农林生物质制备铁炭复合材料及其环境污染治理应用的研究进展
邱 凌,周勤勤,朱铭强,郭晓慧,范琼波
农林生物质制备铁炭复合材料及其环境污染治理应用的研究进展
邱 凌,周勤勤,朱铭强※,郭晓慧,范琼波
(1. 西北农林科技大学机械与电子工程学院,杨凌 712100;2. 农业农村部农村可再生能源开发利用西部科学观测实验站,杨凌 712100)
以农林生物质为原材料,通过加入铁磁性助剂制备铁炭复合材料是农林生物质高值化利用和受污染水体及土壤治理的重要途径。铁炭复合材料具有高比表面积、丰富表面官能团和优异吸附性能,能够通过表面物理吸附和氧化还原作用,快速吸附污水中的重金属离子,并在外部磁场的吸引下实现快速分离回收和循环使用。该研究论述了不同制备方法的铁炭复合材料及其材料性能,对其去除有机染料污染物、治理污水和土壤重金属的研究现状及发展动态进行了分析和讨论。在此基础上,结合铁炭复合材料的结构发育机理和污水治理及土壤改良的产业发展现状,提出了兼顾低成本、易合成、高效益的复合材料制备方式建议,以期为其在环境污染治理中的广泛应用提供理论和实践参考,进而推动农林生物质资源化高值利用,助力生态环境的绿色低碳高质量发展。
生物质;重金属;污染;铁炭复合材料
0 引 言
环境污染主要与城市化和工业化进程中排放的有机污染物及重金属有关[1],特别是染料、药品、农药等有机分子和重金属离子等化学物质具有极强的稳定性[2],此类物质积累对环境及水生生物具有很大的危害性[3],目前30%的工业污水未经处理最终进入湖泊和河流,严重危及人类生存环境和安全用水[4]。近年来,利用农林生物质制备铁炭复合材料成为研究的热点,其在环境污染治理中具有广阔的应用前景[5],目前研究主要聚焦于通过研究和调控高比表面积活性炭(Activated Carbon,AC)材料的制备条件及其方法,探索其微孔和中孔形成机理及其吸附特性等[6]。利用农林生物质制备的活性炭通常具有较高的比表面积、发达的孔隙率和丰富的表面官能团,可作为一种高性能吸附剂[7]。然而,粉末状活性炭在处理过的污水介质中的后续分离过程,通常需要通过离心或过滤来实现,制约了其规模化应用[8]。因此,通过将铁磁性助剂及其氧化物介导进活性炭基质中制备铁炭复合材料[9],利用其铁磁性的特点,能够快速将铁炭复合材料从污水介质中回收,从而可以有效解决制约活性炭材料在环境污染治理中循环利用的技术瓶颈,推进农林生物质资源高值化利用。
1 制备铁炭复合材料的原料和助剂特性
1.1 制备铁炭复合材料的农林生物质特性
生物质通常指农林废弃物,主要包括畜禽粪便、农作物秸秆、城乡有机垃圾、农业加工残余物、林业及林产加工剩余物等。农林生物质作为一种有机含碳物质,通常由纤维素、半纤维素、木质素、灰分、粗蛋白及少量的无机矿物质组成(表1)。其中,纤维素、半纤维素、木质素是植物细胞壁的主要组分,约占木质纤维素总质量的90%[10]。农林生物质各组分之间相互穿插交织,构成复杂的高聚合物体系(图1),是自然界中产量最大的可持续碳资源。
据统计,中国每年产生1 500亿m3农林生物质,其中,畜禽粪便占50%,秸秆占40%,有机垃圾占6%,农业品加工残余物占4%;每年森林采伐量约2.5亿m3,可产生采伐、造材等林业剩余物1.1亿t[11]。以农林生物质为原料,采用先进的转化技术制备的铁炭复合材料,具有含碳量高、稳定性强、孔隙丰富、比表面积大(10~103m2/g)、吸附能力强、耐降解性高、表面带有含氧官能团和负电荷等优点[12],可广泛应用于治理污水和土壤重金属环境污染。

表1 农林生物质成分分析
1.2 制备铁炭复合材料的磁性助剂特性
在制备铁炭复合材料的磁性助剂中,由于Fe元素获取方便、价格低廉、安全无毒,故应用最为广泛。常用的铁磁性助剂包括FeCl3·6H2O、FeSO4·7H2O、Fe(NO3)3·9H2O和FeCl2·4H2O等金属盐。Rodriguez-Sanches等[13]通过将板栗壳在FeCl3中浸渍处理后制备得到铁炭复合材料,用于工业废水处理,结果表明,其对废水中Hg0的去除量为63.62g/g,可有效去除工业过程中Hg0的排放。
活性炭是制备铁炭复合材料的优质多孔吸附载体,其颗粒大小对吸附效果具有重要影响[14]。虽然大颗粒有利于液固分离,但低比重和大颗粒的包裹限制了活性炭的吸附动力学和吸附容量;小颗粒不仅可以增加吸附剂的容量,并能实现快速平衡[15]。传统的分离方法难以实现小颗粒或粉末状活性炭在废水污染治理中的固液分离,限制了其在循环吸附中的大量应用。因此,通过将不同的磁性助剂与活性炭耦合制备铁炭复合材料的工艺和技术,成为农林生物质资源化利用和废水污染治理的研究热点(表2)。
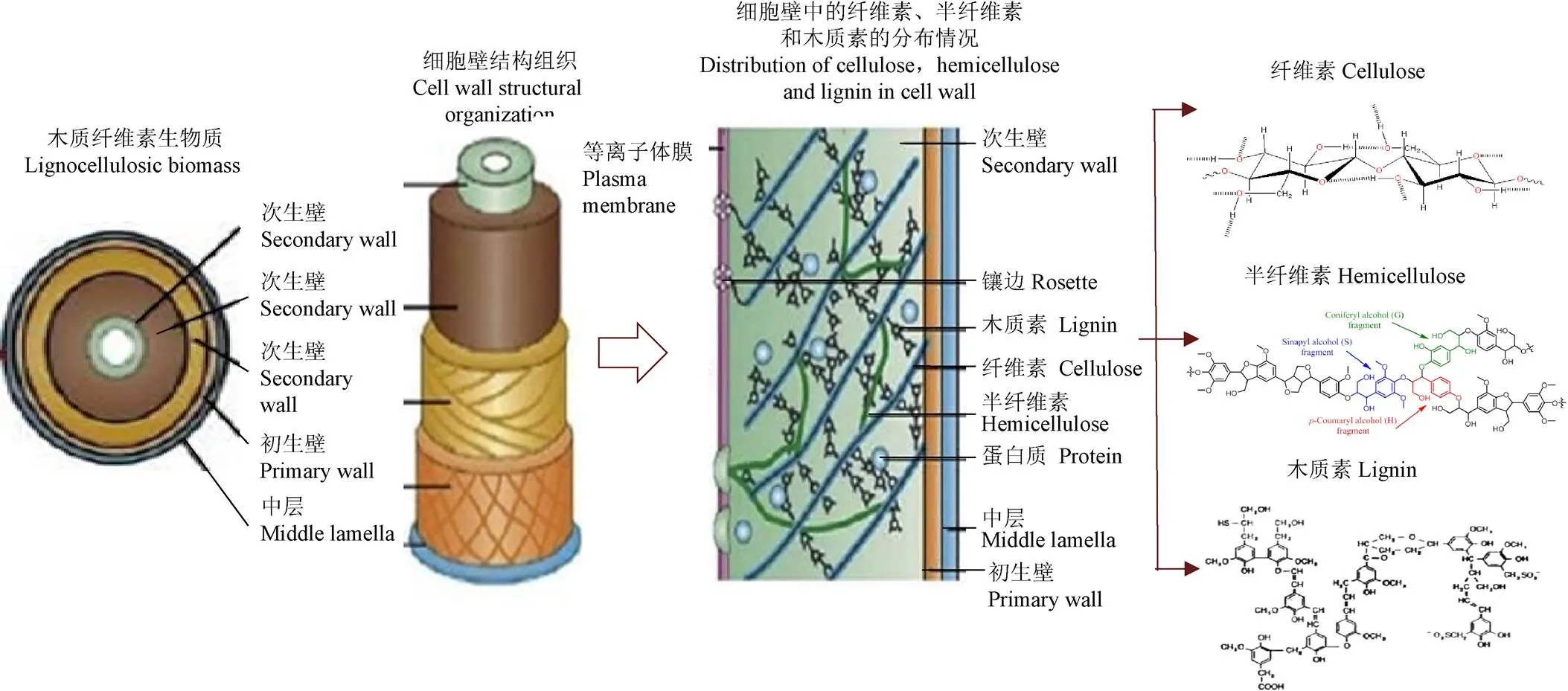
图1 植物细胞壁结构示意图

表2 不同原料和磁性助剂制备铁炭复合材料的反应条件
Costa等[21]用3.0 g Fe(NO3)3·9H2O对20 g大豆皮进行浸渍处理后,采用一步热解法在管式炉中以10 ℃/min的升温速率,在N2氛围下升温至550 ℃热解2 h制备出铁炭复合材料(CM1.3–800),用于废水中酚类化合物的吸附特性研究,结果表明,铁炭复合材料(CM1.3–800)的饱和磁化强度为107 A/m,对咖啡酸的吸附量可达418 mg/g,可有效去除咖啡加工废水中的有机物。在磁分离过程中,具有一定的饱和磁化率,使其在外加磁场的作用下,能够快速分离。
Qu等[26]用FeCl3⋅6H2O对水葫芦进行了浸渍处理,通过水热反应,制备得到铁炭复合材料(MPBCMW3),其比表面积高达2 097.51 m2/g,对六价铬(Cr(VI))和四环素(TC)最大吸附量分别为202.61和202.62 mg/g;经过3次循环吸附后,MPBCMW3对Cr(VI)和TC的脱附率超过83.42%和90.91%,吸附量只下降了11.75%和22.03%,表明MPBCMW3具有良好的可重复使用性。由此可知,农林生物质加入磁性助剂制备为铁炭复合材料后,利用磁分离技术,能够快捷、高效、低成本的分离回收复合材料,进而实现其循环使用。
2 农林生物质制备铁炭复合材料研究进展
2.1 水热合成法制备铁炭复合材料研究进展
