UPLC-QTOF/MS Method for Screening and Determination of 59
Xueliang PANG Lei WANG Biao QI Sining TANG Yanhua YAN Xuesong WANG Lixue DONG Yi ZHANG Aijun LI
Non-labeled Components in Veterinary Drug Preparations
Abstract [Objectives] This study was conducted to provide an accurate and reliable method for rapid high-throughput screening of veterinary drug preparations.
[Methods]For the matrixes of veterinary drug preparations, high-performance liquid chromatography-high resolution quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS) was used to establish a fast screening method for 59 non-standard components in five categories of antiviral agents, aminoglycosides, quinolones, sulfonamides, and tetracyclines in veterinary drugs. The target drugs were separated by a Waters ACQUITY UPLC HSS T3 chromatographic column (50 mm×2.1 mm, 1.8 μm), and data were collected in the positive ion mode. Good separation of the 59 drugs was achieved within 7 min.
[Results] In the concentration range of 0-100 ng/ml, each drug showed a good linear relationship, and the correlation coefficients were all greater than 0.999. The detection limits of the 59 drugs were in the range of 0.1-0.5 mg/ml, and the recovery under the addition concentration of 5 mg/ml was in the range of 85.2%-103.8%.
[Conclusions]The method is fast, simple, accurate, and highly sensitive, and is suitable for high-throughput screening and qualitative identification of non-standard components in veterinary drug preparations.
Key words High performance liquid chromatography-tandem time-of-flight mass spectrometry; Veterinary drugs; Non-standard component
Received: October 8, 2021 Accepted: December 3, 2021
Supported by Key R&D Project of Hebei Province (19227516D); Hebei Provincial Phase II Modern Agricultural Industry Technology System Innovation Team Building Project (HBCT2018120207); Hebei Provincial Phase II Modern Agricultural Industry Technology System Grass Industry Innovation Team Building Project (HBCT2018160403); Hebei Provincial Science and Technology Innovation Leading Talents (21130243A); The Fourth Batch of High-end Talent Project in Hebei Province.
Xueliang PANG (1987-), male, P. R. China, engineer, devoted to research about agricultural product quality and safety monitoring.
Lei WANG (1982-), male, P. R. China, senior agronomist, devoted to research about food inspection.
#These authors contributed equally to this work.
*Corresponding author.
The Guiding Opinions of the Ministry of Agriculture and Rural Affairs on Promoting the Healthy Development of the Veterinary Drug Industry pointed out that the structure of the veterinary drug industry should be further optimized and the quality of veterinary drugs should be improved. According to the current situation of the quality and safety inspection of agricultural products by the Ministry of Agriculture and Rural Affairs, the main reasons for the unqualified veterinary drugs are: unqualified content, undetected active ingredients of traditional Chinese medicine and serious illegal addition of drugs, among which illegal addition accounts for about 3%. There are many kinds of illegally added drugs in veterinary drugs, and various methods and random injections exist and are concealed. However, current detection modes are about known drugs, and most of them only detect one type of veterinary drug or a few types of veterinary drugs. The situation of missed detection is very serious, and the detection methods are time-consuming and reagent-consuming, and have high detection costs[1-3].
There are currently 24 announcements[4], and the inspection methods include microscopic inspection, thin layer chromatography, liquid chromatography-photo-diode array detection (HPLC-PDA), of which HPLC-PDA is mainly used[5]. The tested drugs involve antipyretic analgesics, quinolones, sulfonamides, agonists, antivirals, etc.[6]. Liquid chromatography has strong specificity and accurate quantification, but can detect few types. Moreover, it is also time-consuming, and the detection limits are difficult to reduce[7]. In this study, the pretreatment method and mass spectrometry feature database of 59 non-standard components in veterinary drug preparations were established by HPLC-QTOF/MS. The Full Scan/MSE mode for scanning not only improved the reliability of rapid screening of non-standard components, but also obtained high-sensitivity quantitative results, thereby providing an accurate and reliable method for rapid and high-throughput screening of veterinary drug preparations.
