Corrosion Resistance of Ni-P Composite Coating on Mg Alloy:Critical Role of the Intermediate Phosphate Conversion Coating Layer for Suppressing Galvanic Couple Formation
WANG Yuhang,GUO Liangshuai,2,ZHOU Peng,ZHANG Tao,WANG Fuhui
(1.Shenyang National Laboratory for Materials Science,Northeastern University,Shenyang 110819,Liaoning,China;2.Tianjin Aerospace Long March Rocket Manufacturing Co.,Ltd.,Tianjin 300462,China)
Abstract:Electroless nickel(EN)plating can give rise to the severe galvanic corrosion of the magnesium(Mg)alloy matrix,owing to its nobler electrochemical potential than Mg alloy.To hinder the formation of galvanic couple,an intermediate phosphate conversion coating(PCC)layer is introduced between the EN layer and the Mg alloy matrix.Since the ceramic-like PCC layer cannot be catalyzed,a low-cost Ag-activation technique is used to process the PCC layer before electroless plating.The cross-section morphology and element distribution of the PCC-EN composite coating indicate that the PCC intermediate layer can effectively separate the Mg alloy from the EN layer.Moreover,the results of electrochemical tests suggest that the PCC-EN composite coating has a better corrosion resistance in comparison with the EN coating and AZ91D Mg alloy.
Key words:AZ91D magnesium(Mg)alloy;phosphate conversion coating;electroless plating;corrosion resistance;composite coating
0 Introduction
Magnesium(Mg)alloy is the optimum material for the weight reduction of equipment manufactur‑ing,owing to its low-density,high strength-toweight ratio,high dimensional stability,high damp‑ing capacity,high thermal conductivity,and high electricity conductivity.Attributed to these advan‑tages,Mg alloy becomes one of the most concerned structural and electronic materials in aerospace and telecommunication industries.
However,the high chemical activity of Mg al‑loy limits its widespread application,since the activi‑ty makes Mg alloy have poor corrosion resistance un‑der hash working conditions.To improve the cor‑rosion resistance of Mg alloy,numerous protection methods have been developed,e.g.,electroless plating,chemical conversion coating,plasma electro‑lytic oxidation,organic coating,and anodizing.
Though the above protection methods can im‑prove the corrosion resistance of Mg alloy to some ex‑tent,the surging functional requirements are still hard for the applications of Mg alloy in the aerospace and telecommunication industries.With the increase in the number of electronic devices,the electromagnetic ra‑diation in the frequency band of system operation has significantly increased,which might disturb the opera‑tion systems such as electronic control systems and wireless communication systems.The high-phos‑phorus electroless nickel(EN)plating coating is elec‑trically conductive and low magnetic,and can effec‑tively shield the electronic systems from electromag‑netic interference.Moreover,the EN layer with high hardness can provide wear resistance,which can be used to extend the service life of Mg alloy.
However,there are several urgent problems to be solved before plating nickel on Mg alloy.First,owing to the tremendous electrochemical potential difference between the Mg alloy and the EN layer,severe galvanic corrosion will take place on the Mg alloy matrix when some pores penetrate the EN lay‑er.Second,MgO is generated on the surface of Mg alloy,giving rise to a weak adhesion strength be‑tween the EN layer and the Mg alloy matrix.Third,the large amount of the second phase in Mg alloy will make the EN layer grow unevenly.Therefore,an intermediate phosphate conversion coating(PCC)layer is introduced to enhance the pro‑tective ability.To ensure the unrestricted growth of the EN layer on the ceramic-like PCC layer,the Agactivation technique is used to process the PCC layer before electroless plating,with a lower cost in com‑parison with the Pd-activation process.
In the present study,a nickel-phosphate(Ni-P)composite coating is developed to improve the corro‑sion resistance of Mg alloy.The PCC intermediate layer,hindering the formation of galvanic couple be‑tween the EN layer and the Mg alloy matrix,is pre‑pared prior to the electroless plating.In addition,the PCC intermediate layer is processed by the Ag-activa‑tion technique to facilitate the electroless plating.The chemical composition,microstructure,and corrosion resistance of the composite coating are discussed.
1 Experiment
1.1 Materials and sample preparation
The samples employed in this work are cut from commercial AZ91D Mg alloy,and an inductively coupled plasma-atomic emission spectroscopy(X Se‑ries Π,Thermofisher,US)is used to analyze the chemical composition of the alloy.The results are dis‑played in Tab.1.

