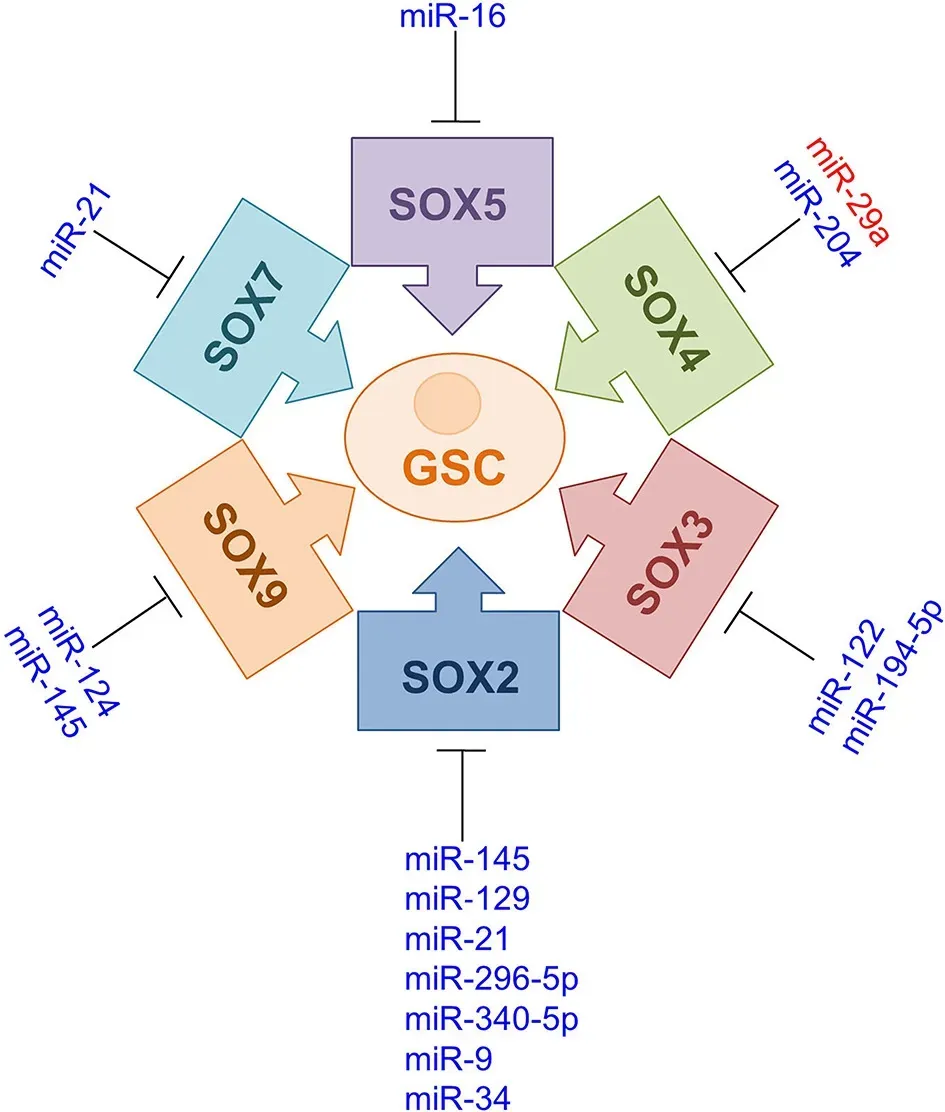
Figure 4|Schematic representation of miRNAs modulating SOX protein
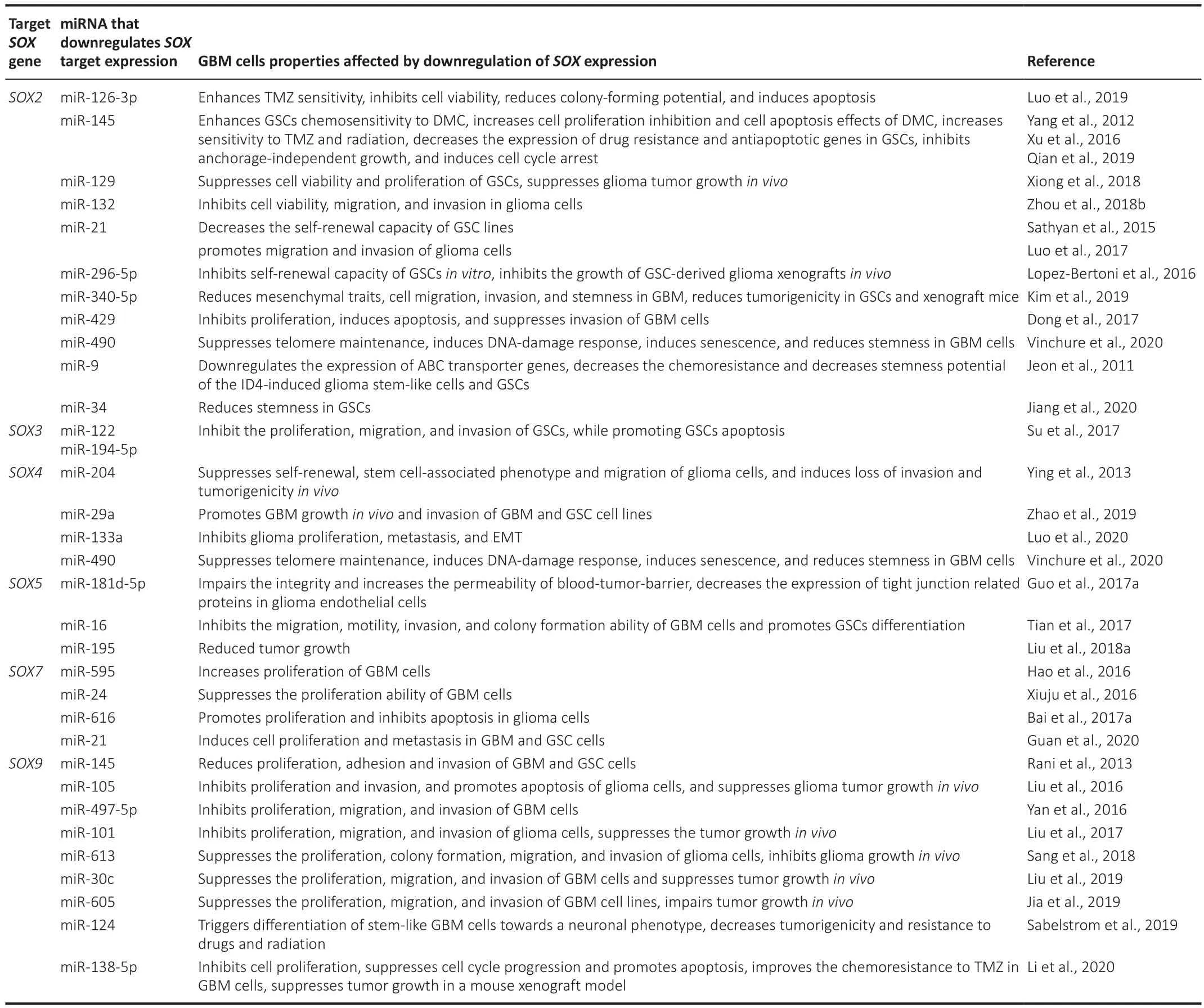
Table 3 |miRNAs and their SOX targets down-regulated in glioblastoma
Besides the roles of miRNAs in regulatingSOXexpression in GSCs, SOX2 has a reciprocal activity, regulating the expression of selected miRNAs in GSCs.Lopez-Bertoni et al.showed that SOX2, together with OCT4, induces promoter hypermethylation and silencing of a subset of miRNAs (miR-124, miR-148a, miR-17, miR-200a, miR-217, miR-296-5p, and miR-30c) by direct transactivation of the DNMT (DNA methyltransferase) promoter and consequent global DNA methylation (Lopez-Bertoni et al., 2015).In the same study, the authors revealed that miR-148a, one of the miRNAs whose expression was down-regulated by SOX2 and OCT4, inhibits GBM cell stem-like properties and their tumor-propagating potential (Lopez-Bertoni et al., 2015).Another down-regulated miRNA, miR-296-5p, directly targets HMGA1 (High mobility group AT-hook 1), which is associated with histone H1 displacement