MicroRNAs as biomarkers in glaucoma and potential therapeutic targets
Bridget Martinez , Philip V.Peplow
Abstract Glaucoma is a neurodegenerative disease in which optic nerve damage and visual field defects occur.It is a leading cause of irreversible blindness.Its pathogenesis is largely unknown although several risk factors have been identified, with an increase in intraocular pressure being the main one.Lowering of intraocular pressure is the only treatment available.Open-angle glaucoma is the most common form of the condition, accounting for ~90% of all cases of glaucoma, with primary open-angle glaucoma and exfoliation glaucoma being the most frequent types.There are strong indications that microRNAs play important roles in the pathogenesis of primary open-angle glaucoma.Most of the recent studies reviewed had performed microRNA profiling in aqueous humor from glaucoma patients compared to controls who were chiefly cataract patients.A very large number of microRNAs were dysregulated but with limited overlap between individual studies.MiRNAs in aqueous humor that could be possible targets for therapeutic intervention are miR-143-3p, miR-125b-5p, and miR-1260b.No overlap of findings occurred within the dysregulated miRNAs for blood plasma, blood serum, peripheral blood mononuclear cells, and tears of primary open-angle glaucoma patients.Several important limitations were identified in these studies.Further studies are warranted of microRNA expression in aqueous humor and blood samples of primary open-angle glaucoma patients in the early stages of the disease so that validated biomarkers can be identified and treatment initiated.In addition, whether modifying the levels of specific microRNAs in aqueous humor or tears has a beneficial effect on intraocular pressure and ophthalmic examination of the eyes should be investigated using suitable animal models of glaucoma.
Key Words: aqueous humor; biomarkers; blood plasma; blood serum; glaucoma; intraocular pressure;microRNA; peripheral blood mononuclear cells; tears; therapeutic targets
Introduction
Glaucoma is a neurodegenerative disease in which optic nerve damage and visual field defects occur.It is a leading cause of irreversible blindness (Jonas et al., 2017) affecting more than 60 million people worldwide and predicted to affect 112 million by 2040 (Tham et al., 2014).The number of undetected cases will also increase.Vision loss is due to loss of retinal ganglion cells(RGCs) and degeneration of the optic nerve, which has a pronounced effect on independent living and quality of life.Its pathogenesis is largely unknown although several risk factors have been identified, including intraocular pressure (IOP), age, race, family history, medical conditions, physical injuries to the eye, corticosteroid use, and other eye-related risk factors such as retinal detachment (AGIS Investigators, 2000; Leske et al., 2001; Gordon et al., 2002; Janssen et al., 2013; Tham et al., 2014; Jonas et al., 2017; American Optometric Association, 2021).The main risk factor is an increase in IOP caused by decreased outflow of aqueous humor through the angle of the anterior chamber, which consists of the trabecular meshwork, uvea, and sclera.While the disease in most cases is connected with raised IOP (> 21 mm Hg), there are cases with low IOP values and continuous progression.The normal IOP range in healthy individuals is 8 to 18 mm Hg (Wang et al.,2018).The lowering of IOP of glaucoma patients is currently the only proven treatment strategy (Lusthaus and Goldberg, 2019), and which has an effect in most patients but at the end of life, approximately 24% of patients are unilaterally blind and 10% bilaterally blind (Mokhles et al., 2016).
Open-angle glaucoma is the most common form of the condition, accounting for ~90% of all cases of glaucoma (Healthline 2018), with primary open-angle glaucoma (POAG) and exfoliation glaucoma (XFG) being the most frequent types (Ritch, 1994; Gupta and Weinreb, 1997; Weinreb and Khaw, 2004).POAG is the most common form of glaucoma, appearing in two-thirds of all cases (Auckland Eye) and has the presence of the glaucomatous optic neuropathy without any identifiable secondary cause (Kwon et al., 2009; Liu et al., 2013).XFG is the most common identifiable secondary form of openangle glaucoma.It occurs in patients with exfoliation syndrome (XFS), an age-related systemic disease characterized by abnormal fibrillary deposits within the eye and various other organs (Mitchell et al., 1999; Challa, 2009).For both POAG and XFG, the only modifiable risk factor is elevated IOP (AGIS Investigators, 2000; Leske et al., 2003; Weinreb and Khaw, 2004; Kwon et al.,2009; Liu and Allingham, 2011).Closed-angle glaucoma is much less common and comprises less than 20% of cases in the United States.Primary angle closure glaucoma (PACG) is the most severe stage of primary angle closure disease (Xu et al., 2018).
The term pseudoexfoliation (PXF) has been used by some groups to describe the accumulation of exfoliative material in different parts of the eye causing glaucomatous neurodegeneration and irreversible blindness (Ekström and Alm, 2008).Abnormal extracellular matrix remodeling together with the accumulation of protein aggregates in the ocular tissues gradually causes progressive fibrosis leading to trabecular meshwork dysfunction, obstruction to aqueous humor outflow, and raised IOP (Ekström and Alm, 2008;Aboobakar et al., 2017).Clinically, an eye with PXF disease may originally lack obvious PXF deposits (unmanifest disease).This progresses to manifest PXF with normal IOP, then the stage with raised IOP (ocular hypertension,OHT), and lastly to pseudoexfoliative glaucoma (PEXG) with irreversible optic nerve/visual field damage.PXF and PEXG are associated with changes in the peripheral blood, therefore making it possible to identify putative markers indicating progression to the next stage and identifying “eyes at risk” (Vessani et al., 2003; Aboobakar et al., 2017; Rao et al., 2020).With reference to the previous paragraph, PXF is equivalent to XFS (Mitchell et al., 1999) and PEXG to XFG (Álvarez et al., 2015).To be consistent hereon in the manuscript, the terms XFS and XFG have been used.