Tab.1 Chemical composition of AZ91D Mg alloy(%)
The Mg alloy is cut into small pieces with a size of 20 mm × 20 mm × 5 mm.The samples used forcoating preparation are sequentially ground with sili‑con carbide papers to 1 000 grits,while the samples used for the microstructure characterization are se‑quentially ground with silicon carbide papers to 2 000 grits,and are polished with a 2.5 μm diamond paste.Finally,the preprocessed samples are cleaned by de‑ionized water and ultrasonically rinsed in ethanol.
1.2 Composite coating preparation
The flow chart for the preparation of the com‑posite coating is shown in Fig.1,and the involved electrolytes and detailed experimental conditions are listed in Tab.2.The first step is to prepare the PCC coating on the Mg alloy by immersing the sample in the phosphate conversion solution.The second step is Ag-activation,which can be divided into sensitiza‑tion and activation.The last step is EN plating by im‑mersing the Ag-activation sample in the alkaline elec‑troless plating solution.To investigate the effect of the PCC-intermediate layer on the corrosion resis‑tance of the coating,the EN plating layer is prepared on the bare Mg alloy.

Tab.2 Composition of the processing solution and the experimental condition of the coating preparation

Fig.1 Flow chart for the preparation of the PCC-EN composite coating
1.3 Characterization of coatings
The surface and cross-section morphology of thecoatings are characterized with a scanning electron microscope(SEM,XL-30 FEG,Philips,the Neth‑erlands),and the element distributions of the coat‑ings are measured with an energy dispersive spec‑trometry(EDS,Ultim Max 100,Oxford,UK)affil‑iated to the SEM.To guarantee the conductivity of the samples used for morphology observation,the samples are plated with a carbon layer before the SEM observation.The X-ray diffraction(XRD,PW1700,Philips,the Netherlands)is used to deter‑mine the phase compositions of the coating.During the test,a Cu target is used,with the Kradiation of=0.154 06 nm,the acceleration voltage of 40 kV,the current of 30 mA,and the 2range from 10° to 80°.The XRD results are analyzed with the Jade 6.0 software and ICDD pdf 2004 database.
1.4 Electrochemical tests
The potentiodynamic polarization and electro‑chemical impedance spectroscopy(EIS)tests are conducted in(NaCl)=3.5% solution at(30±1)℃via an electrochemical workstation(Zennium E,Zahner,Germany).During the tests,the conven‑tional three-electrode cell is composed by a counter electrode of platinum foil,a reference electrode of saturated calomel(SCE),and a working electrode of the coated sample.
After 20 min of open circuit conditioning,the samples are polarized from the open circuit potential to anodic and cathodic,respectively,with a sweep rate of 0.333 mV/s.The polarization curves are used to measure the corrosion current density()and corrosion potential()obtained by means of the Tafel extrapolation method.The EIS tests are conducted with a frequency range from 100 kHz to 10 mHz and a sinusoidal amplitude of 10 mV.The ZSimWin software(Version 3.5)is used to analyze the impedance spectra.
2 Results and discussion
2.1 Microstructure and chemical compositions of the PCC coating before and after Agactivation
The surface and cross-section morphology of the PCC coating after conversion processing is shown in Fig.2.The double-layered PCC coating can be di‑vided into an inner layer and an outer layer.The bot‑tom layer with net-shaped cracks is defined as the inner layer,and the spherical particles on it are con‑sidered as the outer layer(see Fig.2).After 2 min conversion processing,part of the inner layer is cov‑ered by sphere-like particles of an uneven size.However,network-like cracks can still be observed(see Figs.2(a)and 2(d)).The EDS results in Tab.2 indicate that the inner layer is composed of(Mg)=69.19%,(Al)=8.80%,and(O)=19.13%,and the outer layer is composed of(Mn)=17.52%,(P)=15.86%,(O)=66.62 %.When the pro‑cessing time is extended to 4 min(see Figs.2(b)and 2(e)),the number density and dimension of the sphere-like particles obviously increase,but a small portion of the inner layer is still exposed.After 10 min processing(see Figs.2(c)and 2(f)),the num‑ber density of the particles slightly increases,and the growth of particles is suppressed,owing to the im‑pingement of the adjacent particles.Furthermore,al‑most all spherical particles are transformed into lamellar structures with a size between 5 μm to 15 μm,and the net-shaped cracks cannot be observed.The EDS results of the coating after 10 min process‑ing reveal that the weight concentrations of the coat‑ing compositions are(Mn)=19.81%,(P)=15.35%,and(O)=64.84%(see Tab.3).There is no appreciable signal about Mg,indicating that the in‑ner layer is completely covered with the dense outer layer.The cross-section morphology of the PCC coat‑ing after 10 min conversion processing is shown in Fig.2(g).It can be seen that the thickness of the coat‑ing ranges from 10 μm to 12 μm.There are only a few cracks in the outer layer,and no through pores are ob‑served.The compactness of the PCC coating signifi‑cantly enhances along with the processing time.Therefore,the PCC coating after 10 min processing is used as an intermediate layer of composite coating.