from theSOX2promoter and inhibition ofSOX2expression (Lopez-Bertoni et al., 2016).Presented miR-296-5p-HMGA1-SOX2 axis functions as a negative regulator of the GSC phenotype (Lopez-Bertoni et al., 2016).In the study of de la Rocha et al.(2020), forced expression of SOX2 increased the expression ofmiR-128b and miR-425-5p in GSCs.SOX2 controls the transcriptional activity of miR-425-5p by direct binding to the promoter of this miRNA (de la Rocha et al., 2020).The authors also revealed that miR-425-5p is involved in the regulation of the proliferation and apoptosis of GSCs (de la Rocha et al., 2020).Papagiannakopoulos et al.(2012) analyzed the tumor-suppressive role of miR-128 in genetically defined primary glioma-initiating NSCs [NSCs transformed with oncogenic EGFRvIII (Epidermal growth factor receptor variant III) and lacking tumor suppressor genes, p16/p19] and revealed that miR-128 induced repression of mitogenic signaling of glioma-initiating NSCs and enhance their differentiation.miR-128 promoted differentiation of glioma-initiating NSCs by down-regulation ofNestinandSOX2expression (Papagiannakopoulos et al.,2012).
Lopez-Bertoni et al.(2020) also revealed that SOX2 induced activation of miR-486-5p through SOX2-binding sites within its putative promoter region.The resulting SOX2-miR-486-5p axis inhibits tumor suppressor pathways and promotes the stemness of GSCs (Lopez-Bertoni et al., 2020).
In addition, global expression analysis of miRNAs obtained by comparing GSCs and non-stem GBM cell cultures revealed a subset of miRNAs that correlated withSOX2expression (Sana et al., 2018).Among all analyzed GSC samples,GSC cell cultures with the highest tumorigenic potential and pronounced multilineage differentiation showed up-regulation of the expression of a subset of nine miRNAs (miR-9-3p, miR-93-3p, miR-93-5p, miR-106b-5p, miR-124-3p, miR-153-3p, miR-301a-3p, miR-345-5p, and miR-652-3p)compared to their expression in non-stem cell cultures (Sana et al., 2018).The expression of these miRNAs is positively correlated withSOX2expression,suggesting their association with the stem-like characteristics of GSCs (Sana et al., 2018).