将农林生物质和水在180~350 ℃、1 MPa~1 GPa的高压釜中,通过热化学反应即可合成化学物质[27]。水热反应形成的微环境可加速生物质与溶液之间的理化作用,促进离子与酸/碱的反应,降解木质纤维素细胞壁中的碳水化合物,保留原有碳骨架,进而形成多孔结构的复合碳骨架材料[28]。Prasannamedha等[24]以甘蔗渣为原料,采用两步水热炭化法,在Fe(NO3)3·9H2O的作用下制备铁炭复合材料。检测表明,所制备的铁炭复合材料存在Fe/Fe3C/γ-Fe2O3,磁化效果为32.84 A/m,对磺胺甲恶唑的最大吸附量为169.49 mg/g。Wu等[29]用废纸箱的木质纤维素与FeCl3·6H2O进行两步水热处理,在高压反应釜中温度200 ℃反应10 h制备铁炭复合材料。吸附试验发现,在pH值为2的酸性条件下,对蓝色染料(DB 56)和黄色染料(RY 3)的去除率分别达到81.53%和96.77%,且经过5次循环吸附后,去除率仍达到70%以上。Cai等[30]以花生壳为原料,在FeCl3·6H2O和六亚甲基二胺的作用下,采用一步水热法,成功制备了对Cr(VI)具有良好吸附性能的氨基功能化铁炭复合材料,其吸附量为142.86 mg/g,可循环吸附3次。王森等[31]对水热过程中原料种类、反应温度、停留时间、催化剂和溶液的循环利用时间等因素进行了研究,表明水热反应条件对水热炭化过程和最终炭化产物的结构及性质产生较大影响,水介质氛围有助于炭化材料表面含氧官能团的形成,因此,水热炭化产物含有丰富的表面官能团。
由此可见,通过两步和一步水热炭化法可以将金属盐通过浸渍传导到农林生物质的表面或内部,通过炭化制备出表面官能团丰富、多孔结构、能够低成本分离回收的铁炭复合材料,有效解决环境污染治理技术瓶颈,助力生态环境高质量发展。
2.2 溶剂热法制备铁炭复合材料研究进展
将水热法中的水溶液换成有机溶剂或非水溶媒(例如:有机胺、醇、氨、四氯化碳或苯等),通过金属盐的水解作用,生成金属氧化物晶粒负载到生物炭上。该法能较好的控制磁性晶粒在活性炭表面的生长,从而提供更多的吸附位点,使铁炭复合材料具有更好的吸附性能[32]。Fei等[33]利用氧化石墨烯(GO)和Fe3+做原料,采用一锅溶剂热法制备铁炭复合材料,并测定其对亚甲基蓝(Methylene Blue,MB)的吸附性能,研究表明,用铁炭复合材料吸附30 min后,其对MB的吸附量可达9.73 mg/g,且经过5次循环重复使用后,对MB的吸附量仍大于5.79 mg/g。由此可见,采用该法制备的铁炭复合材料,具有优良的可重复使用和较强的吸附去除MB的性能。
溶剂热法除了采用生物炭做载体外,还可将磁性助剂介导入农林生物质,进行耦合反应,以达到提质增效的目的。Wang等[34]将磁性助剂MnFe2O4直接加入椰壳中,通过一步溶剂热法,成功制备了铁炭复合材料(MnFe2O4@AC),在25 ℃条件下,0.2 g吸附剂的吸附量约为226 mg/g,表明铁炭复合材料(MnFe2O4@AC)具有吸附量大、磁性强的优点,对乙草胺等有机污染物的吸附、分离、降解等方面具有良好的应用前景。Ren等[35]以Fe(NO3)3和环糊精为原料,尿素为碱源,通过一步溶剂热法,合成了比表面积为112.91 m2/g的磁性纳米吸附剂(Fe3O4@C)。研究表明,磁性纳米吸附剂(Fe3O4@C)对六价铬(Cr(VI))和刚果红(CR)的最大吸附量分别为33.35和262.72 mg/g,具备较强的去除能力。
由此可见,与其他制备方法相比,溶剂热合成工艺和技术的显著特点在于能够有效抑制产物的氧化[36],防止空气中氧的污染,对制备高品质铁炭复合材料表现出非常显著的优势。
2.3 浸渍热解法制备铁炭复合材料研究进展
将农林生物质在含过渡金属盐的溶液中浸渍处理后,对热稳定性不同、能形成相分离结构的聚合物进行炭化,热稳定性高的聚合物(炭前驱体聚合物)经过高温炭化形成炭基体,热稳定性低的聚合物(造孔剂)经热解挥发后,在炭基体中留下大量的孔隙,进而可得到高品位的铁炭复合材料[37]。Zhu等[38]以黑液木质素和污泥为原料,以KOH为活化剂,采用一步热解制备出磁性活性炭(Magnetic activated carbon,MAC)。经过5次吸附/解吸后,MAC的回收率均大于84.0%,表明MAC具有良好的再生能力。Ahmed等[39]以微孔三嗪类聚合物(COP)为原料,浸渍Zn(OH)2后,在N2氛围中,以1 000 ℃热解制得新型炭材料。负载Zn(OH)2后,由于中孔体积的增加,使碳的比表面积和总孔体积增大,其对磺胺甲恶唑和磺胺氯吡啶的最大吸附量分别达到514和430 mg/g,在经过4次吸附/解析后,吸附容量仍保持95%。上述结果分析表明,用聚合物浸渍热解法制备的磁性吸附剂,具有优异的吸附净化能力,能够有效去除污水中残留的污染物。
2.4 化学共沉淀法制备铁炭复合材料研究进展
在沉淀剂及配位剂作用下,含有2种或多种阳离子的金属盐溶液生成难溶性的盐或氧化物,均一地沉积到活性炭上,再经过烘干或者煅烧处理进而制备出铁炭复合材料[40]。Feng 等[41]将金属盐FeCl3·6H2O和FeSO4·7H2O加入到木基活性炭中,采用化学共沉淀法制备铁炭复合材料用于处理造纸污水的吸附,其最大饱和磁化强度可达29.68 A/m,在pH值为2时,对化学需氧量(Chemical Oxygen Demand,COD)的去除可达65 mg/g。
共沉淀法与吸附法的区别在于共沉淀法直接将活性炭与金属盐溶液混合,将金属离子和金属氧化物先吸附在表面或者进入孔道内部,再原位沉淀实现负载的目的。Gao等[42]以黑木耳废渣为原料,采用化学共沉淀法和浸渍-热解法制备了2种类型的铁炭复合材料(MBC-1和MBC-2)用于污水中四环素(TC)的去除,结果表明,化学共沉淀法制备的铁炭复合材料MBC-1对污水中TC的去除量达到42.31 mg/g,比浸渍-热解法制备的铁炭复合材料MBC-2对TC的去除量24.31 mg/g提高了1.74倍,表现出了更高的吸附能力。由此可知,以化学共沉淀法得到的铁炭复合材料,磁性相对固定且稳定,过程相对简单。
2.5 电弧放电法制备铁炭复合材料研究进展