Materials and Methods
Instruments and equipment
API 4000 high performance liquid chromatograph-mass spectrometer equipped with TurboV ion source produced by AB SCIEX in the United States was used. Other pretreatment instruments used were a GENIUS 3 vortex oscillator produced by IKA, a Sorvall LYNX 4000 centrifuge produced by Thermo Scientific, a Milli-Qultrapure water machine produced by Millipore in the United States, and electronic balances (one ten-thousandth, hundred thousandth) produced by Shimadzu.
Materials and reagents
The reagents used mainly included methanol (Merck KGaA, USA, chromatographically pure), acetonitrile (Merck KGaA, Chromatographically pure, USA), formic acid (Thermo Fisher, chromatographically pure), and ammonium formate (Dikma, purity>99.0%). The 59 screened drug standard reference substances used in this experiment were all produced by Tianjin Alta Scientific Co., Ltd. Specifically, the mixed standard antiviral product containing ribavirin, amantadine, rimantadine hydrochloride, memantine hydrochloride, acyclovir, imiquimod, moroxydine hydrochloride and oseltamivir had a concentration of 100 μg/ml, and was sold under the product number of 1ST45033-100M; the mixed standard of aminoglycosides containing spectinomycin pentahydrate dihydrochloride, hygromycin B, dihydrostreptomycin sulphate, streptomycin sulfate, amikacin dihydrate, kanamycin sulfate, tobramycin, gentamicin C1A solution and neomycin sulfate had a concentration of 100 μg/ml and was sold under the product number of 1ST9217-100MW; the netilmicin sulfate standard had a concentration of 100 μg/ml, and was sold under the product number of 1ST7713B-100W; the mixed standard of quinolones containing enrofloxacin, ciprofloxacin, norfloxacin, ofloxacin, difluoxacin hydrochloride, oxolinic acid, afloqualone, sarafloxacin hydrochloride, sparfloxacin, danofloxacin, fleroxacin, marbofloxacin, enoxacin, orbifloxacin, pefloxacin mesylate, lomefloxacin, cinoxacin and nalidixic acid had a concentration of 100 μg/ml, and was sold under the product number of 1ST47513-100M; the mixed standard of sulfonamides containing sulfadiazine, sulfathiazole, sulfapyridine, sulfamerazine, sulfadimidine, sulfamonomethoxine, sulfamethizole, sulfameter, sulfachloropyridazine, sulfamethoxypyridazine, sulfadoxine, sulfadimethoxine, sulfamethoxazole, sulfisoxazole, sulfabenzamide, sulfaquinoxaline, sulfacetamide, trimethoprim, sulfaphenazolum had a concentration of 100 μg/ml and was sold under the product number of 1ST9239-100M; the tetracycline standard was sold under the product number of 1ST4102 (solid powder); the chlortetracycline standard was sold under the product number of 1ST4110 (solid powder); the oxytetracycline standard was sold under the product number of 1ST4111 (solid powder); and the doxycycline standard was sold under the product number of 1ST4112 ( solid powder).
The test veterinary drug preparations were sampled by Tangshan Food and Drug Comprehensive Inspection and Testing Center (Tangshan Agricultural Products Quality and Safety Inspection and Testing Center, Tangshan Inspection and Testing Institute).
Experimental methods
Sample pretreatment
Solid veterinary drug preparations were first crushed and mixed with a homogenizer for later use, while liquid veterinary drug preparations were used directly. First, a 2 g or 2 ml of veterinary drug preparation sample was accurately weighed or accurately measured into a 50 ml centrifuge tube, added with 20 ml of acetonitrile reagent, and shaken well. Then, 1 ml of 1% formic acid solution was added, and the obtained solution was shaken again and ultrasonically treated for 15 min. The treated solution was centrifuged at 8 000 r/min for 5 min. The supernatant was pipetted and filtered through a 0.22 μm organic filter membrane into a sample injection vial. The treated sample was diluted step by step according to the packaging content of the veterinary drug to be tested, and tested on the machine.