Contribution of SOX TFs in Inverse Regulation of MicroRNAs in Neurodegeneration and Cancer
Results from numerous epidemiological studies revealed an inverse correlation between certain NDs and cancers (Seo and Park, 2020).Neurodegeneration results in the premature death of postmitotic neurons,while cancer is characterized by enhanced resistance to cell death.However,the progression of these two chronic physiological ailments results from molecular mechanisms that are either complementary deregulated or share overlapping signaling pathways, including epigenetic and post-transcriptional modifications (Plun-Favreau et al., 2010; Seo and Park, 2020).Compared to other mammalian organs, the highest levels of miRNAs are detected in the brain.Moreover, a significant increase or decrease in miRNAs expression was detected during the early stages of nerve deterioration and oncogenesis,respectively.Thus, recent studies suggested that these two conditions may be regulated by common miRNAs pathways involved in proliferation,differentiation, or cell death (Plun-Favreau et al., 2010; Godlewski et al., 2019;Seo and Park, 2020).This review presents a possible interplay between SOX TFs and miRNAs in these shared regulatory networks.The increase of miR-9, which is involved in the regulation of NSCs proliferation and differentiation in adult neurogenesis, has been associated with PD, HD, and AD pathology(Godlewski et al., 2019).However, in GBM, by targetingSOX2expression,miR-9 decreases the chemoresistance and stemness potential of the inhibitor of differentiation 4-induced glioma stem-like cells and GSCs (Jeon et al.,2011).Next, miR-34a, a target of p53, induces cell cycle arrest, senescence,and apoptosis and is associated with PD pathology (Godlewski et al., 2019).However, by decreasing theSOX2expression, miR-34a also reduces the stemness in GSCs (Jiang et al., 2020).miR-124, the most abundant miRNA in the brain, regulatesSox9expression during embryonic and adult neurogenesis(Stevanovic et al., 2021) as well as in GBM cells (Sabelstrom et al., 2019).In various NDs, including AD and PD, a decreased level of miR-124 was detected and associated with an increased percentage of cell death (Godlewski et al.,2019).Godlewski with authors summarized the miRNAs deregulated in brain cancer, NDs, and ischemia (Godlewski et al., 2019).By comparing the lists of miRNAs currently investigated as therapeutic targets in these three brain pathologies, the authors identified four common miRNAs (miR-21, let-7, miR-210, and miR-128) (Godlewski et al., 2019).Three of them (miR-21, let-7, and miR-128) are involved in the interplay with SOX (Sathyan et al., 2015; Morgado et al., 2016; Luo et al., 2017).We compared results on SOX/miRNAs interplay between GBM and other brain pathologies, including neurodegenerative diseases.Interestingly, as shown in Figure 5, GBM shared two SOX/miRNAs interplay with NDs (miR-124/SOX9 and miR-204/SOX4), one with TBI (miR-21/SOX2), and one with ischemic stroke (miR-145/SOX2).

Figure 5|SOX/miRNAs interplay shared between glioblastoma and other brain pathologies (traumatic brain injury, neurodegenerative diseases, and ischemic stroke).
RNA interference-based approaches (including miRNAs) for targeting translation of TFs have been broadly investigated in the last two decades and recently reached clinical trials (Mullard, 2019; Dammes and Peer, 2020;Mullard, 2020).However, many obstacles still have to be overcome to ensure safe, effective, and durable miRNA-based strategies for treating brain pathologies (Dammes and Peer, 2020; Laham-Karam et al., 2020).
The complexity of miRNAs binding to target mRNAs represents a huge challenge in exploiting miRNAs for therapeutic approaches that selectively target particular pathophysiological mechanisms.A short binding sequence enables a single miRNA to have many potential mRNA targets and potentially simultaneously regulates different pathways, thus shaping cell transcriptomic landscape (Zhang and Wang, 2017).As elaborated in this review, several miRNAs target multipleSOXgenes (e.g., miR-21 targetsSOX2andSOX7; miR-145 targetsSOX2andSOX9; miR-490 targetsSOX2andSOX4) (Rani et al.,2013; Sathyan et al., 2015; Qian et al., 2019; Guan et al., 2020; Vinchure et al., 2020).It is interesting to point out that multiple binding sites overlapping between the same miRNA on the mRNAs enhance the down-regulation of specific targets (Zhang and Wang, 2017) while multiple miRNAs with similar characteristics can cooperatively target individual mRNA, thus synergistically amplifying target repression (Rinck et al., 2013).For example, miR-126 inhibitsSOX2expression by targeting two binding sites in the 3′-UTR ofSOX2mRNA(Otsubo et al., 2011), while miR-122 and miR-194-5p inhibitSOX3expression by targeting its 3′-UTR thus mediating the effect of lncRNA-SOX2OT in GBM cells (Su et al., 2017).These data indicate that the SOX/miRNAs interplay is rather complex.Detailed elucidation of the cellular mechanisms involved in the restriction of mRNA targets (cell-specific mRNA and miRNA expression,RNA compartmentalization) and current advances in “omics” technologies and computational methods will help in the identification of specific miRNAs involved in the pathogenesis of a particular condition (Morris et al., 2021).Due to the dual nature of SOX proteins in cancer, acting both as oncogenes and tumor suppressors depending on cell context (Ikushima et al., 2009;Zhang et al., 2014), restricted targeting of a particular brain area is required to preventSOXexpression modulation in unaffected brain tissue.