用较粗的石墨棒为阴极,较细石墨棒为阳极,在真空反应室中充入氦气、氩气等惰性气体,在电弧放电过程中,碳原子和填充在阳极石墨棒内的金属催化剂蒸发,碳原子在多重因素的作用下重组形成碳纳米材料,同时在石墨阴极上沉积出产物[43]。Hu等[44]采用一步法直流电弧放电,在不同的气体压力下,将不同摩尔比的NH3加入到He/CH4气体混合物中,合成了具有高浓度氨基功能化的石墨包覆磁性纳米颗粒。
Jagannatham等[45]采用旋转阴极电弧放电法在自然环境下合成了碳纳米管,在不同的沉积时间对CNTs进行化学镀镍,发现化学沉积镍的量随沉积时间的增加而增加,且随着沉积时间的延长,金属离子还原增强,当沉积时间为60 min时,可获得均匀的Ni涂层。Hu等[46]通过调整电弧等离子体的合成参数,对封装石墨壳的表面形貌和质量进行了系统研究,发现当CH4的浓度从0增加到50%时,涂层石墨壳的最外层趋于光滑和清洁,通过距圆心1~10 cm的收集距离,可获得石墨化程度较高的表面形貌;当工作气体压力从13 332 Pa降低到3 333 Pa时,最外层的碳纳米环在径向尺度上呈膨胀演化,甚至出现连续的非晶态覆盖层。
由此可见,电弧放电法的优势在于能够通过调整电极电势与电流密度来精确控制纳米颗粒的合成,为表面形貌和性能可调的铁炭复合材料的制备提供新思路和途径。
2.6 微波法制备铁炭复合材料研究进展
微波加热电磁波与制备材料的分子以更均匀的方式相互作用,有助于在材料内部均匀和更快地传递能量,从而实现节约能源,提高效益的目的。Salem等[47]采用微波辅助修饰氧化铁纳米颗粒的方法,将杏仁壳和核桃壳粉末复合浸渍,研究了ZnCl2、FeCl3和FeCl2混合物对阳离子染料吸附效率的影响,发现在中性条件下,FeCl3浸渍所得的铁炭复合材料对阳离子染料的最大吸附量为130 mg/g,且在动态吸附体系中可提供约1 000 m2/g的比表面积。Yang 等[48]以稻秆为原料,利用微波和蒸汽活化技术制备了一种磁性钴铁多孔炭,用于去除燃煤烟气中的单质汞。结果表明,钴氧化物和铁氧化物是除汞的主要活性成分,制备的钴-铁炭复合材料中的Co3+/ Co2+和Fe3+/ Fe2+参与了Hg0的捕获过程,对Hg0的最大吸附量为60.14g/g,同时钴-铁炭复合材料多孔碳具有良好的再生性能,经过6次重复吸附之后,其对Hg0的去除率仍可达到60.12%。
3 铁炭复合材料治理环境污染研究进展
农林生物质通过水热合成法等不同的技术制备出的铁炭复合材料,不仅具有丰富的孔隙结构和含氧官能团,同时是一种具有高密度吸附位点的吸附剂,展现出优良的环境污染治理能力,在环境污染治理中具有非常广阔的应用前景,图2展示了用不同农林生物质和磁性助剂制备的铁炭复合材料在去除有机染料污染、治理污水和土壤重金属中的综合应用示意。

图2 铁炭复合材料在环境污染治理中的综合应用
3.1 铁炭复合材料治理染料污水污染研究进展
中国每年产生染料污水约40亿t,位列全国工业排放污水第五[49]。由于染料污水色度深、有机污染物含量高、难生物降解,尤其是印染废水存在着对人类健康和海洋生物具有致癌和诱变作用的芳香环[50],已成为污水治理的研究重点,目前大量的研究聚集于通过吸附[51]、絮凝[52]、光催化降解[53]和膜过滤[54]等技术,去除染料污水中的有害因子的探索。
膜过滤技术能耗低,处理过程容易控制,可对印染污水进行深度处理,但存在着潜在的膜污染风险,增加了后续处理的难度[55]。光催化降解技术在经济上可行,但会导致不完全降解[56]。活性炭吸附技术工艺简单,成本低且环保,满足环境绿色可持续发展,是目前治理印染废水污染的通用技术。
Foroutan等[57]以海藻为原料,与Fe3O4纳米颗粒进行复合,制备了一种可循环利用的高效铁炭复合材料,对印染废水中的亚甲基蓝(MB)和甲基紫(MV)进行吸附特性及机理研究。结果表明,其饱和磁化强度为26.57 A/m,比表面积(BET)为126.77 m2/g,对亚甲基蓝(MB)和甲基紫(MV)的最大吸附量分别达到60.60和59.88 mg/g。经过7次重复使用后,该铁炭复合材料对印染废水中亚甲基蓝(MB)和甲基紫(MV)染料的多次回收处理效果良好,且不显著降低其对印染物的去除率,由此可见,铁炭复合材料在印染废水净化方面具有广阔的应用前景。
由于铁炭复合材料具有较高的比表面积和发达的孔隙结构及丰富的表面化学官能团,对印染废水中的亚甲基蓝(MB)、甲基紫(MV)、刚果红(CR)、甲基橙(MO)、孔雀石绿(MG)和结晶紫(CV)等污染成分具有良好的去除效果(表3)。通常情况下,铁炭复合材料对染料污染成分的去除是通过物理吸附与化学吸附的协调作用完成的,具有极强的选择性,其脱色能力依次为碱性染料、直接染料、酸性染料和硫化染料。这主要是铁炭复合材料的具有丰富的介孔和微孔,亲水性强,能快速实现吸附脱色过程[58]。因此,制备高品质的铁炭复合材料对治理印染废水污染具有重要意义。
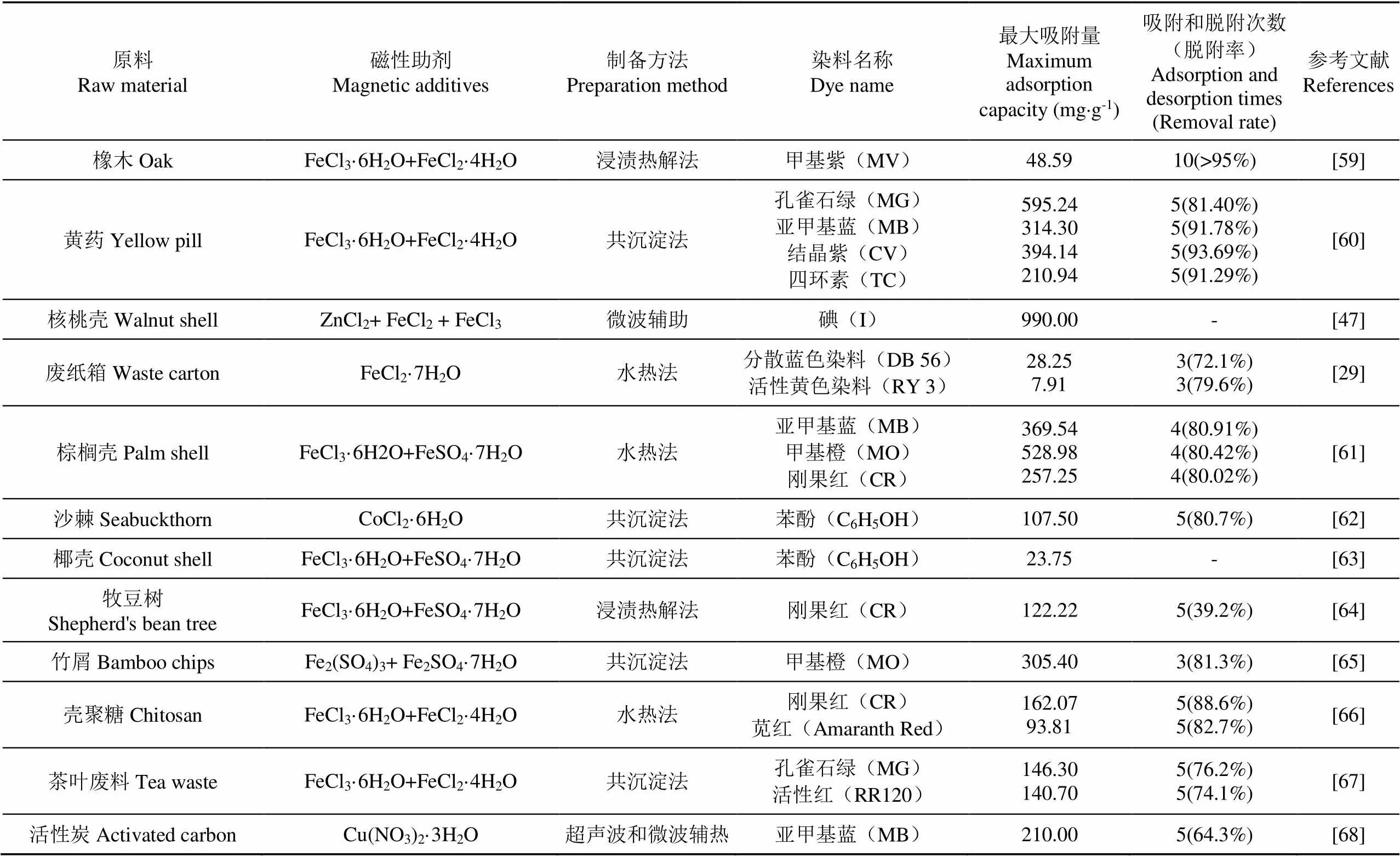
表3 铁炭复合材料对印染污水污染成分的去除效果
3.2 铁炭复合材料去除污水重金属研究进展
铬、汞、镉、铅、砷、铜、镍等重金属离子进入人体后,与体内高分子物质(如蛋白质等)发生反应从而使其失去活性,在人体器官中逐渐积累,造成慢性中毒,严重威胁人类健康。重金属吸附机理涉及多种机理的结合,因前驱体材料和重金属的不同类型而异。铁炭复合材料对重金属的物理吸附是由活性炭表面分子与重金属离子之间发生的范德华力引起的[69],其中重金属在较高的价态下携带更多的电荷,从而产生更强的静电相互作用和吸附能力,通过氧化还原反应,改变元素的存在形态,特别是价态变化影响元素化学行为、生物有效性和重金属的迁移能力[70]。
Fang等[71]通过高强度研磨对椰壳进行预处理后,制备出活性炭(HAC),在酸碱溶液洗涤的含铬HAC上建立了2种铬离子去除机理,包括六价铬(Cr(VI))还原为三价铬(Cr(III)),铬酸根(CrO42-)与羧基(COOH)表面官能团氢键结合,随后在HAC表面和孔隙内析出氢氧化铬(Cr(OH)3),证实了Cr(VI)在HAC表面的吸附是多层吸附和化学吸附的综合结果。