The production and outflow of aqueous humor in the anterior segment of the eye maintains IOP levels.This clear fluid is responsible for maintaining the shape and optical properties of the eye while also providing nutrients and removing waste from the anterior segment tissues.Aqueous humor is secreted by the ciliary epithelium and exits the eye via two outflow pathways: the conventional pathway and the unconventional pathway (Coca-Prados and Escribano,2007; Goel et al., 2010).The conventional pathway consists of the trabecular meshwork and Schlemm’s canal tissues and accounts for about 80% of the aqueous humor drainage in older adults (Goel et al., 2010).Decreased outflow through these tissues is the main contributor to the elevated IOP levels in glaucoma (Goel et al., 2010; Stamer and Acott, 2012).With XFG, the abnormal fibrillary material found in the anterior segment can accumulate along the conventional outflow pathway, leading to disorganization and degeneration of the trabecular meshwork and Schlemm’s canal and elevation of IOP (Schlötzer-Schrehardt and Naumann, 2006).Such elevated IOP levels are a major risk factor to RGC degeneration and, if left untreated, visual impairment.
To date, the early diagnosis of POAG remains unsatisfactory which aggravates the global burden of glaucoma.Although many linkage analyses and genomewide association studies (GWAS) have attempted to unravel the genetics of glaucoma, these genetic associations account for less than 10% of all glaucoma cases (Shastry, 2013; Wang and Wiggs, 2014; Liu and Allingham,2017).Therefore, other genetic factors such as microRNAs (miRNAs) are likely to be involved in the disease pathogenesis (Gonzalez et al., 2014; Jayaram et al., 2018).MiRNAs are present in human aqueous humor, both in solution associated with RNA-binding proteins and contained within extracellular vesicles including exosomes (Dunmire et al., 2013; Tanaka et al., 2014;Dismuke et al., 2015; Wecker et al., 2016).Since extracellular vesicles released from the ciliary body may contribute to outflow pathway signaling, miRNAs could play a role in the outflow pathway (Lerner et al., 2017).Identification of such biomarkers may help to characterize and stratify the severity of outflow dysfunction and responses to treatment.Also, biomarkers of this type may identify novel therapeutic targets to modulate IOP and be used to identify the phenotype of a specific individual’s outflow facility and perhaps predict responses to therapeutic intervention.
MicroRNAs (miRNAs) are single-stranded non-coding RNA molecules approximately 22 nucleotides long that recognize sequences in the 3’-untranslated regions (3′-UTR) of target mRNAs and either induce mRNA degradation (Bagga et al., 2005) or inhibit their translation (He and Hannon,2004; Meister, 2007).MiRNAs have been found to be dysregulated in a variety of diseases and disorders (Peplow et al., 2019), and may play important roles in the pathogenesis of POAG (Kong et al., 2014; Ran et al., 2015; Zhang et al.,2015; Molasy et al., 2017).For example, miR-29b and miR-24 are involved in gene regulation in trabecular meshwork cells (Luna et al., 2011).Moreover, the miRNA expression levels have been linked to maintaining the balance of the aqueous humor, the change in the trabecular meshwork, and the apoptosis of RGCs (Jayaram et al., 2015, 2017; Drewry et al., 2016).Several miRNAs(e.g., miR-29b, miR-200c, miR-204, and miR-24) are also reported as potential diagnostic biomarkers or therapeutic targets for glaucoma (see reviews:Gonzalez et al., 2014; Molasy et al., 2017).Recent reviews have described the mechanism of miRNAs in POAG regarding elevated IOP and optic nerve damage (Wang et al., 2021) and the relationship between miRNA and the trabecular meshwork (Guo et al., 2017).
Only a very small volume of aqueous humor (~100 µL) can be collected at ophthalmic procedures (e.g., cataract or glaucoma surgery).Blood plasma or serum is more suitable for the identification of possible biomarkers in the early detection of glaucoma and response to therapy, as it can be obtained via a minimally invasive procedure and can be collected at frequent intervals to monitor changes in the levels of biomarkers with disease progression and response to treatment.Recently, elevated serum antibodies against autoantigens in ocular tissues have been suggested as biomarkers for diagnosing glaucoma and distinguishing between normal-tension and hightension glaucoma patients (Shin et al., 2021).In addition, autotaxin and transforming growth factor-β levels in aqueous humor were promising diagnostic biomarkers for distinguishing open-angle glaucoma subtypes(Igarashi et al., 2021).We chose to analyze recent literature on the expression levels of miRNAs measured in aqueous humor, blood samples, and tears in glaucoma which could serve as diagnostic biomarkers to distinguish from controls and between subtypes, monitor disease severity, and as potential therapeutic targets.
MicroRNAs in Glaucoma
We performed a PubMed search for original research articles published during January 2014-October 2021 on possible miRNA biomarkers of glaucoma compared to healthy controls (usually cataract patients) in aqueous humor,blood plasma, blood serum, and tears.In addition, we examined these articles for whether they could distinguish between various glaucoma subtypes.The steps involved in the review and its contents are shown (Figure 1).A total of 15 articles were found for this review.Of these, 8 had used aqueous humor, 3 aqueous humor and blood plasma, 1 blood serum, 1 tears, 1 peripheral blood mononuclear cells, and 1 GWAS data.The relevant findings in the research articles from the PubMed search are summarized as follows.
