Boron at tera-Pascal pressures
Peiju Hu(胡佩菊) Junhao Peng(彭俊豪) Xing Xie(谢兴) Minru Wen(文敏儒)Xin Zhang(张欣) Fugen Wu(吴福根) and Huafeng Dong(董华锋)
1School of Physics and Optoelectronic Engineering,Guangdong University of Technology,Guangzhou 510006,China
2School of Materials and Energy,Guangdong University of Technology,Guangzhou 510006,China
3Guangdong Provincial Key Laboratory of Information Photonics Technology,Guangdong University of Technology,Guangzhou 510006,China
Keywords: boron,high-pressure prediction,first principles,USPEX
1. Introduction
The light element boron, which has only three valence electrons, has attracted extensive interest because of its susceptibility to external pressure.Under different pressure,there are at least 16 allotropes,[1-3]e.g.,α-B,[4,5]β-B,[6]γ-B,[7]t-B.[8]These allotropes are mostly composed of an icosahedron unit and have outstanding physical characteristics, such as superconducting properties.[9,10]Boron compounds, such as B6O,B6P,B13C2,[11,12]MgB2,[13]and Na2B30,[14]containing icosahedrons, have similar physical properties. Because of these diverse structures and special properties,the study of boron under high pressure becomes an interesting topic.
In 2009,Oganovet al.[7]determined orthorhombicγ-B28under 10-12 giga-Pascal (GPa) and found its semiconductor and super-hard properties.[11]Recently,in 2013,Parakhonskiyet al.[15]synthesizedδ-B andε-B at 9 GPa in the laboratory.However,these phases are metastable states. In 2017,ζ-B[16]was discovered at 115 GPa in experiments, and was thought to be a product with the same space group (Cmce) asα-Gatype boron. High-pressure hexagonal B10(P63/mcm), predicted by Pickardet al,[17]is more stable thanα-Ga-B above 383 GPa.[17]High pressure causes B10to lose the common building block(icosahedral unit B12)and form a linear atomic chain arrangement together with an isosceles triangle arrangement.Even though the pressure of boron has reached 400 GPa,the structure under tera-Pascal pressure had not been studied.Exploration of the possible existence of stable but hitherto undiscovered high-pressure structures can advance our understanding of theoretical physics and chemistry. In this paper,we use a first-principles crystal structure prediction method to predict a new boron structure under ultra-high pressure. We found a dense boron structure at 1.317 TPa,in which there is a boron atom embedded in the icosahedron.
2. Computational details
Here, we used theab initioevolutionary algorithm USPEX,[18-24]which can simultaneously find crystal structures of all stable elemental crystals in multi-pressures. In our searches at 500 GPa,1500 GPa,2000 GPa,and 5000 GPa pressure points, the initial population included 120 structures with up to 28 atoms per primitive cell, with all subsequent generations consisting of 50 structures produced by heredity(30%), transmutation (20%), soft mutation (20%), and a random symmetric generator(30%).
The VASP[25-27]code is applied to perform density functional theory (DFT) calculations under the Perdew-Burke-Ernzerhof (PBE) functional of the generalized gradient approximation (GGA).[28,29]We used the projector augmented wave(PAW)[30]method with 1.1 Bohr core radius and 2s22p1electrons treated as valence electrons under high pressure.The plane-wave kinetic energy cutoff of 900 eV was used. Brillouin zone sampling was carried out using uniformΓ-centeredkmeshes with 2π×0.03 °A-1spacing for reciprocal space sampling. Structure relaxations and total energy calculations were carried out when the convergence criteria for energy were set to 1×10-5eV and 1×10-3eV, respectively. All of the structures were optimized under the pressure.
