Non-canonical role of the ATR pathway in axon regeneration as a mechanosensitive brake
Feng Li , Yuanquan Song
DNA damage has been linked to neuropathology.Diverse DNA damage response (DDR) pathways help preserve DNA integrity in the nervous system during both the developmental and mature stages.Mutations of factors in various signaling pathways responsive to different types of DNA damage have been associated with developmental syndromes with neurological symptoms (McKinnon, 2009; Araújo and Kuraoka, 2019; Khokhlova et al., 2020).For example, mutations in a single gene encoding ataxia-telangiectasia mutated(ATM), which is activated by DNA doublestrand breaks for checkpoint initiation, can lead to ataxia telangiectasia (McKinnon,2004).It is believed that neuropathology can result from cellular DNA damage, or even DNA damage within the mitochondria, as has been shown to correlate with aging and Parkinson’s disease (Bender et al., 2006).However, attributing all the mechanisms underlying neurological diseases caused by DDR gene deficiencies to the accumulation of genetic alterations is an oversimplification.In particular, non-canonical DDRs have exhibited examples of uncoupling of upstream triggers (DNA damage) and downstream events.Other than single/double-strand breaks of DNA, for instance,telomere maintenance and oxidative stress in cells can also activate DDR (Burgess and Misteli, 2015).The consequences of DDR have been typically characterized as cellcycle arrest, expression of repair genes,apoptosis, etc.However, the specific effects of DDR pathway activation in various types of cells, especially the terminally differentiated cells, warrant further exploration.Whether the DDR machinery can function beyond its traditional role remains an open question.
A recent study identified a novel role of the DDR pathway in neurons.Using an axonal injury and repair paradigm inDrosophila melanogaster, this study uncovered the ATM and rad3-related (ATR) pathway as a key regulator in axon regeneration (Li et al., 2021).As a serine/threonine kinase,ATR responds to DNA single-strand breaks and replication stress.ATR, together with ATM, mediates the two major checkpoints to protect the integrity of the genome.The ATM pathway is dispensable for cell survival and mutations of its components are frequently observed in tumor cells.The ATR pathway appears more critical for viability, as mice embryos bearing ATR loss-of-function mutations die at an early developmental stage, confounding investigations into ATR’s role out of the cell cycle (Yazinski and Zou,2016).In this study, gene candidates were specifically manipulated in subtypes of sensory neurons, revealing a new function of the ATR pathway.After knocking down the fly homolog of ATR, meiotic 41, in class III dendritic arborization sensory neurons,the authors noticed increased capacity of these neurons to regrow axons after injury,and the same effect was observed in ATR null flies (Li et al., 2021).This finding suggests that, in non-dividing cells, DDR pathways have developed non-canonical functions to help cells adapt to their environment and cope with their unique perturbations.It is intriguing to ask what the upstream signal is that activates the ATR pathway in the scenario of axon injury, whether it is still DNA damage, and whether other components in the same pathway are also involved.
A large range of genomic aberrations can activate the ATR pathway.In addition, it has been reported that mechanical stress on the nuclear envelope can relocalize ATR to the nuclear membrane to protect the perinuclear chromatin, suggesting that ATR directly or indirectly senses mechanical stimuli to prevent cells from mechanical insults (Kumar et al., 2014).In the context of axon regeneration, it has been shown that a mechanosensitive ion channel, Piezo, inhibits axon regrowth after injury by sensing the mechanical force in the environment around the axon tip (Song et al., 2019).While possible mechanisms for Piezo activation to generate a regenerative halt have been proposed, it is exciting to find that ATR is implicated in the Piezo-induced inhibition of axon regeneration.This study demonstrated that, instead of DNA damage, ATR responds to mechanical stimuli from the environment.γH2AX (phosphorylation of the Ser-139 residue of the histone variant H2AX) staining,which detects DNA-damage eventsin situ,showed no difference between injured and uninjured fly sensory neurons.Moreover,targeting the replication protein A complex,the machinery that monitors single-stranded DNAs and recruits ATR, did not result in a similar axon regeneration phenotype as ATR ablation.These results thus suggested that DNA damage is not directly induced by axon injury and that a signal transduction pathway is activated downstream of ATR, but not by DNA damageper se.In the following experiment using a cultured cell model of osmotic stress to induce ATR clustering in the nucleus, PIEZO1 was demonstrated to be required and act upstream, as ATR clustering was significantly reduced in the PIEZO1 knock-out cell line.By genetic interaction analyses in flies, Piezo and ATR were demonstrated to function in the same genetic pathway during axon regeneration.Furthermore, nitric oxide, produced by nitric oxide synthases, was suggested to act as the signal from Piezo to ATR (Li et al.,2021).Previous work has established that calcium elevation immediately after axotomy is critical for the initiation of growth cone reformation and that calcium, as a second messenger, is derived from extracellular and internal sources and spreads rapidly to the whole axon and cell body (Mahar and Cavalli,2018).However, the identity of a possible signal communicating between the axon and nucleus when regenerating axons elongate remains unknown.This study presented evidence that nitric oxide is a strong candidate, as it is produced in the axon tip and propagates to the nucleus, enabling neurons to respond to the mechanical force from the extracellular matrix (Li et al., 2021)(Figure 1).