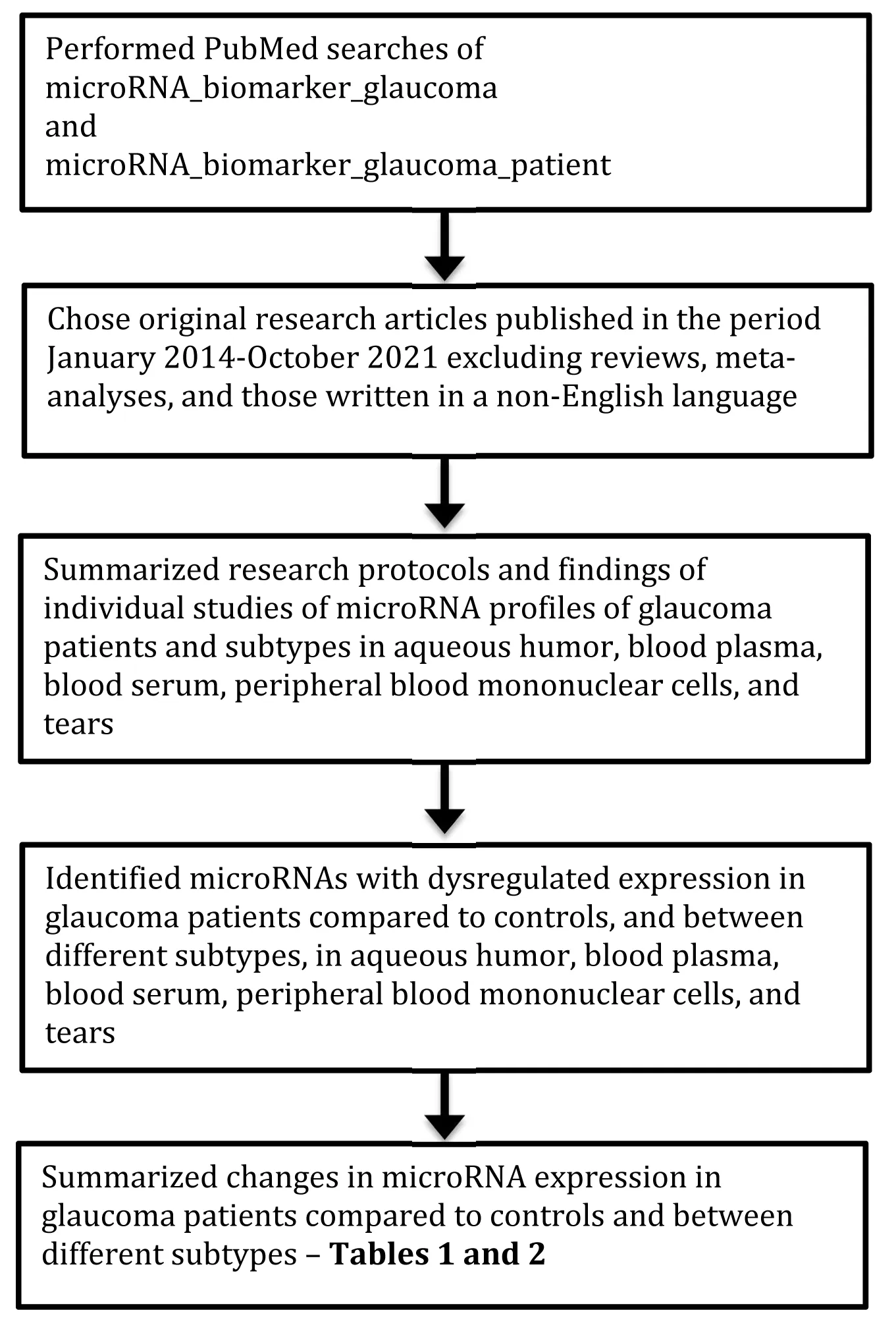
Figure 1|Flow diagram to indicate how the review was performed and its contents.
Aqueous humor
RNA sequencing was used by Seong et al.(2021) to analyze aqueous humor from 6 normal-tension glaucoma patients and 7 control subjects.Each glaucoma patient was using one topical medication.Eight miRNAs were significantly upregulated compared to controls: let-7a-5p, let-7c-5p, let-7f-5p,miR-192-5p, miR-10a-5p, miR-10b-5p, miR-375, miR-143-3p.No significantly downregulated miRNAs were found.The results of RNA sequencing were verified by analyzing let-7c-5p by qPCR which showed significantly increased let-7c-5p expression compared to controls.
Kosior-Jarecka et al.(2021) selected 22 miRNAs for analysis based on previous publications that showed them to be the most abundant in aqueous humor.The groups studied were 19 POAG, 14 XFG, 9 PACG, and 36 cataract patients as control.Using RT-PCR and microarray assay, 8 miRNAs were detected in at least 20% of samples in at least one of the studied groups.Three miRNAs miR-1202, miR-1260b, miR-4634 were detected in at least 20% of samples in all studied groups.The most frequently expressed miRNA in the studied panel was miR-1260b, which was detected in 19 POAG (100%), 13 XFG (92%), and 7 PACG (77%) samples.Seven out of eight of the most frequently expressed miRNAs were detected in a minimum of three samples of two glaucoma subgroups and cataract group, enabling differential expression analysis.No significant differences were observed in the frequency of expression and level of expression for these miRNAs.However, there was a tendency for different expressions of miR-184, miR-1260b, and miR-6722-3p between the various glaucoma subtypes, with miR-184 and miR-6722-3p tending to be expressed more frequently in XFG patients.The expression of miR-6515-3p tended to be lower in POAG patients compared to controls.MiR-1260b was the most abundantly expressed among the studied miRNAs and may be a potential biomarker of clinical status in XFG and PACG.The expression of miR-1260b was correlated with the level of maximum IOP in the PACG group.In the XFG group,the expression levels of miR-1260b were correlated with the stage of glaucoma assessed as mean defect index in visual field examination.The level of miR-1260b tended to decrease with a more advanced stage of glaucoma.
Using small RNA sequencing, Hubens et al.(2021) examined samples from 9 POAG and 10 cataract patients.The POAG group included patients with early, moderate, advanced, and severe glaucoma (Mills et al., 2006), and most POAG patients were on at least two types of topical IOP lowering medication.262 miRNAs were identified of which 62 were detected in at least 60% of the samples.Of these, 7 miRNAs were significantly differentially expressed, with 4 being upregulated miR-30a-3p, miR-143-3p, miR-211-5p, miR-221-3p, and 3 being downregulated miR-451a, miR-486-5p, miR-92a-3p in POAG.Of the expression levels of the 7 differentially expressed miRNAs, that of miR-143-3p correlated weakly with IOP.None of them correlated significantly with disease severity (mean deviation) or disease progression (mean deviation loss per year).By receiver operating curve (ROC) analysis, the upregulated miRNAs had AUC values between 0.77 (miR-211-5p) and 0.89 (miR-143-3p, sensitivity 89%, specificity 89%) suggesting they might have potential as diagnostic biomarkers of POAG.The downregulated miRNAs had AUC values between 0.51 and 0.56 and did not distinguish glaucoma patients from controls.The combination miR-143-3p with miR-221-5p had AUC 0.96, sensitivity 89%,specificity 100%, identifying it as a good test to distinguish between POAG and controls.