Chromatography-mass spectrometry analysis conditions
Chromatographic conditions: HSS T3 chromatographic column (Waters ACQUITY UPLC, 2.1 mm×50 mm, 1.8 μm).
Mobile phase preparation: ① Mobile phase A was an aqueous solution of 5 mmol/L ammonium acetate+0.1% formic acid. First, 0.394 g of ammonium acetate was accurately weighed into a 1 L volumetric flask, dissolved with water, added with 1 ml of formic acid, and diluted to the mark with ultrapure water. The obtained mixture was treated with an ultrasonic cleaning machine for dissolution and mixing well. Then, the solution was filtered with a filter membrane to remove impurities and added into a solution bottle. Next, the filtrate was degassed ultrasonically for 10 min for later use. ② The mobile phase B was acetonitrile, which was degassed ultrasonically for 10 min for later use.
Mass spectrometry conditions: MRM scanning mode; mass spectrum acquisition time 10 min; collision gas pressure 10 psi; curtain gas pressure 35 psi; ion source gas pressure 1∶50 psi; ion source gas pressure 2∶50 psi; injection voltage 10 eV; ion spray voltage 5 500 V; ion source temperature 550 ℃.
Results and Analysis
Optimization of chromatographic conditions and mass spectrometry conditions
Because trifluoroacetic acid is used as an ion-pairing reagent, which interacts with the hydrophobic bonding phase of chromatographic columns and the polar surface of drugs in multiple modes. Although it can effectively improve the peak shape and increase the peak resolution, long-term use will increase the pressure of chromatographic columns and greatly shorten the service life of chromatographic columns[8-12], so 1% formic acid and acetonitrile were used as mobile phases.
In addition, the veterinary drug preparations for injection have no interference of protein and fat, and the contents are high, so there is no need to use solid phase extraction column for concentration and purification[13-15], and direct dilution can eliminate interference.
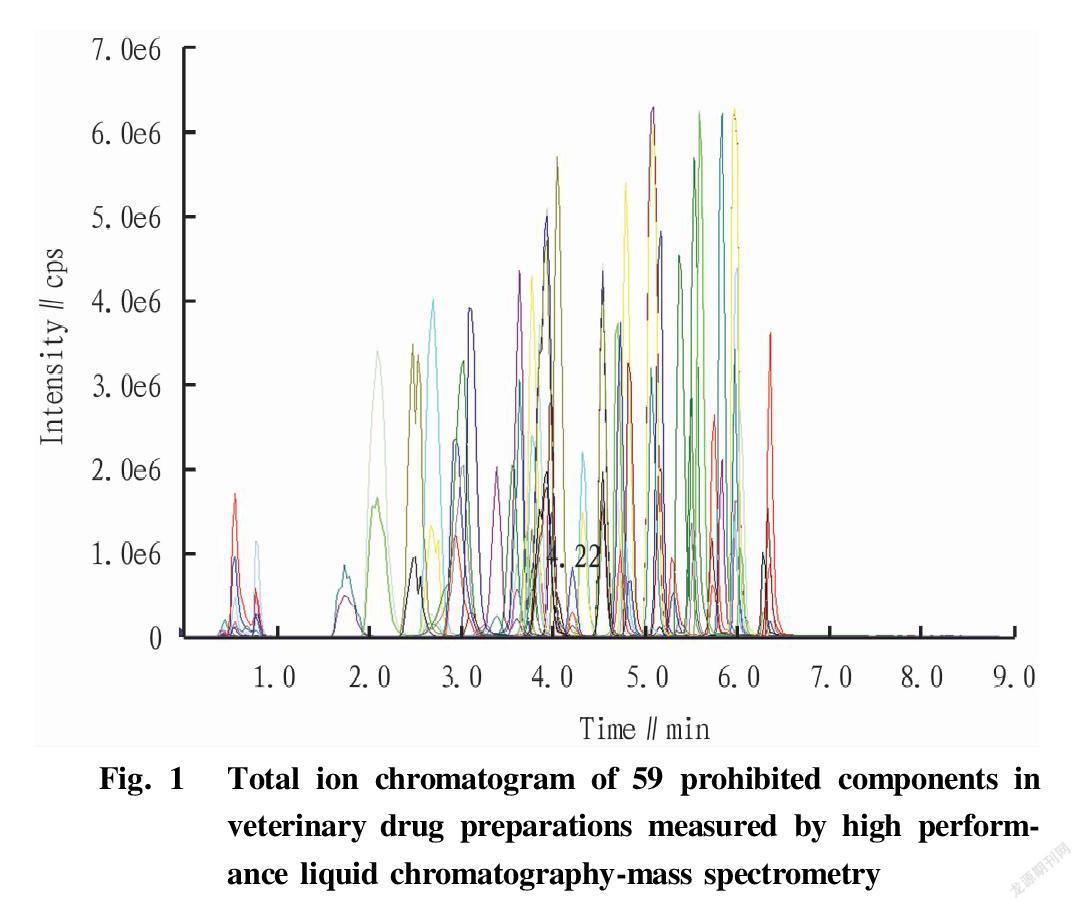
The mixed standard solution including 59 drugs of five major types with a concentration of 1 μg/ml was injected into the mass spectrometer at a rate of 20 μl/min. The positive ion scan mode was used to collect first-level mass spectra. Through the second-level mass spectra, the ion with the first strongest kurtosis was selected as the quantitative ion, and the ion with the second strongest kurtosis was used as the qualitative ion. After that, the multi-reaction monitoring mode was used to optimize the mass spectrometry parameters such as collision energy and collision cell outlet voltage to obtain the optimal mass spectrometry conditions, and the total ion chromatogram obtained were shown in Fig. 1.
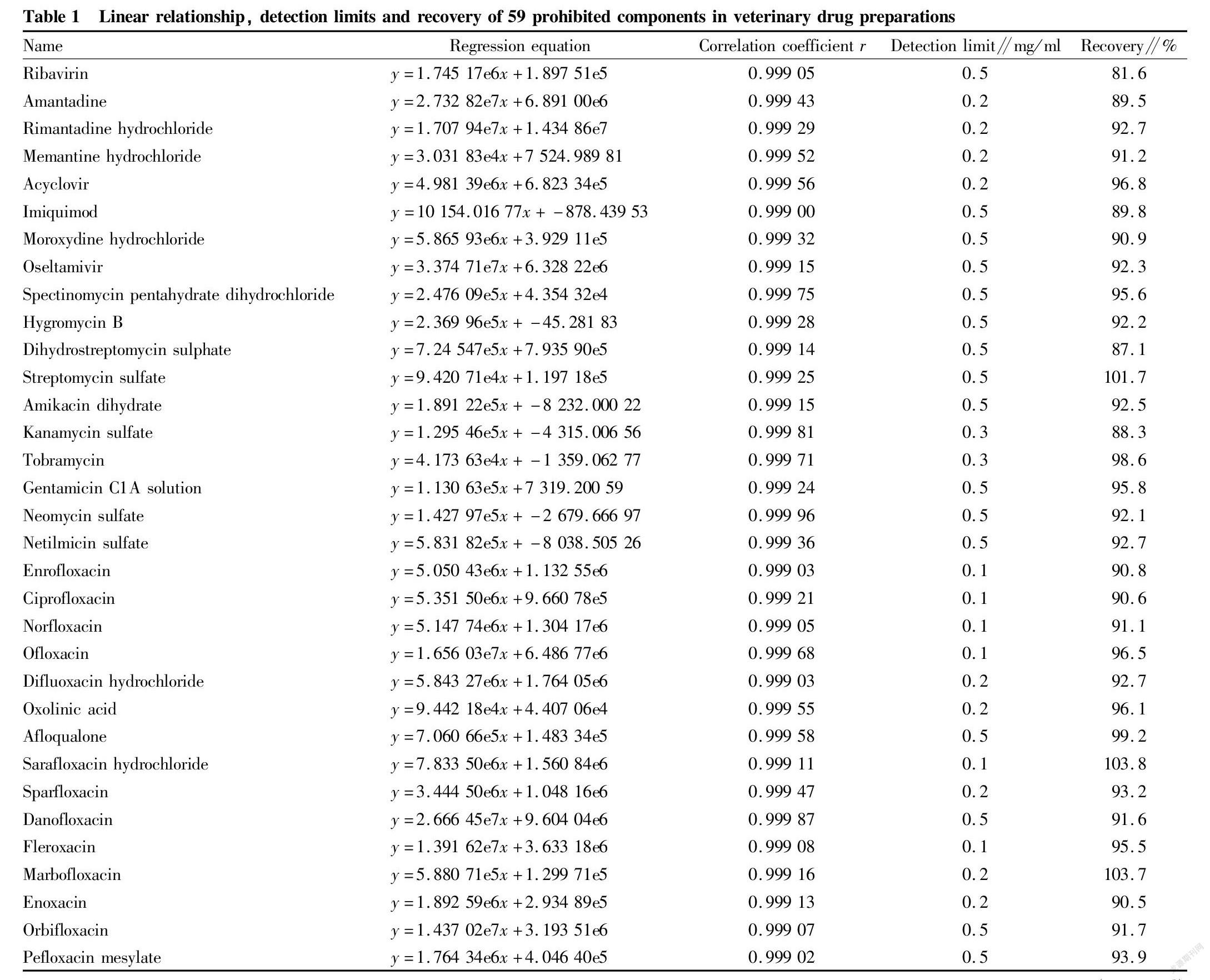