Although ATR relocalization at the nuclear envelope induced by osmotic stress is independent of replication protein A-single-stranded DNA nucleofilaments,phosphorylated CHEK1 and ATR interacting protein were found at the nuclear envelope with ATR (Kumar et al., 2014).During axon regeneration, fly homologs of CHEK1/grapes and ATR interacting protein/mus304 were also found to participate in the ATR-activation-induced regeneration inhibition.In the fly sensory neurons, knockdown of CHEK1/grapes or ATR interacting protein/mus304 recapitulated the axon regeneration enhancement caused by ATR deficiency.Moreover, overexpression of CHEK1/grapes sufficiently inhibited axon regeneration (Li et al., 2021).These results indicate that other components of the ATR pathway, mainly in the execution steps, cooperate to carry out this non-canonical function.In DNA repair, after ATR recruitment to replication protein A-single-stranded DNA, its activity is regulated by TOPBP1 (DNA Topoisomerase II Binding Protein 1), which is mediated by the 9-1-1 complex composed of RAD9-HUS1-RAD1 and RAD17.After CHEK1 is phosphorylated by ATR, it can in turn phosphorylate and inhibit CDC25, which is a phosphatase for CDK1.Although RAD17 and TOPBP1 were not found to respond to theosmotic-stress-induced ATR relocalization(Kumar et al., 2014), they are indispensable for axon regeneration inhibition.Knockdown of fly homologs of RAD17, TOPBP1, and RAD1 in the sensory neurons all significantly increased axon regeneration, while loss of function of fly homologs of CDC25 and CDK1 reduced axon regeneration (Li et al.,2021).It is thus demonstrated that ATRCHEK1-CDC25-CDK1 is an axis regulating axon regeneration in response to the mechanical force from the extracellular matrix (Figure 1).CDK1 phosphorylation at the end of this axis typically leads to cell cycle arrest.However, this study provided evidence that, in terminally differentiated cells, such as neurons, the ATR-activation machinery, together with its downstream effectors, modulates cellular regeneration when encountering axonal injury.Therefore,cells are capable of utilizing or repurposing seemingly “redundant/obsolete” regulatory pathways to adapt to unusual scenarios, such as cellular stress.It remains a fascinating question when/how such a functional switch was made, from an evolutionary standpoint.
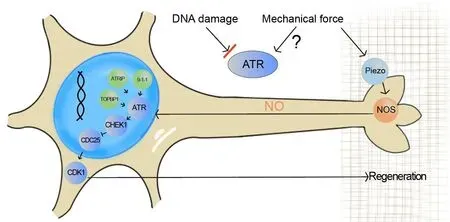
Figure 1|The ataxia-telangiectasia mutated and rad3-related (ATR) pathway responds to mechanical force to inhibit axon regeneration.
Discovering novel neuronal intrinsic regulators for axon regeneration is one of the key areas for the development of new therapeutics for nervous system trauma(Mahar and Cavalli, 2018).The ATR pathway identified inDrosophilathus provides potential targets for treatments.This study further tested the conservation of the ATR pathway in axon regeneration in mammalian injury models, confirming that manipulations of ATR and CHEK1 in mice neuronsin vitroandin vivoalso affect axon regeneration.Lastly, ATR and CHEK1 pharmacological inhibitors have been developed to treat various types of cancers, and they were found to promote axon regeneration in cultured mammalian neurons and flies.Other DNA repair pathways, such as ATM,were proved not essential for regulating axon regeneration in the injured fly sensory neurons, demonstrating the specificity of the ATR pathway (Li et al., 2021).However,we cannot rule out the possibility that the ATM-CHEK2 pathway may be involved under other injury paradigms in other cell types.To conclude, this study raises the possibility that pathways with conventionally known functions may exhibit non-canonical roles in unexpected situations, and suggests that utilizing the screening platform inDrosophilawill be a fruitful strategy to identify more factors controlling axon regeneration.
The present work was supported by the National Institutes of Health Grant,1R01NS107392 (to YS).
Feng Li*, #, Yuanquan Song*, #
Institute for Translational Brain Research, Fudan University, Shanghai, China (Li F)Raymond G.Perelman Center for Cellular and Molecular Therapeutics, The Children’s Hospital of Philadelphia, and Department of Pathology and Laboratory Medicine, University of Pennsylvania,Philadelphia, PA, USA (Song Y)#Both authors contributed equally to this work.
*Correspondence to:Feng Li, PhD,feng@fudan.edu.cn; Yuanquan Song, PhD,songy2@email.chop.edu.
https://orcid.org/0000-0002-5568-7431(Feng Li)
https://orcid.org/0000-0001-7699-2059(Yuanquan Song)
Date of submission:September 13, 2021
Date of decision:October 27, 2021
Date of acceptance:December 10, 2021
Date of web publication:March 23, 2022
https://doi.org/10.4103/1673-5374.335807
How to cite this article:Li F, Song Y (2022)Non-canonical role of the ATR pathway in axon regeneration as a mechanosensitive brake.Neural Regen Res 17(11):2423-2424.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Interplay of SOX transcription factors and microRNAs in the brain under physiological and pathological conditions
- Cerebellar pathology in motor neuron disease:neuroplasticity and neurodegeneration
- Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease
- An atypical ubiquitin ligase at the heart of neural development and programmed axon degeneration
- The endogenous progenitor response following traumatic brain injury: a target for cell therapy paradigms
- The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer’s disease