Using the limma package of the R software for statistical analysis to establish differentially expressed lncRNA and mRNA, Zhou et al.(2020) analyzed data from the Gene Expression Omnibus with 10 aqueous humor samples collected from POAG patients and 10 aqueous humor samples from cataract patients.POAG patients did not receive any glaucoma medication 4 months before surgery and had uncontrolled IOP.Both lncRNAs and mRNAs that were negatively correlated with certain common miRNAs were defined as candidate ceRNA (competitive endogenous RNA) pairs.A total of 4130 differentially expressed RNAs were identified.Among them, 1041 were lncRNA (508 up- and 533 down-regulated) and 3089 were mRNA (2135 upand 954 down-regulated).9 miRNAs (miR-20b-5p, miR-761, miR-17-5p, miR-338-3p, miR-24-3p, miR-125b-5p, miR-3619-5p, miR-129-5p, and miR-27a)and 4 lncRNAs (DNAJC27-AS1, AF121898, OIP5-AS1, and SNX29P2) were established as hub RNAs in the ceRNA network.Comparing the differences in the degree, closeness, and betweenness centrality among lncRNAs, miRNAs,and mRNAs showed that lncRNAs and miRNAs had a higher degree, closeness,and betweenness centrality than mRNAs indicating that lncRNAs and miRNAs tended to be pivotal to the risk of POAG.
Two cohorts of patients were recruited by Hindle et al.(2019) with cohort 1 being Caucasian and consisting of 17 POAG and 11 cataract patients, while cohort 2 was Japanese and comprised 13 XFS, 3 XFG, and 22 cataract patients.In the initial miRNA selection analysis, 9 samples from cohort 1 were included;in the complete analysis, all of cohort 2 plus additional 19 participants from cohort 1 were included.By RT-PCR, 73 miRNAs were detectable in every sample of aqueous humor.6 of the 20 miRNAs shown to be elevated in theblood plasma of glaucoma and XFS patients (described in the sectionblood plasma) were also significantly increased in aqueous humor from the same subjects: miR-637, miR-99b-3p, miR-4725-3p, miR-4724-5p, miR-4358, miR-433-3p compared to controls.An improved correlation was shown between miRNA levels in aqueous humor and blood plasma when only the top 6 aqueous humor biomarkers and top 20 plasma biomarkers were plotted.
Liu et al.(2018) analyzed aqueous humor samples from 3 POAG patients with moderate visual field defect (M-POAG), 3 POAG patients with severe visual field defect (S-POAG), and 6 cataract controls using next-generation sequencing (NGS).88 miRNAs had significantly different expression with a fold change of ≥ 4 and a diverge probability of ≥ 0.8 when comparing the POAG group with the cataract group.Of these, 73 miRNAs were upregulated and 15 were downregulated.When comparing the S-POAG with M-POAG group, 16 miRNAs were significantly upregulated with a fold change of ≥ 4 and a diverge probability of ≥ 0.8: miR-205-5p, miR-206, miR-16-5p, miR-501-3p, miR-409-3p, miR-200a-3p, miR-200b-3p, miR-382-5p, miR-543, miR-136-3p, miR-30c-2-3p, miR-139-5p, miR-340-5p, miR-488-3p, miR-202-5p, miR-369-5p.12 of the 16 differentially expressed miRNAs between S-POAG and M-POAG were also significantly upregulated between POAG and cataract groups: miR-205-5p, miR-200b-3p, miR-136-3p, miR-488-3p, miR-200a-3p, miR-139-5p, miR-369-5p, miR-206, miR-501-3p, miR-30c-2-3p, miR-543, miR-16-5p.Three miRNAs were analyzed by qPCR in 18 independent samples.The expression of miR-184, miR-486-5p, and miR-93-5p using qPCR agreed with the sequencing results.
A NanoString assay was used in a discovery set of 12 POAG, 12 XFG and 11 cataract patients by Drewry et al.(2018).Two miRNAs were differentially expressed in POAGvs.control: miR-125b-5p (downregulated), miR 302d-3p and miR-451a (upregulated).Five miRNAs were differentially expressed in XFGvs.control: miR-122-5p, miR-3144-3p, miR-320e, miR-630 (upregulated),miR-320a (downregulated).Two miRNAs were differentially expressed in XFGvs.POAG: miR-125b-5p (upregulated), miR-302d-3p (downregulated).Using PCR in a validation set of 17 POAG, 14 XFG and 10 cataract patients, 3 miRNAs were differentially expressed in POAGvs.control: miR-451a, miR-302d-3p(upregulated), miR-125b-5p (downregulated).Five miRNAs were differentially expressed in XFGvs.control: miR-122-5p, miR-320e, miR-3144-3p, miR-630(upregulated), miR-320a (downregulated).Three miRNAs were differentially expressed in XFG vs.POAG: miR-125b-5p (upregulated), miR-320a, miR-302d-3p (downregulated).
Jayaram et al.(2017a) analyzed samples from 6 POAG and 8 cataract patients using PCR.The POAG patients had a mean of 1.2 topical medications; 2 had dry AMD (age-related macular degeneration) as ocular comorbidity and 1 had retinal detachment repair.None of the cataract patients were using topical medications; 1 had dry AMD as ocular comorbidity and 1 had retinal detachment repair.MiR-518d and miR-143 were significantly upregulated and miR-660 significantly downregulated in samples from all POAG subjects compared with controls.Three miRNAs miR-135a, miR-9, and miR-128a were consistently expressed in controls but not detected in POAG patients.No miRNAs were expressed solely in the POAG samples.
Aqueous humor from 4 cataract Caucasian subjects was analyzed by Wecker et al.(2016).By NGS, the most abundant miRNA detected in the samples was miR-451a, followed by miR-184, miR-16-5p, and miR-4468.By qPCR, 3 miRNAs were analyzed in 5 independent aqueous humor samples.The ones chosen were miR-451a as the most abundant in NGS, miR-144 as of intermediate abundance, and miR-202 which was not detected by NGS in the samples but has been described as the most abundant miRNA in aqueous humor in pooled samples using qPCR (Dunmire et al., 2013).The mean C(t) values obtained were 27.9, 32.7, and 37.5 for miR-451a, miR-144, and miR-202, respectively,thereby confirming the relative expression patterns obtained using NGS.