铁炭复合材料因其比表面高、孔径结构丰富,分离方便、成本低等优点,被广泛应用于去除污水重金属的研究(表4)。由表4可见,不同原料及方法制备的铁炭复合材料对水污染中铅、汞、镉、铬、镍、锌、铜等重金属均表现出较好的去除效果,表明铁炭复合材料在去除污水中重金属方面具有优良的应用价值。
Zhang等[72]研究了在不同热解温度下,水热法制备的油菜秸秆铁炭复合材料对污水中Pb(II)和Cd(II)的去除特性和机理,结果表明,油菜秸秆铁炭复合材料对Pb(II)和Cd(II)的最大吸附量分别为253.2和73.3 mg/g,通过热力学分析,表明其吸附Pb(II)和Cd(II)是自发的吸热反应,且主要取决于材料表面不均匀的活性位点。

表4 铁炭复合材料去除污水中重金属的效果
3.3 铁炭复合材料治理土壤重金属污染研究进展
铁炭复合材料因其具有致密的微孔结构和巨大的比表面积,对铅、汞、铬、镉等重金属表现出较强的吸附能力,同时因其制备生产成本低、生态安全、无污染、可大面积推广等显著特点,成为一种高效、廉价的土壤重金属修复剂。李鸿博等[80]研究发现,通过铁炭复合材料的物理吸附、静电吸附、离子交换、络合、沉淀和氧化还原等作用,可以直接吸附重金属离子。闫翠侠等[81]用鸡粪为原料制备铁炭复合材料,对土壤中Cd、Pb进行了修复,结果表明,添加鸡粪铁炭复合材料,能显著提高土壤pH值(<0.05),对土壤 Pb、Cd最大吸附量分别达到52.02(BC600)和242.59 mg/g(BC800),表现出较强的钝化作用和效果。
铁炭复合材料作为土壤重金属钝化剂的有效性取决于原料类型、热解条件、改性材料性质等物理化学特性,以及土壤环境条件、化学形态和金属离子浓度。铁炭复合材料用于修复土壤时,通过其氧化物、氢氧化物和碳酸盐和土壤元素的反应,改变土壤的理化特性,固定土壤金属离子,提高土壤质量和生产力。除此之外,铁炭复合材料在土壤系统中具有多种作用,如调节土壤pH值、氧化还原电位及释放还原离子等,进而固定并降低土壤重金属的生物利用度和危害,提高酸性土壤的pH值和养分,改变土壤微生物群落结构,改善土壤性质,提高土壤持水保肥能力,促进农作物持续健康生长(表5)。Xu等[82]以水稻秸秆为原材料制备铁炭复合材料,用于修复土壤重金属和提高土壤微生物丰富度和多样性研究,结果表明,水稻秸秆铁炭复合材料有效固定了土壤重金属,降低了土壤铁的生物有效性,提高了土壤锰的生物有效性,从而潜在地影响了土壤微量养分的肥力和土地生产能力。

表5 铁炭复合材料对土壤重金属的修复效果
4 结论与展望
利用农林生物质与磁性助剂反应制备的铁炭复合材料,具有孔隙结构发达、比表面积高、表面官能团丰富的优点。通过分析可知,水热合成法有助于炭化材料表面含氧官能团的形成;溶剂热法能够有效地抑制产物的氧化,有利于高品位铁炭复合材料的制备;浸渍热解法可在炭活化产物中生成大量的孔隙结构;化学共沉淀法可将金属离子和金属氧化物附着在炭材料表面或者孔道内部;电弧放电法可通过调整电极电势与电流密度精确控制纳米颗粒的合成;微波法可实现内部加热,缩短铁炭复合材料合成时间。铁炭复合材料致密的微孔结构和发达的比表面积,通过范德华力的物理吸附和氧化还原反应,对铅、汞、镉等重金属离子具有较强的吸附和固定能力,也可有效去除污水中的染料污染物。因此,铁炭复合材料在去除有机染料污染、治理污水和土壤重金属吸附等领域的应用,可助力生态环境绿色低碳高质量发展。
铁炭复合材料不仅可以有效吸附各类污染物和重金属,而且能够通过快速固液分离,达到重复利用的目的。虽然目前的研究丰富了铁炭复合材料的制备技术和应用,但仍需进一步研究和探索以下科学和技术问题:
1)不同的制备工艺和参数对铁炭复合材料微观结构和性能的影响特性;
2)铁炭复合材料的孔径结构形成机理与吸附性能之间的耦合关系;
3)污染物释放和回收难题导致的二次污染的长效检测和治理机制;
4)铁炭复合材料用于环境污染治理的技术经济综合评价。
[1] Li K, Wang J, Zhang Y. Heavy metal pollution risk of cultivated land from industrial production in China: Spatial pattern and its enlightenment[J]. Science of the Total Environment, 2022, 828: 154382.
[2] 田雨,刘晓刚,赵玉,等. 稻壳炭制备工艺参数对吸附性能的影响[J]. 农业工程学报,2020,36(24):211-217.
Tian Yu, Liu Xiaogang, Zhao Yu, et al. Effects of preparation process parameters of rice husk carbon on adsorption performance[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(24): 211-217. (in Chinese with English abstract)
[3] Huang G, Zhang M, Liu C, et al. Heavy metal(loid)s and organic contaminants in groundwater in the Pearl River Delta that has undergone three decades of urbanization and industrialization: Distributions, sources, and driving forces[J]. Science of the Total Environment, 2018, 635: 913-925.
[4] Sacdal R, Madriaga J, Espino M P. Overview of the analysis, occurrence and ecological effects of hormones in lake waters in Asia[J]. Environmental Research, 2020, 182: 109091.
[5] 王立宁,韩鑫宇. 生物质炭化技术的农林生物质技术中的运用[J]. 资源节约与环保,2019,1(8):112.