Fig.2 Surface morphology of the PCC coating at the processing time of 2 min,4 min,and 10 min,and the cross-section morphology of the PCC coating at the processing time of 10 min
To further investigate the phase components of the PCC coating,the XRD is used.The XRD pat‑terns of the inner and outer layers are shown in Fig.3.In the spectra,Mg,MgAl,MnHPO,and MgH‑POpeaks can be detected.The Mg and MgAlpeaks indicate that X-rays can penetrate through the PCC coating to reach the Mg alloy matrix.In com‑bine with the EDS analysis(see Tab.3),the inner layer can be identified as MgHPOand the outer lay‑er can be identified as MnHPO.

Fig.3 XRD patterns of the PCC layer and PCC-EN composite coating
The surface morphology of the PCC coating af‑ter Ag-activation is shown in Fig.4.After the Ag-activation process,the lamellar structure parti‑cles on the outer layer are still intact.Meanwhile,sub-micrometer agglomerate particle masses are gen‑erated on the lamellar structure with a size ranging from 100 nm to 1 μm.The agglomerate particles are analyzed with SEM/EDS to gain more insight into the activation process,the locations A and B are signed on Fig.2(see Tab.3 and Fig.5).The PCC coating is composed of Mn,P,O,and Ag,and Ag is evenly distributed on the PCC coating.Hence,the PCC coating can retain the compact lamellar struc‑tures after Ag-activation,and a mass of Ag active site is generated on it.

Tab.3 Chemical composition of the PCC layer before and after Ag-activation

Fig.4 Surface morphology of the PCC coating after Agactivation

Fig.5 Element distributions of the PCC coating after Agactivation processing
2.2 Microstructure and chemical composition of the PCC-EN composite coating
The surface and cross-section morphology of the PCC-EN composite coating is characterized with the SEM to gain a further insight into the microstruc‑tures,and the SEM images are displayed in Fig.6.After the EN process,the nodular morphological compact structures with a homogeneous size distribu‑tion completely cover the PCC coating(see Fig.6(a)).From Fig.7,it can be seen that the surface of the EN layer is a relatively smooth patch,and no large bulges are detected.According to the cross-section SEM image in Fig.6(b),the PCC-EN composite coating has a double-layer structure,which is composed of the outer EN layer and the in‑ner PCC layer.The thickness of the EN layer and the PCC layer are approximately 8 μm and 10 μm,re‑spectively.Therefore,the PCC compact intermedi‑ate layer can effectively separate the Mg alloy matrix from the EN layer,inhibiting the galvanic corrosion.

Fig.6 Surface and cross-section morphology of the PCCEN composite coating

Fig.7 3D Topographical image of the PCC-EN composite coating
The element distributions of the PCC-EN com‑posite coating are displayed in Fig.8,where elements Ni and P are evenly distributed on the surface.The cross-section element distributions of the PCC-EN composite coating are displayed in Fig.9.It is obvi‑ously that element Ni is not detected in the PCC layer and Mg alloy matrix.The PCC intermediate layer can be a barrier to separate the Mg alloy matrix from the EN layer.The strong broad hump at 2=45° of the PCC-EN composite coating in the XRD spectra indicates that the EN layer is mainly composed of amorphous Ni.Moreover,the amorphous structure can reduce the risk of intergranular corrosion,which is in favor of enhancing the corrosion resistance of the PCC-EN composite coating.

Fig.8 Element distributions of the PCC-EN composite coating

Fig.9 Element distributions of the cress-section of the PCC-EN composite coating
2.3 Corrosion resistance
The potentiodynamic polarization test is used to evaluate the effect of the PCC-intermediate layer on the corrosion resistance of electroless plating sam‑ples.The polarization curves are displayed in Fig.10.To avoid the negative difference effect on the electrochemical parameters,the corrosion current density()and corrosion potential()are de‑termined from the cathodic branch by the Tafel ex‑trapolation method.As shown in Tab.4,the cor‑rosion potentials of the PCC-EN and EN coatings are obviously higher than that of AZ91D Mg alloy.However,the corrosion current density of the EN coating is significantly enlarged,which might be at‑tributed to the galvanic couple between the EN coat‑ing and the Mg alloy matrix.The extremely small corrosion current density of the PCC-EN composite coating indicates that the PCC-intermediate layer can greatly improve the corrosion resistance of the elec‑troless plating coating.