Buys et al.(2015) analyzed samples from 6 POAG patients of which 3 had peripheral visual field loss (PVFL) and 3 had early paracentral visual field loss(ePaVFL), a POAG subtype associated with vascular dysregulation, together with 3 cataract patients using the Qiagen MIHS-3218Z platform.Supervised clustering separated the 3 sample types and identified 2 miRNAs among the top classifying input variables, miR-4738-3p and miR-4740-3p.Abundance of miR-4738-3p in aqueous humor was higher in PVFL compared to cataract control, and abundance of miR-4740-3p in aqueous humor was lower in ePaVFL compared to cataract control and PVFL.Subsequently, samples from 3 PVFL patients and 4 control patients were analyzed on a custom-built array(CMHS-02263) which separately clustered PVFLversuscontrol using the 5 top classifying miRNAs: miR-3181, miR-4469, miR-760 (increased abundance),miR-4798-3p, miR-940 (decreased abundance).
Microarray analysis of samples from 10 glaucoma patients (7 POAG, 1 PACG and 2 XFG), together with those from 10 controls (5 cataract, 5 epineural membrane) was performed by Tanaka et al.(2014).121 miRNAs were detected in all 20 patients.Among these, 18 miRNAs showed a statistically significant difference in expression level between glaucoma and control patients, 8 were upregulated (miR-4484, miR-6515-3p, miR-3663-3p,miR-4433-3p, miR-6717-5p, miR-4725-3p, miR-1202, miR-3197) and 10 downregulated (miR-4507, miR-3620-5p, miR-5001-5p, miR-6132, miR-4467,miR-187-5p, miR-6722-3p, miR-4749-5p, miR-1260b, miR-4634) in glaucoma patients.ROC analysis of the 8 upregulated and 10 downregulated miRNAs in glaucoma patients showed they all had an AUC ≥0.8 and would be fair tests to distinguish glaucoma patients from controls.
There are several important observations regarding these studies on aqueous humor samples.Those performed by Tanaka et al.(2014), Hubens et al.(2021),Kosior-Jarecka et al.(2021), and Seong et al.(2021) should be considered as pure discovery studies as there was no validation cohort.In the study by Hindle et al.(2019), patients from cohort 1 were included with all of cohort 2,and there was no independent validation group.While an independent group was utilized by Liu et al.(2018), only three miRNAs were analyzed.Similarly,Wecker et al.(2016) included an independent group but only three miRNAs were analyzed.A discovery and validation set of patients were included by Drewry et al.(2018) and this study probably has the more reliable findings.The studies by Buys et al.(2015) and Jayaram et al.(2017a) are best regarded as pilot studies.
Blood plasma
Hubens et al.(2021) obtained blood plasma samples from 9 POAG and 10 cataract patients.POAG patients were on at least two types of topical IOP lowering medication and included those with early, moderate, advanced, and severe glaucoma (Mills et al., 2006).By small RNA sequencing, none of the miRNAs identified in blood plasma were significantly differentially expressed between POAG patients and controls.The miRNA composition differed from that in aqueous humor.MiR-184, the most abundant miRNA in aqueous humor, was not detected in blood plasma, nor was miR-124-3p.Conversely,several of the highly abundant miRNAs in blood plasma such as miR-126-3p and miR-16-5p were not detected in any of the aqueous humor samples.Other abundant blood plasma miRNAs e.g., miR-103a-3p/107 had a much lower abundance in aqueous humor.
Hindle et al.(2019) examined samples from two cohorts of patients.Cohort 1 was Caucasian and consisted of 17 POAG and 11 cataract patients, while cohort 2 was Japanese and comprised 13 XFS, 3 XFG, and 22 cataract patients.In the initial miRNA selection analysis, 9 samples from cohort 1 were included;in the complete analysis, all of cohort 2 plus additional 19 participants from cohort 1 were included.By RT-PCR, 20 miRNAs were significantly more abundant in blood plasma from glaucoma or XFS patients than from control subjects: miR-4667-5p, miR-99b-3p, miR-637, miR-4490, miR-1253, miR-3190-3p, miR-3173-3p, miR-608, miR-4725-3p, miR-4448, miR-323b-5p, miR-4538, miR-3913-3p, miR-3159, miR-4663, miR-4767, miR-4724-5p, miR-1306-5p, miR-181b-5p, miR-433-3p.There were no interaction effects in two-way ANOVA comparison between disease and cohort factors for any of miRNAs examined in the blood plasma samples, indicating that miRNAs responded similarly to disease across the two ethnicities.ROC analysis was performed for each of the 20 miRNAs.MiR-4448 had the highest AUC 0.83, sensitivity 94%, specificity 80%.A combination of 3 miRNAs miR-637, miR-1306-5p, miR-3159 produced the highest AUC 0.91, sensitivity 85%, specificity 88%, and distinguished glaucoma patients from controls.
Blood plasma samples from 3 ePaVFL and 3 control patients were analyzed on the Qiagen miFinder384HC platform by Buys et al.(2015).Two miRNAs in blood plasma separated ePaVFL from cataract control, miR-150-5p, miR-720 (having decreased abundance), and may be diagnostic biomarkers of the ePaVFL subtype of POAG.
Important observations can be made regarding these studies on blood plasma samples.That performed by Hubens et al.(2021) should be considered as a pure discovery study as there was no validation cohort.Also, Hindle et al.(2019) included patients from cohort 1 with all of cohort 2, and there was no independent validation group.