The specificity of miRNAs binding is also a major obstacle in the translation of preclinical animal research into clinical treatments for human brain diseases.Although both miRNAs andSOXgenes are evolutionarily conserved between rodents and humans, miRNAs binding sites on target mRNAs show species-specific and organ-specific sequence variations making rodent-based miRNAs therapies ineffective in humans (Miura et al., 2013).Testing on large animal models of human diseases (Potschka, 2013; Potschka et al., 2013) or employing human models based on pluripotent stem cells can help overcome this obstacle (Morris et al., 2021).
The transcriptional landscape of the brain is changing dramatically with age(Ziats and Rennert, 2014).The same occurs in different neural cell types during neurogenesis in the embryonic and adult brain.Consequent changes in miRNAs expression profiles and the mRNAs target pool raise safety issues regarding unknown potential interactions and put in question theeffectiveness of the therapies.SOX proteins have dynamic expression profiles during adult neurogenesis (Ferri et al., 2004; Steiner et al., 2006; Haslinger et al., 2009; Venere et al., 2012) and in the course of different brain pathologies(Holmberg et al., 2011).In addition, SOX proteins show different expression profiles in GSCs and differentiated GBM cells (Holmberg et al., 2011), which makes the development of SOX targeted therapy for gliomas particularly challenging.It has also been shown that expression profiles of specific miRNAs changed during neurodegeneration (Wang et al., 2008; Sheedy,2015).The expression status of several miRNAs, including miR-9 and mirR-124a, is altered between primary and recurrent GBM tumors (Matos et al.,2018).
Recently, it has been revealed that miRNAs belong to the group of genderspecific biomarkers of NDs (Piscopo et al., 2021).It does not come as a surprise since gender represents a significant factor in the prevalence,incidence, development, and progression of NDs (Loke et al., 2015;Buoncervello et al., 2017).For example, miR-132 is overexpressed in males with PD (Olsen et al., 2009), miR-29a and miR-29c are up-regulated in females with PD (Bai et al., 2017b), while miR-145 is up-regulated in serum samples of female patients with ALS (Toivonen et al., 2014).
The impact of a single miRNA on gene regulation may be insufficient for the therapy of GBM, and recent results suggest that targeting miRNA should be considered as a part of combination therapy (Baumann and Winkler, 2014;Liu and Tu, 2015; Banelli et al., 2017).Bhaskaran et al.(2019) demonstrated that a combination miRNA strategy using simultaneous expression of miR-124, miR-128, and miR-137 (Cluster 3) delivered via extracellular vesicles,showed anticancer synergism and increase survival when combined with chemotherapy in murine GBM models.Corsten et al.(2007) also showed synergistic cytotoxicity of miR-21 and NPCs derived secretable form of TRAIL in a glioma mouse model.
Despite all the challenges discussed above, many miRNAs are currently under investigation as potential therapeutic targets.Some of them even reach preclinical or clinical trials (Aloizou et al., 2020).Among miRNAs targetingSOXgenes, miR-21 is the most prominent therapeutic target in gliomas (Aloizou et al., 2020).
miR-21 activity leads to increased proliferation, invasiveness, and treatment resistance in GBM cells.Its down-regulation significantly suppresses malignant properties and sensitizes GBM cells to radiation (Sathyan et al., 2015; Luo et al., 2017; Guan et al., 2020).The ultimate goal for the clinical translation is to develop a therapy that will target and suppress miR-21 or up-regulate its downstream targets.Several techniques have been developed for miRNAs inhibition, including chemically enhanced antisense molecules, RNA sponges,anti-miRNAs hammerhead ribozymes, and DNA-zymes targeting specific miRNAs and their precursors (Belter et al., 2016; Li and Zamore, 2018; Liu et al., 2018b).