Wang Lining, Han Xinyu. Application of biomass carbonization technology in agricultural and forestry biomass technology[J]. Resource Conservation and Environmental Protection, 2019, 1(8): 112. (in Chinese with English abstract)
[6] 顾洁,周建斌,马欢欢,等. 油茶壳热解产物特性及热解炭制备活性炭工艺优化[J]. 农业工程学报,2015,31(21):233-239.
Gu Jie, Zhou Jianbin, Ma Huanhuan, et al. Characteristics of camellia shell pyrolysis products and optimization of preparation parameters of activated carbon[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2015, 31(21): 233-239. (in Chinese with English abstract)
[7] 杨晶,李丽,季必霄,等. 生物炭吸附废水中重金属研究进展[J]. 能源环境保护,2020,34(6):1-7.
Yang Jing, Li Li, Ji Bixiao, et al. Research progress on absorption of heavy metals in wastewater by biochar[J]. Energy Environmental Protection, 2020, 34(6): 1-7. (in Chinese with English abstract)
[8] 向速林,龚聪远. 金属改性生物炭对磷的吸附研究进展[J]. 应用化工,2022,51(4):1-8.
Xiang Sulin, Gong Congyuan. Research progress of phosphorus adsorption by metal modified biochar[J]. Applied Chemical Industry, 2022, 51(4): 1-8. (in Chinese with English abstract)
[9] Li X, Wang C, Zhang J, et al. Preparation and application of magnetic biochar in water treatment: A critical review[J]. Science of the Total Environment, 2020, 711: 134847.
[10] 刘亦陶,魏佳,李军. 废弃生物质水热炭化技术及其产物在废水处理中的应用进展[J]. 化学与生物工程,2019,36(1):1-10.
Liu Yitao, Wei Jia, Li Jun. Progress in hydrothermal carbonization of waste biomass and application of biochar in wastewater treatment[J]. Chemistry & Bioengineering, 2019, 36(1): 1-10. (in Chinese with English abstract)
[11] 邱凌. 生物炭介导厌氧消化特性与机理[M]. 杨凌:西北农业科技大学出版社,2020:74-78.
[12] Zhu R, Yu Q, Li M, et al. Analysis of factors influencing pore structure development of agricultural and forestry waste-derived activated carbon for adsorption application in gas and liquid phases: A review[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 105905.
[13] Rodriguez-Sanchez S, Diaz P, Ruiz B, et al. Food industrial biowaste-based magnetic activated carbons as sustainable adsorbents for anthropogenic mercury emissions[J]. Journal of Environmental Management, 2022, 312(1): 114897.
[14] 霍丽丽,姚宗路,赵立欣,等. 典型农业生物炭理化特性及产品质量评价[J]. 农业工程学报,2019,35(16):249-257.
Huo Lili, Yao Zonglu, Zhao Lixin, et al. Physical and chemical properties and product quality evaluation of biochar from typical agricultural residues[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(16): 249-257. (in Chinese with English abstract)
[15] Gayathiri M, Pulingam T, Lee K T, et al. Activated carbon from biomass waste precursors: Factors affecting production and adsorption mechanism[J]. Chemosphere, 2022, 294(5): 133764.