Blood serum
Liu et al.(2019) performed NGS on samples from 9 POAG and 9 controls and showed that miR-210-3p, miR-885-5p, and miR-3149 were differentially expressed in POAG group (all upregulated).Using qRT-PCR the expressions of miR-210-3p, miR-885-5p were consistent with those of sequencing but miR-3149 could not be detected.qRT-PCR was then used to examine the expression levels of miR-885-5p and miR-210-3p in an independent screening sample of 26 POAG and 26 controls.MiR-210-3p was significantly upregulated in the serum of POAG patients but miR-885-5p was not significantly differentially expressed in the serum of POAG patients compared to controls.The levels of miR-210-3p were validated in an independent sample of 33 POAG and 33 controls.Again, miR-210-3p showed significantly increased expression levels in POAG patients compared to controls.On ROC analysis, in the screening set for miR-210-3p AUC was 0.846, sensitivity 81%, specificity 85%, and for miR-885-5p AUC was 0.527, sensitivity 58%, specificity 54%.In the validation set, AUC of miR-210-3p was 0.813, sensitivity 85%, specificity 70%, for distinguishing POAG patients from controls.
The important observation regarding blood serum samples is that Liu et al.(2019) used a discovery and two independent sample groups, and therefore the findings would be considered to be reliable.
Peripheral blood mononuclear cells
17 XFS, 11 XFS with OHT, 11 XFG, and 11 cataract controls were recruited by Rao et al.(2020).Patients with XFS, raised IOP and normal optic nerve/visual field were classified as XFS with OHT, while those with optic nerve and corresponding visual field damage were labeled as XFS with XFG.The patients with XFG were significantly older than earlier forms of XFS or controls.To avoid bias due to long-term medications, patients naïve to ocular or systemic medical therapy were chosen for analysis.Only bilateral cases were considered which excluded patients with eyes in different XFS stages or clinically unilateral disease.In the discovery stage by qPCR, 12 of 84 mRNAs were significantly upregulated in XFG compared to XFS with OHT, those with> 2.5-fold differential expression were miR-96-5p, miR-302b-3p, miR-223-3p,miR-124-3p, miR-424-5p, miR-143-3p, miR-302a-3p, miR-122-5p.
Comparing phenotypes of XFS (classical and radial pigmentary) with controls,miR-144-3p showed differential downregulation in pigmentary phenotype of XFS.In the validation stage by qPCR, the miRNAs related to fibrosis were validated in various disease stages which confirmed the role of miR-122-5p which was upregulated in XFG compared to XFS with OHT.A total of 19 other miRNAs were found related to fibrosis or TGF-β1 pathway and were significantly upregulated in later stages of XFG (miR-26a-5p, miR-101-3p, miR-107, miR-18a-5p, miR-199a-5p, miR-129-5p, miR-133a-3p, miR-211-5p, miR-223-3p, miR-1-3p, miR-200a-3p, miR-204-5p, miR-208a-3p, miR-215-5p, miR-338-5p, miR-449a, miR-449b-5p, miR-5011-5p, miR-661).MiR-19a-3p and miR-30a-5p related to proteoglycans were significantly upregulated in XFG compared to XFS with OHT.
In the study by Rao et al.(2020) it was not reported what the group sizes were for the discovery and validation stages or whether patients in the discovery stage were also included in the validation stage.
Tears
Raga-Cervera et al.(2021) collected reflex tears from the inferior meniscus of the eye without instilling anesthetics from 20 POAG and 22 OHT as controls(eyes with elevated IOP but not displaying optic disc damage or altered visual field).Using NGS, 95 miRNAs were identified as present in tears of POAG and OHT patients.Of these, 6 miRNAs were upregulated (miR-26b-5p, miR-27a-3p, miR-152-3p, miR-30e-5p, miR-125b-2-5p, miR-224-5p), and 2 miRNAs downregulated (miR-151a-3p, miR-1307-3p) in tears from POAG patients compared to OHT patients.By ROC analysis, AUC value of 4 of the 8 miRNAs(miR-26b-5p, miR-30e-5p, miR-151a-3p, miR-152-3p) was > 0.75, so they could be considered as fair tests to distinguish POAG from OHT patients.
The study by Raga-Cervera et al.(2021) should be considered as a pure discovery study as there was no validation cohort.
Genome-wide scan
Data from the recent GWAS in glaucoma endophenotypes provided by International Glaucoma Genetics Consortium was used by Ghanbari et al.(2017) to examine the association of miRNA-related genetic variants with POAG endophenotypes.The association was examined of 411 miRNA variants(in 332 miRNA genes) with IOP, VCDR (vertical cup-to-disc ratio), cup area and disc area.Two miRNA variants passed the Bonferroni-corrected significance threshold of 1.22 × 10-4(0.05/411).Genetic variants in the miR-612 precursor and in the miR-4707 seed region were significantly associated with VCDR and cup area.The variant in miR-612 has been previously demonstrated to increase miR-612 expression (Kim et al., 2012).While the variant in miR-4707 does not influence the miRNA expression, it affects the binding of miR-4707 to one of its glaucoma-associated target genes,CARD10.
Those miRNAs found to have altered expression in aqueous humor, blood plasma, blood serum, peripheral blood mononuclear cells, and tears in glaucoma and its subtypes are summarized in Tables 1 and 2.
Discussion
Primary open-angle glaucoma, the most common form of glaucoma, develops slowly and usually without any symptoms.Many people are unaware they have this condition until they have significant vision loss, with peripheral vision being initially affected but which may advance to central vision loss.If left untreated, glaucoma can lead to significant vision loss in both eyes and may even lead to blindness.Angle-closure glaucoma is a less common type of glaucoma that can progress slowly or abruptly, and severe vision loss can occur quickly (American Optometric Association).At present, glaucoma can only be diagnosed by a comprehensive eye examination using specialized equipment/technology such as optical coherence tomography (Bussel et al.,2014) performed by an ophthalmologist or optometrist.It clearly would be advantageous to have a blood-based screening test for glaucoma that could be used when specialized eye examination facilities are unavailable.Several studies have attempted to identify biomarkers of glaucoma in aqueous humor but collecting such fluid from patients is associated with high risk and cannot be performed regularly.Also, there is possible contamination with blood or tears during its collection.