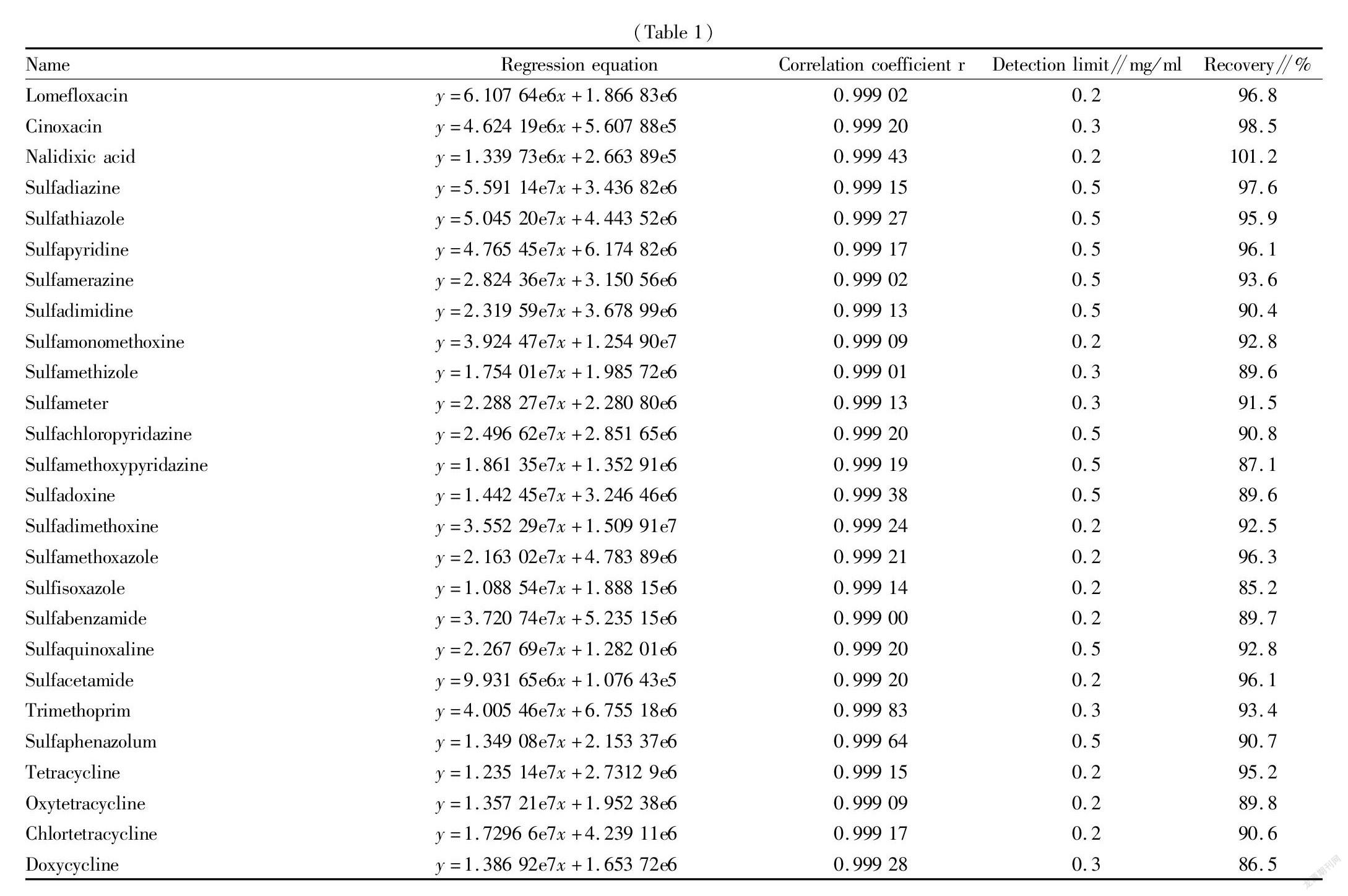
Linear relationship, detection limit and recovery rate
Standard curves were drawn with the concentrations of the standard working solutions of 59 drugs in 5 categories, namely antiviral agents, aminoglycosides, quinolones, sulfonamides and tetracyclines as the x coordinates, and the areas of the quantitative ion peaks as the y coordinates. The concentration of each drug in the range of 0-100 ng/ml showed a good linear relationship, and the correlation coefficients were greater than 0.999. Based on the method of adding standard solutions to a blank matrix sample, the detection limits of the 59 drugs obtained were in the range of 0.1-0.5 mg/ml, and through the addition of the matrix standard sample with a concentration of 5 mg/ml, the calculated recovery was in the range of 85.2%-103.8%, as shown in Table 1.
Conclusions and Discussion
① The pretreatment method for the detection of non-standard components in veterinary drug preparation samples was determined. Each sample was extracted by 20 ml of acetonitrile + 1 ml of 1% formic acid solution + ultrasonic extraction for 15 min. The sample liquid was centrifuged at 8 000 r/min for 5 min, and the supernatant was filtered with a 0.22 μm organic filter membrane. After the initial determination of the concentration on the machine, the test solution was diluted by appropriate multiples for the quantitative detection with UPLC-QTOF/MS.
② A quantitative detection method for the simultaneous and accurate quantification of 59 non-standard components in 5 categories of veterinary drug preparations by UPLC-QTOF/MS was successfully established. The linear concentration range was 0-100 ng/ml, and the correlation coefficients were all greater than 0.999. The detection limits were in the range of 0.1-0.5 mg/ml, and the recovery of the matrix standard sample with a concentration of 5 mg/ml was 85.2%-103.8%.
References
[1] Announcement No. 1848 of the Ministry of Agriculture of the People’s Republic of China. Inspection method for illegal addition of 11 kinds of chemical drugs (substances) such as antipyretics, antiviral agents, antibiotics and quinolones in Astragalus polysaccharide injection and inspection method for illegal addition of fluoroquinolone chemical drugs (substances) to Chinese veterinary powders such as Feizhu Powder, Jianwei Powder and Yinqiao Powder[S]. (in Chinese).
[2] Announcement No. 2508 of the Ministry of Agriculture of the People’s Republic of China. Announcement on the issuance of inspection methods for 11 illegally added chemical drugs (substances) of veterinary drugs including "Inspection method for illegal addition of antipyretic and analgesic drugs to penicillin potassium (sodium) for injection"[S]. (in Chinese).