[16] Yağmur H K, Kaya İ. Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue[J]. Journal of Molecular Structure, 2021, 1232(5): 130071.
[17] Kumar A, Patra C, Kumar S, et al. Effect of magnetization on the adsorptive removal of an emerging contaminant ciprofloxacin by magnetic acid activated carbon[J]. Environmental Research, 2022, 206: 112604.
[18] Zhang M, Gao B, Varnoosfaderani S, et al. Preparation and characterization of a novel magnetic biochar for arsenic removal[J]. Bioresource Technology, 2013, 130: 457-462.
[19] Wang S, Gao B, Zimmerman A R, et al. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite[J]. Bioresource Technology, 2015, 175: 391-395.
[20] Theydan S K, Ahmed M J. Adsorption of methylene blue onto biomass-based activated carbon by FeCl3activation: Equilibrium, kinetics, and thermodynamic studies[J]. Journal of Analytical and Applied Pyrolysis, 2012, 97: 116-122.
[21] Costa L F, Ruotolo L A M, Ribeiro L S, et al. Low-cost magnetic activated carbon with excellent capacity for organic adsorption obtained by a novel synthesis route[J]. Journal of Environmental Chemical Engineering, 2021, 9(2): 105061.
[22] 秦洁,常薇,杜燕萍,等. 磁性椰壳活性炭的制备与吸附性能[J]. 西安工程大学学报,2021,35(5):7-11.
Qin Jie, Chang Wei, Du Yanping, et al. Preparation and adsorption property of magnetic coconut shell activated carbon[J]. Journal of Xi'an Polytechnic University, 2021, 35(5): 7-11. (in Chinese with English abstract)
[23] Liu Z, Zhang F S, Sasai R. Arsenate removal from water using Fe3O4-loaded activated carbon prepared from waste biomass[J]. Chemical Engineering Journal, 2010, 160(1): 57-62.
[24] Prasannamedha G, Senthil Kumar P, Shankar V. Facile route for synthesis of Fe(0)/Fe3C/gamma-Fe2O3carbon composite using hydrothermal carbonization of sugarcane bagasse and its use as effective adsorbent for sulfamethoxazole removal[J]. Chemosphere, 2022, 289: 133214.
[25] Thaveemas P, Chuenchom L, Kaowphong S, et al. Magnetic carbon nanofiber composite adsorbent through green in-situ conversion of bacterial cellulose for highly efficient removal of bisphenol A[J]. Bioresource Technology, 2021, 333: 125184.
[26] Qu J, Wang S, Jin L, et al. Magnetic porous biochar with high specific surface area derived from microwave-assisted hydrothermal and pyrolysis treatments of water hyacinth for Cr(Ⅵ) and tetracycline adsorption from water[J]. Bioresource Technology, 2021, 340: 125692.
[27] Zhuang X, Liu J, Zhang Q, et al. A review on the utilization of industrial biowaste via hydrothermal carbonization[J]. Renewable and Sustainable Energy Reviews, 2022, 154: 111877.
[28] Sun D, Lv Z W, Rao J, et al. Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemicelluloses and lignin: A review[J]. Carbohydrate Polymers, 2022, 281: 119050.
[29] Wu Y, Yang X T, Fang X, et al. Hydrothermal conversion of waste cartons into a magnetic carbon-iron composite for use as an efficient and recyclable dye adsorbent[J]. Journal of Colloid and Interface Science, 2020, 578: 717-725.
[30] Cai W, Wei J, Li Z, et al. Preparation of amino-functionalized magnetic biochar with excellent adsorption performance for Cr(VI) by a mild one-step hydrothermal method from peanut hull[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 563: 102-111.
[31] 王森,袁娇娇,易佩,等. 水热炭化技术及其在废水处理中的应用研究进展[J]. 工业水处理,2022,42(3):1-8.
Wang Sen, Yuan Jiaojiao, Yi Pei, et al. Hydrothermal carbonization technology and its application research progress in wastewater treatment[J]. lndustrial Water Treatment, 2022, 42(3): 1-8. (in Chinese with English abstract)
[32] Mruthunjayappa M H, Kotrappanavar N S, Mondal D. New prospects on solvothermal carbonisation assisted by organic solvents, ionic liquids and eutectic mixtures-A critical review[J]. Progress in Materials Science, 2022, 126: 100932.
[33] Fei P, Wang Q, Zhong M, et al. Preparation and adsorption properties of enhanced magnetic zinc ferrite-reduced graphene oxide nanocomposites via a facile one-pot solvothermal method[J]. Journal of Alloys and Compounds, 2016, 685: 411-417.
[34] Wang Y, Lin C, Liu X, et al. Efficient removal of acetochlor pesticide from water using magnetic activated carbon: Adsorption performance, mechanism, and regeneration exploration[J]. Science of the Total Environment, 2021, 778: 146353.
[35] Ren L, Lin H, Meng F, et al. One-step solvothermal synthesis of Fe3O4@Carbon composites and their application in removing of Cr (VI) and Congo red[J]. Ceramics International, 2019, 45(7): 9646-9652.
[36] 魏明真. 溶剂热法合成纳米材料的研究进展[J]. 四川化工,2007,3(3):22-24.
Wei Mingzhen. Reasearch progress on solvothernal synthesis of nanomaterials[J]. Sichuan Chemical Industry, 2007, 3(3): 22-24. (in Chinese with English abstract)
[37] 牛姣姣,崔灿,谢雅典,等. 磁性生物炭的制备及其对重金属吸附研究进展[J]. 山东化工,2022,51(1):87-90.
Niu Jiaojiao, Cui Can, Xie Yadian, et al. Pesearch progress on preparation of magnetic biochar and its adsorption of heavy metals[J]. Shandong Chemical Industry, 2022, 51(1): 87-90. (in Chinese with English abstract)
[38] Zhu R, Xia J, Zhang H, et al. Synthesis of magnetic activated carbons from black liquor lignin and Fenton sludge in a one-step pyrolysis for methylene blue adsorption[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106538.
[39] Ahmed I, Lee H J, Jhung S H. Covalent-organic polymer-derived carbons: An effective adsorbent to remove sulfonamide antibiotics from water[J]. Chemical Engineering Journal, 2022, 437: 135386.
[40] Nkurikiyimfura I, Wang Y, Safari B, et al. Temperature-dependent magnetic properties of magnetite nanoparticles synthesized via coprecipitation method[J]. Journal of Alloys and Compounds, 2020, 846: 156344.
[41] Feng Z, Chen H, Li H, et al. Preparation, characterization, and application of magnetic activated carbon for treatment of biologically treated papermaking wastewater[J]. Science of the Total Environment, 2020, 713: 136423.
[42] Gao F, Xu Z, Dai Y. Removal of tetracycline from wastewater using magnetic biochar: A comparative study of performance based on the preparation method[J]. Environmental Technology & Innovation, 2021, 24: 101916.
[43] Sivaranjanee R, Kumar P S. A review on cleaner approach for effective separation of toxic pollutants from wastewater using carbon sphere’s as adsorbent: Preparation, activation and applications[J]. Journal of Cleaner Production, 2021, 291: 125911.
[44] Hu R, Furukawa T, Wang X, et al. Highly concentrated amino group-functionalized graphite encapsulated magnetic nanoparticles fabricated by a one-step arc discharge method[J]. Carbon, 2016, 110: 215-224.