The phonon dispersion curves were produced using the supercell approach (3×3×3 supercell). Force constants of supercells were obtained using the VASP and PHONOPY codes.[31]Images of the structures were produced using VESTA software.[32]ture withFm3msymmetry, as depicted in Fig. 2(c), which is the boron allotrope with the highest symmetry so far. The lattice parameters ofFm3m-B (fcc-B) area=b=c=2.078 A°at 1.4 TPa. There are equivalent atoms occupying the crystallographic 4bWyckoff site in the unit cubic cell, which is the(0.5,-0.5, 0.5) position. The phase transition, from B10to fcc-B, occurs at 1.317 TPa, which is highlighted in the illustration in Fig.1. B10with the lowest enthalpy energy,is most stable under the pressure from 0.5 TPa to 1.317 TPa. Then,over 1.317 TPa,fcc-B has lower enthalpy than Band steadily
3. Results and discussion
By employing the evolutionary algorithm, we searched for new boron allotropes under 0.5-5 TPa pressure. With the given information of the boron crystal structure predicted,we were able to correctly reproduce the reported structure B10(stable above 383 GPa[17]) andα-Ga-B (stable at 100-300 GPa[33]),which proves our search method is feasible.Under ultra-high pressure,our results reveal a novel cubic struc-10occupies a wide pressure range. In addition, two hitherto unknown metastable phases exist:P63/mmc-B andI4/mmc-B.The lattice parameters and fractional atomic coordinates of these stable and metastable phases are listed in Table 1.
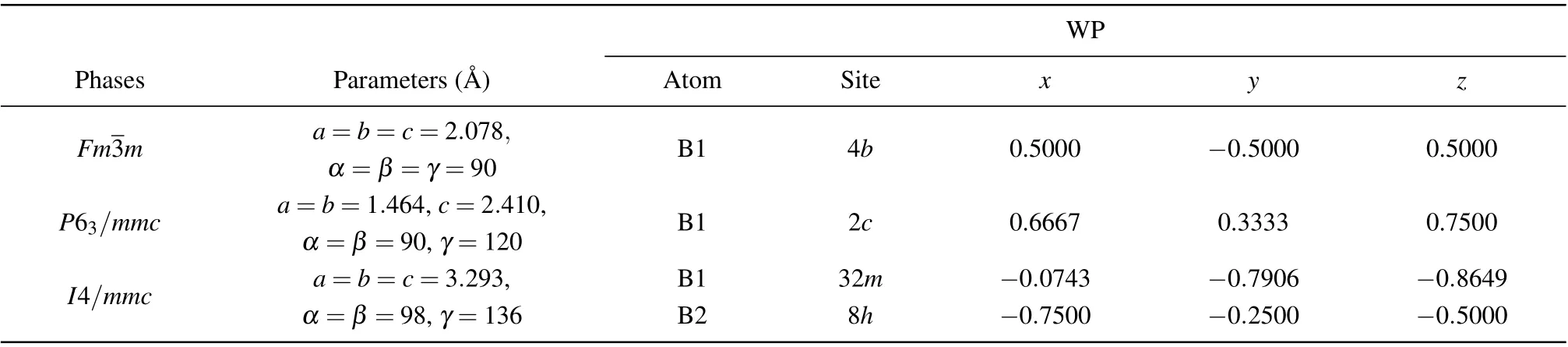
Table 1. Lattice parameters and fractional atomic coordinates of boron at 1.4 TPa.
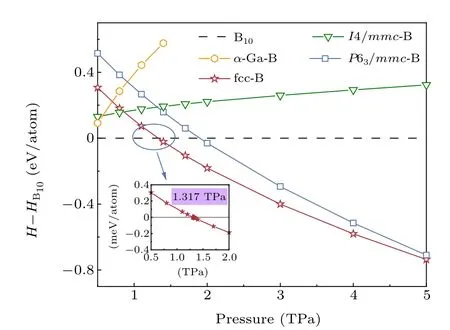
Fig. 1. Enthalpy curves of boron. Enthalpies per atom are shown relative to B10 (P63/mcm) phase. The phase transition (B10 to fcc-B) occurs under 1.317 TPa. Metastable phases(P63/mmc and I4/mmc)are also found.