Tab.4 Fitted electrochemical parameters of the polarization curves in Fig.10

Fig.10 Potentiodynamic polarization curves of the bare Mg alloy,EN coating,and PCC-EN composite coating in w(NaCl)=3.5% solution
The EIS results of the PCC-EN and EN coat‑ings are displayed in Fig.11.The Nyquist spectra of the PCC-EN composite coating exhibit two capaci‑tive loops(see Fig.11(a)).The low frequency(LF)loop represents the dielectrical diffusion behav‑ior of the electrolyte within the composite coating,while the high frequency(HF)loop represents the di‑electrical behavior of the double electrical layer be‑tween the Mg alloy and the electrolyte.
However,the inductance loop is observed in the Nyquist spectra of the EN coating,indicating the breakdown of the EN coating.Besides,the nota‑bly higher impedance module of the PCC-EN com‑posite coating can also prove that the corrosion resis‑tance of the PCC-EN composite coating is better than the EN coating(see Fig.11(b)).

Fig.11 EIS spectra of the PCC-EN and EN coatings in 3.5% NaCl solution
To acquire the accurate fitting results,the elec‑trical equivalent circuit(EEC)is introduced to ap‑proach the EIS plots.The spectra of the EN coating are fitted by the EEC displayed in Fig.12(a),whereis the electrolyte resistance,andare the charge transfer resistance and the constant phase ele‑ment used to simulate the non-ideal behavior of the double electrical layer between the Mg alloy and the electrolyte,andare the resistance and the constant phase element of the EN coating,Ris the resistance of inductanceandis the inductance,which are used to simulate the corrosion product ad‑sorption during the localized corrosion.The spectra of the PCC-EN composite coating are fitted by the EEC displayed in Fig.12(b),whereandare the resistance and the constant phase element of the PCC intermediate layer,andare the resistance and the constant phase element of the EN layer.

Fig.12 Electrical equivalent circuits used to fit the EIS data
The fitted values of these components are listed in Tabs.5 and 6.In addition,the intercept on the-axis of the Nyquist plots at indefinitely low fre‑quency is defined as the polarization resistance().The polarization resistances of the PCC-EN()and EN()coatings are calculated by

It can be obtained thatis 1 400.30 Ω·cm,andis 3 137.81 Ω·cm.The results are accor‑dant with the fitted results of the potentiodynamic po‑larization tests displayed in Tabs.5 and 6.The results of all experiments show that the corrosion resistance of the PCC-EN composite coating is much more su‑perior to the corrosion resistance of the EN coating.
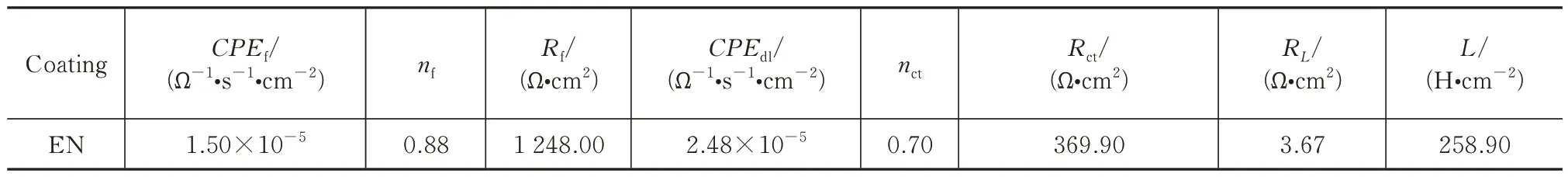
Tab.5 Fitted results of the EN coating impedance spectra

Tab.6 Fitted results of the PCC-EN coating impedance spectra
3 Conclusions
In this work,the effect of the phosphate conver‑sion coating intermediate layer on the corrosion resis‑tance of the Ni-P composite coating is investigated.The conclusions are summarized as follows.
1)After Ag-activation,the compact lamellar structure of the PCC layer is intact,and sub-mi‑crometer Ag particle masses are generated on the PCC layer.
2)The introduced intermediate PCC layer can be a barrier to separate the Mg alloy matrix from the EN layer,which effectively improves the corrosion resistance of the PCC-EN composite coating.
- 上海航天的其它文章
- Development and Application of Ti-based Alloy Casting Technologies in the Field of Aerospace
- Applications of Magnesium Alloys in Aerospace and Aviation
- Repair Welding of Casting Magnesium Alloys:A Review
- Research on Residual Stress Measurement of Magnesium Alloy Cabin Castings
- Microstructure and Hot Deformation Behavior of Mg-9Al-3Si-0.375Sr-0.78Y Alloy
- Effect of Semi-solid Isothermal Heat Treatment on Microstructure of VW63Z Alloy