[45] Jagannatham M, Sankaran S, Prathap H. Electroless nickel plating of arc discharge synthesized carbon nanotubes for metal matrix composites[J]. Applied Surface Science, 2015, 324: 475-481.
[46] Hu R, Furukawa T, Wang X, et al. Morphological study of graphite-encapsulated iron composite nanoparticles fabricated by a one-step arc discharge method[J]. Applied Surface Science, 2017, 416: 731-741.
[47] Salem S, Teimouri Z, Salem A. Fabrication of magnetic activated carbon by carbothermal functionalization of agriculture waste via microwave-assisted technique for cationic dye adsorption[J]. Advanced Powder Technology, 2020, 31(10): 4301-4309.
[48] Yang W, Chen H, Han X, et al. Preparation of magnetic Co-Fe modified porous carbon from agricultural wastes by microwave and steam activation for mercury removal[J]. Journal of Hazardous Materials, 2020, 381: 120981.
[49] Zhou J, Lü Q F, Luo J J. Efficient removal of organic dyes from aqueous solution by rapid adsorption onto polypyrrole–based composites[J]. Journal of Cleaner Production, 2017, 167: 739-748.
[50] Dotto G L, Santos J M N, Tanabe E H, et al. Chitosan/polyamide nanofibers prepared by Forcespinning® technology: A new adsorbent to remove anionic dyes from aqueous solutions[J]. Journal of Cleaner Production, 2017, 144: 120-129.
[51] Wang F, Li L, Iqbal J, et al. Preparation of magnetic chitosan corn straw biochar and its application in adsorption of amaranth dye in aqueous solution[J]. International Journal of Biological Macromolecules, 2022, 199: 234-242.
[52] Han G, Du Y, Huang Y, et al. Study on the removal of hazardous Congo red from aqueous solutions by chelation flocculation and precipitation flotation process[J]. Chemosphere, 2022, 289: 133109.
[53] Cheng S, Zhao S, Xing B, et al. Preparation of magnetic adsorbent-photocatalyst composites for dye removal by synergistic effect of adsorption and photocatalysis[J]. Journal of Cleaner Production, 2022, 348: 131301.
[54] Dong M, Guo J, Wang Y, et al. Humic acid non-covalent functionalized multi-walled carbon nanotubes composite membrane and its application for the removal of organic dyes[J]. Journal of Environmental Chemical Engineering, 2022, 10(2): 107320.
[55] Wang Z, Zhang Y, Li K, et al. In situ coupling of electrochemical oxidation and membrane filtration processes for simultaneous decontamination and membrane fouling mitigation[J]. Separation and Purification Technology, 2022, 290: 120918.
[56] Kang S, Zhang H, Dou M, et al. The microthermal construction of Z-scheme CdS@g-C3N4composite: Efficient tetracycline photodegradation, reaction mechanism and possible degradation pathway[J]. Optical Materials, 2022, 125: 112092.
[57] Foroutan R, Mohammadi R, Razeghi J, et al. Performance of algal activated carbon/Fe3O4magnetic composite for cationic dyes removal from aqueous solutions[J]. Algal Research, 2019, 40: 101509.
[58] Cheng S, Zhao S, Guo H, et al. High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed[J]. Bioresource Technology, 2022, 343: 126081.
[59] Foroutan R, Mohammadi R, Ahmadi A, et al. Impact of ZnO and Fe3O4magnetic nanoscale on the methyl violet 2B removal efficiency of the activated carbon oak wood[J]. Chemosphere, 2022, 286: 131632.
[60] Yang Z, Zhao Z, Yang X, et al. Xanthate modified magnetic activated carbon for efficient removal of cationic dyes and tetracycline hydrochloride from aqueous solutions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 615: 126273.
[61] Kittappa S, Jais F M, Ramalingam M, et al. Functionalized magnetic mesoporous palm shell activated carbon for enhanced removal of azo dyes[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104081.
[62] Mohammadi S Z, Darijani Z, Karimi M A. Fast and efficient removal of phenol by magnetic activated carbon-cobalt nanoparticles[J]. Journal of Alloys and Compounds, 2020, 832: 154942.
[63] Hao Z, Wang C, Yan Z, et al. Magnetic particles modification of coconut shell-derived activated carbon and biochar for effective removal of phenol from water[J]. Chemosphere, 2018, 211: 962-969.
[64] Priyan V V, Kumar N, Narayanasamy S. Toxicological assessment and adsorptive removal of lead (Pb) and Congo red (CR) from water by synthesized iron oxide/activated carbon (Fe3O4/AC) nanocomposite[J]. Chemosphere, 2022, 294: 133758.
[65] Zhang H, Li R, Zhang Z. A versatile EDTA and chitosan bi-functionalized magnetic bamboo biochar for simultaneous removal of methyl orange and heavy metals from complex wastewater[J]. Environmental Pollution, 2022, 293: 118517.
[66] Li Y, Dong X, Zhao L. Application of magnetic chitosan nanocomposites modified by graphene oxide and polyethyleneimine for removal of toxic heavy metals and dyes from water[J]. International Journal of Biological Macromolecules, 2021, 192: 118-125.
[67] Kaveh R, Bagherzadeh M. Simultaneous removal of mercury ions and cationic and anionic dyes from aqueous solution using epichlorohydrin cross-linked chitosan @ magnetic Fe3O4/activated carbon nanocomposite as an adsorbent[J]. Diamond and Related Materials, 2022, 124: 108923.
[68] Jiang X, Xia H, Zhang L, et al. Ultrasound and microwave-assisted synthesis of copper-activated carbon and application to organic dyes removal[J]. Powder Technology, 2018, 338: 857-868.
[69] 李顺顺,李伏虎,张佳,等. 活性炭纤维在工业废水处理中吸附重金属离子的研究[J]. 山东化工,2022,51(5):216-219.
Li Shunshun, Li Fuhu, Zhang Jia, et al. Study on heavy metal lon adsorption of activated carbon fiber in industrial wastewater treatment[J]. Shandong Chemical Industry, 2022, 51(5): 216-219. (in Chinese with English abstract)
[70] Mariana M, Abdul K, Mistar E M, et al. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption[J]. Journal of Water Process Engineering, 2021, 43: 102221.
[71] Fang Y, Yang K, Zhang Y, et al. Highly surface activated carbon to remove Cr(VI) from aqueous solution with adsorbent recycling[J]. Environmental Research, 2021, 197: 111151.
[72] Zhang Z, Wang T, Zhang H, et al. Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism[J]. Science of the Total Environment, 2021, 757: 143910.
[73] Ifthikar J, Wang J, Wang Q, et al. Highly efficient lead distribution by magnetic sewage sludge biochar: Sorption mechanisms and bench applications[J]. Bioresource Technology, 2017, 238: 399-406
[74] Prabu D, Kumar P S, Rathi B S, et al. Feasibility of magnetic nano adsorbent impregnated with activated carbon from animal bone waste: Application for the chromium (VI) removal[J]. Environmental Research, 2022, 203: 111813.
[75] Nejadshafiee V, Islami M R. Adsorption capacity of heavy metal ions using sultone-modified magnetic activated carbon as a bio-adsorbent[J]. Materials Science & Engineering C-Materials for Biological Applications, 2019, 101: 42-52.
[76] Ain Q U, Farooq M U, Jalees M I. Application of magnetic graphene oxide for water purification: Heavy metals removal and disinfection[J]. Journal of Water Process Engineering, 2020, 33: 101044.