Recent research studies have indicated miRNAs as diagnostic and prognostic biomarkers in many neurodegenerative diseases including ocular diseases such as diabetic retinopathy and age-related macular degeneration (AMD)(Martinez and Peplow, 2019, 2021).The majority of the different studies reviewed here performed miRNA profiling in aqueous humor from glaucoma patients compared to controls who were cataract patients.A very large number of miRNAs were found to be dysregulated but with limited overlap between individual studies (Table 1).Comparing glaucoma patients to controls, miR-143-3p was upregulated in three of the studies (Jayaram et al., 2017; Hubens et al., 2021; Seong et al, 2021).In addition, miR-4725-3p was upregulated in POAG + XFS + XFG compared to controls (Hindle et al.,2019) and in POAG + PACG + XFG compared to controls (Tanaka et al., 2014).MiR-125b-5p was shown to be a hub miRNA in the ceRNA network (Zhou et al., 2020).It was downregulated in POAG versus controls but upregulated in XFG versus POAG (Drewry et al., 2018).There were some inconsistencies in the findings.For example, comparing POAG with controls, miR-451a was downregulated (Hubens et al., 2021) but upregulated in an earlier study (Drewry et al., 2018).Also, miR-6515-3p was upregulated in POAG+ PACG + XFG compared to controls (Tanaka et al., 2014) but tended to be downregulated in POAG compared to controls (Kosior-Jarecka et al., 2021).These inconsistencies could be due to a number of variables and which are confounding factors (see later section).Interestingly, a large number of miRNAs were upregulated in patients with severe POAG compared to those with moderate POAG, using visual field defects mean deviation values to categorize the two groups (Liu et al., 2018).Also, patients with PVFL were distinguished from those with ePaVFL and from controls by virtue of different miRNA profiles (Buys et al., 2015).
In miRNA profiling of samples other than the aqueous humor, there was similarity in upregulated blood plasma miRNAs in POAG + XFS + XFG patients versus controls and XFS patients versus controls (Hindle et al., 2019).No overlap of findings occurred within the dysregulated miRNAs for blood plasma, blood serum, peripheral blood mononuclear cells, and tears (Table 2).However, there was some overlap with the findings in aqueous humor.Six miRNAs were upregulated (miR-637, miR-99b-3p, miR-4725-3p, miR-4724-5p, miR-4358, miR-433-3p) in both blood plasma and aqueous humor of POAG + XFS + XFG patients versus controls (Hindle et al., 2019).Also, miR-143-3p was upregulated in the aqueous humor of glaucoma patients (Jayaram et al., 2017; Hubens et al., 2021; Seong et al., 2021) and upregulated in peripheral blood mononuclear cells of exfoliation glaucoma patients (Rao et al., 2020).Furthermore, miR-122-5p was upregulated in aqueous humor of exfoliation glaucoma patients compared to controls (Drewry et al., 2018) and upregulated in peripheral blood mononuclear cells of exfoliation glaucoma patients (Rao et al., 2020).Only one of the miRNAs (miR-29b, miR-200c, miR-204, miR-24) reported as potential diagnostic biomarkers or therapeutic targets for glaucoma (see reviews: Gonzalez et al., 2014; Molasy et al., 2017)was found to be dysregulated in the various samples examined, with miR-204-5p being upregulated in peripheral blood mononuclear cells in the later stages of exfoliation glaucoma (Rao et al., 2020).
Several important limitations were identified in these recent studies.(i) Many had used very small-sized groups with some being as limited as 3 or 6 in number (Table 3).Only one study had performed a sample size calculation and used group sizes of 20 POAG and 22 OHT patients (Raga-Cervera et al., 2021).(ii) Heterogeneity of the glaucoma patients was quite marked in some studies e.g., the POAG group included patients with early, moderate,advanced, and severe glaucoma (Hubens et al., 2021), or the experimental group comprised POAG together with subtypes XFS and XFG (Hindle et al.,2019).(iii) Marked differences in age and gender of groups were seen in some studies e.g., XFG patients were considerably older than earlier forms of XFS or controls (Rao et al., 2020).Glaucoma patients comprised 6 males and 4 females, while the control group included 3 males and 7 females (Tanaka et al., 2014).(iv) Differences in IOP levels and the use of topical medication by glaucoma patients were reported e.g., in one study POAG patients were on at least two types of topical IOP lowering medication (Hubens et al., 2021), while in another study POAG patients did not receive any glaucoma medication 4 months before surgery (Zhou et al., 2020) or were naïve to ocular or systemic medical therapy (Rao et al., 2020) (Table 3).It would be helpful to categorize the patients into normal-tension glaucoma and high-tension glaucoma groups.(v) Patients of different ethnicity were combined e.g., Caucasian and Japanese patients (Hindle et al., 2019).(vi) In some studies patients had significant comorbidities such as dry AMD (Jayaram et al., 2017) while in other studies patients had no severe coexisting diseases (Hubens et al., 2021).(vii)Inclusion and exclusion criteria were not reported in several of the studies(e.g., Jayaram et al., 2017; Zhou et al., 2020).(viii) Many of the studies are best regarded as discovery studies as no validation cohorts were included (see Bauer et al., 2020).(ix) Different analytical methods have been used including next-generation sequencing and PCR, and the findings were not always in agreement (Liu et al., 2019).Validation of data should be performed using PCR.(x) Normalization of miRNA data was not included in one of the studies(Zhou et al., 2020).(xi) ROC analysis to indicate which miRNAs are good or fair tests to distinguish glaucoma patients from controls or separate between different glaucoma subtypes was only performed in five of the studies (Tanaka et al.2014, Hindle et al., 2019, Liu et al., 2019, Hubens et al.2021, Raga-Cervera et al., 2021).Some of these limitations had been indicated previously(Jayaram et al., 2017b; Li and Wang, 2017).