Here,we compare the new structure with the well-knownα-B in Fig. 2(b), which contains the icosahedral unit (B12)consisting of 20 triangles(as shown in Fig.2(d)). The icosahedral unit B12,a common building block,is present in other complex allotropes.[34]The most representative structure isα-B, which is connected by icosahedrons. It is reported that it can be stable up to at least 200 GPa,and its gap-closure pressure ispc=50 GPa.[35]The cause of metallization is a significant contraction of the intericosahedral three-center bond,which brings a higher coordination number to the icosahedron and a transfer of charge from the intraicosahedoral bond to the three-center bond.[35]However,the three-center bonds are significantly shortened and disappear in fcc-B. In fcc-B, the boron coordination number increases to 12, which is consistent with the reported prediction,[35]forming a cuboctahedron with 8 triangles and 6 quadrilaterals.The length of the bonds is 1.47 °A at 1.4 TPa,which is shorter than that of the intraicosahedoral bonds(1.80 °A)inα-B under ambient pressure. Similar to theα-B,the unit cell of this structure can be viewed as a structure with a boron atom embedded in the icosahedron,as shown in Fig. 2(c), which is denser thanα-B. Due to the periodic arrangement,these cuboctahedrons are closely linked to form a densely packed array of a crystalline phase.
To investigate their charge density distribution, we have calculated the charge density differences of fcc-B andα-B(Fig. 2). Orange or yellow indicates electron gain, and blue indicates electron loss. As shown by the arrow in Fig. 2(c),valence electrons of the fcc-B structure gather into the lattice interstices under high pressure. These electrons can be viewed as atoms embedded in the lattice interstices,[36]which can increase the density of the crystalline phase. Theα-B has more obvious charge aggregation than fcc-B.As shown in Fig. 2(c), three equivalent B1 atoms form strong three-center bonds,which connect icosahedrons. As highlighted by the arrow,due to the redistribution of electrons around B1,electrons gather in the middle of the three-center bonds under ambient pressure.
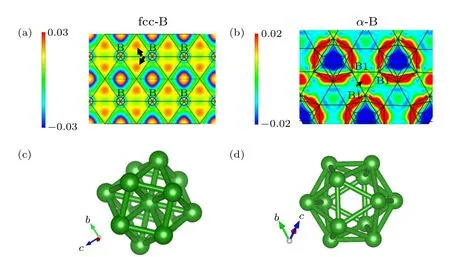
Fig. 2. Charge density differences of (a) fcc-B at 1.4 TPa and (b) α-B under ambient pressure are plotted within the(1-1 0)plane and(1 1 1)plane,respectively. (c)Crystal structure of fcc-B.(d)Schematic diagram of icosahedron(the basic unit of α-B).Orange or yellow indicates electron gain,and blue indicates electron loss. The arrow points to electrons interstice.
To investigate the dynamics stability of fcc-B,phonon vibration modes and frequencies are calculated under different pressure, as shown in Figs. 3(a)-3(c). As the pressure increases, B atoms vibrate violently around their equilibrium positions. The high-frequency vibration mode at 1.4 TPa is mainly 52.98 THz,while the middle frequency part is mainly about 30.97 THz(Fig.3(b)). There are no soft modes of fcc-B at 1.4 TPa and 5 TPa,which indicates it is dynamically stable.However,fcc-B appears in soft mode at 0 TPa(Fig.3(a)).This means the boron atoms cannot return to their original lattice position after moving, resulting in the lattice spreading and forming an unstable state. That is to say, fcc-B could not be quenched under ambient conditions. In contrast,α-B appears in soft modes at 1.4 TPa(Fig.3(d)),indicating thatα-B with the boron icosahedron cannot be stable at 1.4 TPa. High pressure causes the icosahedron to collapse and the structure to recombine. It is also confirmed by the gradual deformation and disappearance of icosahedrons inα-Ga-B (100-300 GPa[33])and B10(383-1317 GPa[17]).