[77] Rahmani-Sani A, Singh P, Raizada P, et al. Use of chicken feather and eggshell to synthesize a novel magnetized activated carbon for sorption of heavy metal ions[J]. Bioresource Technology, 2020, 297: 122452.
[78] Pan J, Gao B, Wang S, et al. Waste-to-resources: Green preparation of magnetic biogas residues-based biochar for effective heavy metal removals[J]. Science of the Total Environment, 2020, 737: 140283.
[79] Hou T, Yan L, Li J, et al. Adsorption performance and mechanistic study of heavy metals by facile synthesized magnetic layered double oxide/carbon composite from spent adsorbent[J]. Chemical Engineering Journal, 2020, 384: 123331.
[80] 李鸿博,钟怡,张昊楠,等. 生物炭修复重金属污染农田土壤的机制及应用研究进展[J]. 农业工程学报,2020,36(13):173-185.
Li Hongbo, Zhong Yi, Zhang Haonan, et al. Mechanism for the application of biochar in remediation of heavy metal contaminated farmland and its research advances[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(13): 173-185. (in Chinese with English abstract)
[81] 闫翠侠,贾宏涛,孙涛,等. 鸡粪生物炭表征及其对水和土壤镉铅的修复效果[J]. 农业工程学报,2019,35(13):225-233.
Yan Cuixia, Jia Hongtao, Sun Tao, et al. Characteristics of chicken manure biochars and its effect on Cd and Pb remediation in water and soil[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(13): 225-233. (in Chinese with English abstract)
[82] Xu Q, Xu Q, Zhu H, et al. Does biochar application in heavy metal-contaminated soils affect soil micronutrient dynamics?[J]. Chemosphere, 2022, 290: 133349.
[83] Liu M, Zhu J, Yang X, et al. Biochar produced from the straw of common crops simultaneously stabilizes soil organic matter and heavy metals[J]. Science of the Total Environment, 2022, 828: 154494.
[84] Nkoh J N, Ajibade F O, Atakpa E O, et al. Reduction of heavy metal uptake from polluted soils and associated health risks through biochar amendment: A critical synthesis[J]. Journal of Hazardous Materials Advances, 2022, 6: 100086.
[85] Jiang S, Dai G, Zhou J, et al. An assessment of integrated amendments of biochar and soil replacement on the phytotoxicity of metal(loid)s in rotated radish-soya bean-amaranth in a mining acidy soil[J]. Chemosphere, 2022, 287: 132082.
[86] Zhang M, Wang Y. Effects of Fe-Mn-modified biochar addition on anaerobic digestion of sewage sludge: Biomethane production, heavy metal speciation and performance stability[J]. Bioresource Technology, 2020, 313: 123695.
[87] Zhang G, Guo X, Zhao Z, et al. Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil[J]. Environmental Pollution, 2016, 218: 513-522.
[88] Chen D, Liu X, Bian R, et al. Effects of biochar on availability and plant uptake of heavy metals-A meta-analysis[J]. Journal of Environmental Management, 2018, 222: 76-85.
Research progress on the preparation of iron-carbon composites from agricultural and forestry biomass and their application in improving environmental pollution
Qiu Ling, Zhou Qinqin, Zhu Mingqiang※, Guo Xiaohui, Fan Qiongbo
(1.,,,712100,; 2...,,712100,)
An ever-increasing number of pollutants have posed serious hazards to human health and ecological environment, especially industrial wastewater, dye wastewater, and heavy-metal carcinogenic substances with the development of industrialization and urbanization in recent years. Fortunately, the iron-carbon composites can be expected to prepare using agricultural and forestry wastes. Among them, ferromagnetic additives have been widely used in the treatment of environmental pollution, due to the high specific surface area, better porosity, and abundant surface functional groups. The current preparation of iron-carbon composites includes hydrothermal synthesis, solvent heat, chemical co-precipitation, arc discharge, impregnation pyrolysis, and microwave. In this review, the latest research progress was summarized for the pros and cons of various syntheses. Among them, the hydrothermal environment accelerated the physicochemical interaction between the biomass and the aqueous solution in the hydrothermal synthesis. In turn, the formation of oxygen-containing functional groups was promoted on the surface of the carbonized materials. The solvent heat method was utilized to effectively inhibit the oxidation of products for the preparation of high-purity substances. The impregnation pyrolysis greatly contributed to a large number of pore structures in the carbonized products. The chemical co-precipitation was able to attach the metal ions and metal oxides to the surface of carbon materials or inside the pore channels. The arc discharge was used to precisely control the synthesis of nanoparticles via the varying electrode potential and current density. The microwave method was applied to realize the internal heating for less reaction time. The prepared iron-carbon composites exhibited excellent adsorption performance, easy separation, and high recycling rate. Extensive application prospects can be expected in the potential treatment of pollutants. A systematic investigation was then focused on the application progress of iron-carbon composites prepared from agricultural and forestry wastes in environmental pollution management, especially, the removal of heavy metals (Zn, Pb, Cd, Cr, Co, Ca, Mg, and Ni) from the wastewater, the treatment of dye wastewater, and the soil heavy metal pollution. More importantly, the removal of heavy metals and dyeing wastewater contained a combination of multiple adsorptions. Among them, the physical adsorption was caused by the Van der Waals forces between the molecules on the surface of iron-carbon composites and heavy metal or dye pollutant ions. The chemisorption was the process in the presence of elements via the redox reactions, especially the change of valence state. The optimal adsorption was achieved under the various chemical behavior, biological effectiveness, and migration ability of heavy metals. As such, the heavy metals were remediated in the soil pollutants, due to the significant effect of the iron-carbon composites on the immobilization of metal ions in the soil. Specifically, the iron-carbon composites with positive surfaces shared a great ability to immobilize anionic pollutants, whereas, the iron-carbon composites with negative surfaces mainly immobilized the cationic pollutants. The modified biochar can be expected to serve as a very promising immobilizer for soil heavy metal pollution. Therefore, the iron-carbon composites were prepared from the agricultural and forestry wastes in the environmental pollution remediation applications. Therefore, the low-cost, high-performance, high-efficiency adsorbent and remediation agent can provide great potential to leading technology and material for future environmental pollution treatment.
biomass; heavy metals; contaminant; iron-carbon composites
10.11975/j.issn.1002-6819.2022.22.019
S216.2
A
1002-6819(2022)-22-0172-11
邱凌,周勤勤,朱铭强,等. 农林生物质制备铁炭复合材料及其环境污染治理应用的研究进展[J]. 农业工程学报,2022,38(22):172-182.doi:10.11975/j.issn.1002-6819.2022.22.019 http://www.tcsae.org
Qiu Ling, Zhou Qinqin, Zhu Mingqiang, et al. Research progress on the preparation of iron-carbon composites from agricultural and forestry biomass and their application in improving environmental pollution[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(22): 172-182. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2022.22.019 http://www.tcsae.org
2022-08-09
2022-11-11
陕西省农业科技创新驱动项目(NYKJ-2022-YL(XN)17):黄河流域农业面源污染防控技术研发与示范;国家自然科学基金青年项目(31900105):磁性铁炭复合材料基材料对厌氧消化微生物种间电子传递的促进效应机制研究
邱凌,教授,博士生导师,研究方向为生物质能源与绿色低碳农业工程技术教学与研究。Email:3037296930@qq.com
朱铭强,教授,博士生导师,研究方向为生物质能源与绿色低碳农业工程技术研究。Email:zmqsx@nwsuaf.edu.cn