All these techniques have been applied for successful down-regulation of miR-21 in GBM cells.Delivering therapies to tumors in the specific brain area presents quite a challenge.In GBM, besides a blood-brain barrier, there is a second blood-brain tumor barrier that further inhibits drug penetration (Dong,2018).In the development of miR-21 therapeutics for glioma, both an uptake via a systemic route and strategies that bypass the blood-brain barrier was tested (Aloizou et al., 2020).All these efforts, including successful delivery of miR-21 therapeutics, paved the way for miR-21 inhibitor RG-012 to be examined in phase II clinical trials in patients with Alport syndrome (Gomez et al., 2015).
miRNAs represent a class of molecules with a broad spectrum of advantages for ideal candidates for biomarkers in various diseases, particularly in cancer and neurological disorders (Condrat et al., 2020).They show a high level of stability in a wide range of biological fluids, easy accessibility, and potential for early detection (Tribolet et al., 2020).These characteristics are important for the early detection of NDs since successful treatment of these diseases istime-critical, and medication administration should begin before neural death appears (Lee et al., 2020).Even though numerous studies and great effort have been made over the past decade, no miRNAs as biomarkers have made it to the clinic yet (Condrat et al., 2020).The search for miRNAs as biomarkers of NDs is ongoing.Many miRNAs remain promising candidate biomarkers for different NDs (Wiedrick et al., 2019; Dong and Cong, 2021).Since identifying a singular biomarker for the disease is questionable, there is a need to establish a group of biomarkers that will clearly distinguish one disease from another,particularly among a group of NDs (Joilin et al., 2019).Using a set of various miRNAs for disease diagnosis and prediction represent a low-cost and noninvasive method (Condrat et al., 2020)
Considering a possible interplay betweenSOXgenes and miRNAs in brain development and pathology, members ofSOXgene family can be used as biomarkers of a specific brain disorder together with miRNAs.It has already drawn attention thatSOXgenes may serve as biomarkers for various diseases.It is known that SOX and miRNAs can have mutually exclusive expression patterns in the brain (Sathyan et al., 2015).Since plasma miR-124 levels may serve as a potential diagnostic biomarker of PD, mutual expression profiles of miR-124 and SOX2 could be used to predict the onset of the diseases and could be utilized in overseeing the rate of progression or response to treatment.Studying the interplay and matching the expression of miRNAs andSOXgenes could increase the sensitivity and specificity of NDs diagnosis.There is no doubt that in the era of improved RNA-seq technology, we might expect that identification of a specific set of miRNAs andSOXgenes and their interplay may enable earlier and better diagnosis.
The interplay of SOX TFs and miRNAs in the brain under physiological and pathological conditions has just begun to be understood and explored.There is no doubt that further elucidation of this interplay will provide new avenues for developing novel and safe miRNA-based strategies for efficiently combatting brain pathologies.
Author contributions:All authors wrote the manuscript and contributed to literature collection and data analysis.MStevanovic designed the concept of the manuscript and supervised and edited the manuscript.MStevanovic,DSN, DD, and MM contributed to the preparation of the tables.MM, DSN, and MSchwirtlich designed the figures.All authors contributed to the article, and approved the submitted version.
Conflicts of interest:There are no conflicts of interest.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Cerebellar pathology in motor neuron disease:neuroplasticity and neurodegeneration
- Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease
- An atypical ubiquitin ligase at the heart of neural development and programmed axon degeneration
- The endogenous progenitor response following traumatic brain injury: a target for cell therapy paradigms
- The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer’s disease
- Telomerase and neurons: an unusual relationship