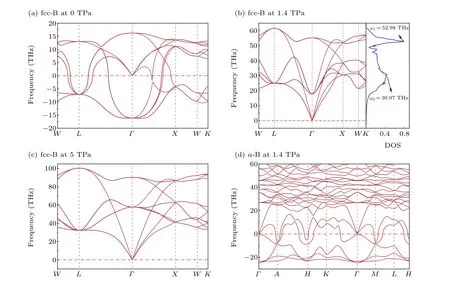
Fig.3. Phonon dispersion curves of(a)fcc-B at 0 TPa,(b)fcc-B at 1.4 TPa,(c)fcc-B at 5 TPa,(d)α-B at 1.4 TPa. Total phonon density of state for fcc-B at 1.4 TPa[(b)right]is performed.
Electronic band structures and the density of states(DOS)of fcc-B under 1.4 TPa are shown in Figs. 4(a) and 4(c).Under pressure, valence electrons gather in the lattice interstices due to repulsion by core electrons.[37]It is found that Na,[37]Ca2N,[36]and Li,[38]etc.[23]metal materials are transformed into insulators after applied pressure, and their electronic structures show the phenomenon of electrons entering the lattice interstices. However, fcc-B shows a similar phenomenon, i.e., electrons entering the lattice interstices, but it is metal. The energy bands of fcc-B cross the Fermi level(Fig. 4(a)), showing metallic properties. These energy bands near the Fermi surface are relatively flat between 0-10 eV,and their electron localization occurs in the region around-5.5 eV(Fig.4(c)). In addition,α-B is a semiconductor with an indirect bandgap (1.798 eV, Fig. 4(b)), which is consistent with previous reports.[9,39]For the B1 atom,the top of the valence band is contributed by a p orbital. The total density of states is concentrated between-13 eV and 0 eV below the Fermi level,which is contributed by s and p orbitals in B1(Fig.4(d)).
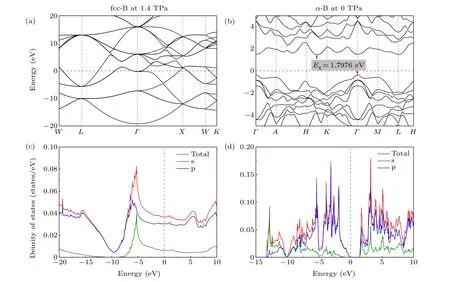
Fig.4. Band structure of(a)fcc-B at 1.4 TPa and(b)α-B at 0 TPa. Density of states of(c)fcc-B at 1.4 TPa and(d)α-B at 0 TPa. Fermi energy(EF)is set to zero. The indirect bandgap(Eg)of α-B is 1.798 eV.
4. Conclusion
In this paper, we propose a new boron structure (space groupFm3m, fcc-B)that is dynamically stable at 1.4 TPa by employing density functional theory. The structure can be regarded as a structure with a boron atom embedded in the icosahedron. Its valence electrons gather in lattice interstices due to repulsion by core electrons under ultra-high pressure.Interestingly,it is metal,unlike Li or Na,with the same electron interstice phenomenon but showing semiconductor character under pressure. The discovery of this new structure expands our comprehension of high-pressure condensed matter and contributes to the further development of high-pressure science.
Acknowledgments
Project supported by the Guangdong Natural Science Foundation of China (Grant Nos. 2017B030306003 and 2019B1515120078) and the National Natural Science Foundation of China(Grant No.11804057).
- Chinese Physics B的其它文章
- Surface modulation of halide perovskite films for efficient and stable solar cells
- Graphene-based heterojunction for enhanced photodetectors
- Lithium ion batteries cathode material: V2O5
- A review on 3d transition metal dilute magnetic REIn3 intermetallic compounds
- Charge transfer modification of inverted planar perovskite solar cells by NiOx/Sr:NiOx bilayer hole transport layer
- A low-cost invasive microwave ablation antenna with a directional heating pattern