[3] Announcement No. 2320 of the Ministry of Agriculture of the People’s Republic of China. Three inspection method standards: Inspection method for illegal addition of lincomycin in Houttuynia cordata injection, inspection method for illegal addition of ofloxacin salicylate in Houttuynia cordata injection, and inspection method for illegal addition of amantadine and rimantadine in Chinese medicine powders[S]. (in Chinese).
[4] LI SG, JIN LS, WANG Y, et al. Analysis of the status quo of off-prescription illegally-added substances of veterinary drugs in China[J]. Gansu Animal and Veterinary Sciences, 2016, 15(46): 24-27. (in Chinese).
[5] HAN NN, XU Y, YU LN, et al. Advantages and disadvantages of HPLC-PDA method used in the inspection of illegal adulteration in veterinary drugs[J]. Chinese Journal of Veterinary Drug, 2016, 50(4): 66-69. (in Chinese).
[6] HU XJ, WU NP, DONG RJ, et al. Determination of atropine illegally added in veterinary drugs by UPLC-MS/MS[J]. Chinese Journal of Veterinary Drug, 2014, 48(11): 49-52. (in Chinese).
[7] WEI XL, GAO YC, ZHANG CJ, et al. Determination of amantadine residues in traditional Chinese veterinary medicine by UPLC-MS/MS[J]. Central South Pharmacy, 2014, 12(4): 363-366. (in Chinese).
[8] DONG LL, YU XH, FAN Q, et al. Research progress on detection technologies and the current situation for chemical drugs illegally adulterated in veterinary preparations[J]. Chinese Journal of Veterinary Drug, 2017, 51(3): 11-14. (in Chinese).
[9] KONG C, ZHOU Z, WANG Y, et al. Screening of chemical drugs in fishery inputs by ultrahigh performance liquid chromatography-orbitrap high resolution mass spectroscopy[J]. Chinese Journal of Analytical Chemistry, 2017, 45(2): 245-252. (in Chinese).
[10] WANG Z, WU CS, WANG GL, et al. Novel strategy for the determination of illegal adulterants in health foods and herbal medicines using high-performance liquid chromatography with high-resolution mass spectrometry[J]. Journal of Separation Science, 2015, 38(6): 925-935.
[11] ZHANG H, AI JT, ZHAO YF. Determination of seven β-agonists residues in pork by high performance liquid chromatography-tandem mass spectrometry[J]. Meat Industry, 2015(11): 42-44, 47. (in Chinese).
[12] HUANG MY, WANG HZ, LU J, et al. Application of QuEChERS-HPLC/MS/MS to the determination of clopidol and abamectin in eggs[J]. Journal of Zhejiang Agricultural Sciences, 2020, 61(9): 1872-1875. (in Chinese).
[13] WEN ZM, LI D, XU YH. Simultaneous determination of various quinolone antimicrobial agents in human plasma by HPLC[J]. China Pharmaceuticals, 2011, 20(14): 25-27. (in Chinese).
[14] GB/T 21312-2007. Analysis of fourteen quinolones in food of animal origin by high performance liquid chromatography tandem mass spectrometry[S]. (in Chinese).
[15] LI PP, GUO YM, CHEN XC, et al. Chromatographic detection of quinolones residues in animal tissues[J]. Food Science, 2013, 34(3): 303-307. (in Chinese).
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Expression Analysis of Heat Shock Protein 70 Gene in Rice (Oryza sativa L.)
- Changes in Physiological and Biochemical Characteristics of Floral Organ
- Research Progress on Lonicera japonica Thunb. Affected by Environmental Stress
- Research Progress on Genetic Diversity of Snap Bean
- Allelopathic Effects of Cedrus deodara Needle Extracts on Seed
- Development Status and Countermeasures of Passiflora spp. Seedling Industry in Qinzhou, Guangxi