Table 1 |Alterations of miRNA expression in aqueous humor in glaucoma and its subtypes

Table 2 |Alterations of miRNA expression in blood plasma, blood serum, tears, PBMCs in glaucoma and its subtypes, and data from genome-wide scan

Table 3 |Number of subjects in glaucoma and control groups, intraocular pressure and use of topical medication by glaucoma patients
Treatment with miRNA mimics (agomirs) or inhibitors (antagomirs) may be a way to increase or lower the expression of selected miRNAs in glaucoma patients and slow the progression of the disease.In preclinical studies, IOP was decreased after intraocular injection of miR-200c in rats (Luna et al.,2012).Moreover, miR-450 increased MyoD, a myogenic transcription factor,and may influence the contractile component of the trabecular meshwork and the outflow of aqueous humor (Izzotti et al., 2015).It was also shown that miR-96 affected the survival and apoptosis of rat RGCs through interaction with caspase-2 (Wang and Li, 2014).Downregulation of miR-100 mediated by lentivirus reduced the apoptosis of rat RGCs and promoted neuronal growth (Kong et al., 2014).Regarding miRNAs present in aqueous humor of glaucoma patients in the studies reviewed, a possible target is miR-1260b.It was downregulated in glaucoma patients (Tanaka et al., 2014), and tended to decrease with a more advanced stage of glaucoma with its expression levels correlated with the stage of glaucoma assessed as mean defect index in visual field examination (Kosior-Jarecka et al., 2021).MiR-1260b has been shown to target genes regulating proliferation and differentiation of neuronal cells such asLMX1B(Yan et al., 2011),SMAD4(Kawaguchi-Niida et al., 2017),WNK1(Sun et al., 2017), andCREB1(Smith et al., 2016) and genetic variants and mutations inLMX1Bwere associated with susceptibility of glaucoma(Cross et al., 2014; Choquet et al., 2018; Gharahkhani et al., 2018).It has alsobeen shown to targetSMAD4andSFRP1(Kosior-Jarecka et al., 2021), which are involved in the outflow regulatory mechanisms in the anterior chamber.Another possible target is miR-143-3p which was upregulated in three of the studies (Jayaram et al., 2017; Hubens et al., 2021; Seong et al, 2021).MiR-143-3p, as part of the miR-143/miR-145 cluster, is important for the regulation of outlow capacity of the trabecular meshwork (Li et al., 2017).MiR-143-3p is located on the long arm of chromosome 5 (5q32), a locus associated with increased risk for developing POAG (Pang et al., 2006).Two linkage loci associated with glaucoma and IOP (GLC1G,GLC1M) involving the region 5q21-32 have been described (Monemi et al., 2005; Kramer et al., 2006; Pang et al.,2006), and an association was found between IOP and copy number variation at this locus (Nag et al., 2013).Also, miR-125b-5p could be a possible target as it was shown to be a hub miRNA in the ceRNA network (Zhou et al., 2020) and pivotal to the risk of glaucoma.It was downregulated in the aqueous humor of POAG patients compared to controls but upregulated for XFG compared to POAG patients (Drewry et al., 2018) and in tears of POAG patients (Raga-Cervera et al., 2021).Gene targets for miR-125b-5p in POAG includeAKT1,ATXN1(Huang et al., 2008; Han et al., 2011),BAK1,BCL2,BCL2L2(Nickells et al., 2008) which are involved in RGC survival.A search of the ClinicalTrials.gov website (U.S.National Library of Medicine) and EU Clinical Trials Register did not indicate any clinical trials that were in progress or recruiting to test miRNA therapeutics in glaucoma patients.
A number of recent genetic studies of glaucoma have been performed.Genes associated with increased IOP or POAG risk includedABCA1,AFAP1,ARHGEF12,ATXN2,CAV1,CDKN2B-AS1,FOXC1,GAS7,GMDS,SIX1/SIX6,TMCO1, andTXNRD2.However, variations in risk and genetic factors based on ethnic and geographic differences were found.While unified molecular pathways accounting for POAG pathogenesis remain undetermined,inflammation and senescence likely play important roles.There are similar ethnic and geographic complexities in PACG, but several genes have been associated with this disorder, includingMMP9,HGF,HSP70,MFRP, andeNOS.Genes implicated in XFG includedLOXL1,CACNA1A,POMP,TMEM136,AGPAT1,RBMS3, andSEMA6A(Zuckerman et al., 2021).Many common variants and associated endophenotypes have been discovered in POAG through GWAS, particularly in ethnically diverse cohorts.Although the functional significance of these common variants is unknown, these advances have increased heritability estimates and helped create polygenic risk scores.In contrast, few variants have been identified in XFS/XFG, hampering efforts to examine endophenotypes, create satisfactory animal models, and establish a firm genetic basis (Tran and Pasquale, 2021).
In conclusion, some progress has been made in identifying miRNAs that have altered expression in POAG patients and their various subtypes.However,there are a large number of limitations and confounding factors in many of these studies that make the comparison of results difficult or unreliable.Future studies are warranted to obtain miRNA expression data for aqueous humor and blood samples of glaucomatous patients in the early stages of the disease, which is usually without any symptoms and can only be detected by ophthalmic examination, so that biomarkers can be identified and treatment started.These studies should be designed to reduce the number of limitations so that they are more compatible.In addition, suitable animal models of glaucoma should be used to test whether modifying the levels of specific miRNAs in aqueous humor or tears has a beneficial effect on IOP and ophthalmic examination of the eyes.Animal models (spontaneous and induced) used to study POAG have included monkeys, dogs, mice, rats, and rabbits (Bouhenni et al., 2012).Laser photocoagulation has been used to develop experimental glaucoma in the rhesus monkey (Burgoyne, 2015).Recently, the development of POAG-like features was reported in a rhesus macaque colony from Southern China (Pasquale et al., 2021) and provides a unique opportunity to test novel therapeutic strategies for the disease.Performing studies in monkeys that spontaneously develop glaucoma-like features would raise fewer ethical concerns than using animals subjected to laser photocoagulation.
Author contributions:Manuscript conception/design, literature retrieval,manuscript preparation, editing and review: BM and PVP.Both authors approved the final version of this manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Interplay of SOX transcription factors and microRNAs in the brain under physiological and pathological conditions
- Cerebellar pathology in motor neuron disease:neuroplasticity and neurodegeneration
- Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease
- An atypical ubiquitin ligase at the heart of neural development and programmed axon degeneration
- The endogenous progenitor response following traumatic brain injury: a target for cell therapy paradigms
- The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer’s disease