Changes in Physiological and Biochemical Characteristics of Floral Organ
Tianyu CUI Xia CAO Zhigang LI Jiayao SUN Peng LIU Pengnian WANG
Development in a Soybean Cytoplasmic-nuclear Male Sterile Line
Abstract [Objectives] This study was conducted to explore the mechanism of soybean cytoplasmic-nuclear male sterility. [Methods] With soybean cytoplasmic-nuclear male sterile line JLCMS9A and its homotype maintainer line JLCMS9B as experimental materials, the activity of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT), malondialdehyde (MDA) content, starch content, soluble protein content, soluble sugar content and free proline content in flower buds, alabastrums and mature flowers were determined, and the contents and changes of auxin (IAA), gibberellin (GA3), isopentenyl adenosine (iPA) and abscisic acid (ABA) at the three stages were analyzed. [Results] The activity of SOD and CAT and the contents of MDA and free proline in the sterile line at the flower bud stage were lower than those of the maintainer line, but the opposite was true at the alabastrum stage and the flowering stage, and their values were higher than those of the maintainer line; the POD activity of the sterile line was significantly lower than that of the maintainer line at the flower bud stage, and the opposite was true at the alabastrum stage and the flowering stage, and its values were higher than those of the maintainer line; and the starch content and soluble sugar content of sterile line 9A showed an overall upward trend, and were significantly lower than those of the maintainer line 9B at the alabastrum stage and the flowering stage. During the whole development process of floral organs, the content of IAA in sterile line 9A showed a trend of first increasing and then decreasing, and the content of iPA increased gradually. The contents of hormones in the sterile line were lower than those in the maintainer line. The ratios of IAA/ABA, IAA/GA3, IAA/iPA and ABA/GA3 in the sterile line were significantly different from those in the maintainer line. It is inferred that the abnormal physiological characteristics of floral organ development are related to the cytoplasmic-nuclear male sterility of soybean. The alabastrum stage may be a critical period for the occurrence of abnormal physiological and biochemical indexes in the floral organs of soybean cytoplasmic-nuclear male sterile lines. [Conclusions] This study provides a theoretical basis for the breeding of fine sterile lines of soybean and the research on the mechanism of sterility.
Key words Soybean; Cytoplasmic-nuclear male sterility; Floral organ; Physiological and biochemical characteristic
Received: November 8, 2021 Accepted: January 7, 2022
Supported by Doctoral Research Start-up Fund (BS514); Inner Mongolia Autonomous Region Science and Technology Reserve Project (2018MDCB02); Inner Mongolia Autonomous Region Science and Technology Planning Project (2018KJJH1702); Scientific Research Project of Inner Mongolia Minzu University (NMDSS2159).
Tianyu CUI (1996-), female, P. R. China, master, devoted to research about genetic breeding of crops.
*Corresponding author. E-mail: 13948651158@126.com.
Soybean originates in China and has a cultivation history of more than 5 000 years. Therefore, China has abundant soybean resources. As one of the main food crops in China, soybean is the main source of protein and edible vegetable oil needed by human beings. With the continuous development of China’s economy, China’s demand for soybeans in all aspects is also increasing. However, due to the low yield and economic benefits of soybeans, the planting area of Soybeans in China is lower than that of major food crops such as maize, rice, and wheat. China’s total soybean production is obviously in short supply. Therefore, cultivating soybeans with high yield, high quality and strong resistance is the primary goal of soybean breeding in China, while the most effective measure to achieve this goal is the utilization of heterosis, and the discovery of soybean cytoplasmic-nuclear male sterile lines provides a basis for the utilization of soybean heterosis[1].
In recent years, many studies have been conducted on the physiological and biochemical characteristics of cotton[2], rice[3], rape[4], tobacco[5], cabbage[6-8] and maize[9], but there are few reports on soybean sterile lines and their physiological and biochemical characteristics. Scholars found that the male sterility of a plant is related to the material metabolism, energy metabolism, endogenous hormones and peroxidase activity of its organs. The substance content in male sterile lines decreases, and the metabolic rate decreases. Studies have pointed out that peroxidases (SOD, POD, CAT) have the effect of scavenging oxygen free radicals generated in the process of plant metabolism, and can maintain the stability of the plasma membrane[10], thereby maintaining the balance of reactive oxygen species in plants. Endogenous hormones are related to plant sterility and are important factors regulating their growth and development[11-12].
The main types of soybean male sterility in China are nuclear male sterility[13-15], cytoplasmic-nuclear male sterility[16-18] and photothermosensitive male sterility[19-21]. In this study, with soybean cytoplasmic-nuclear male sterile line JLCMS9A and its homotype maintainer line JLCMS9B as experimental materials, the activity of SOD, POD and CAT, the contents of MDA, starch, soluble protein, soluble sugar and free proline and changes in the contents and dynamic contents of endogenous hormones such as auxin (IAA), gibberellin (GA3), isopentenyl adenosine (iPA) and abscisic acid (ABA) were determined, and their correlation with sterility was analyzed, aiming to provide a theoretical basis for breeding excellent sterile lines and research on the mechanism of sterility.
Materials and Methods
Experimental materials
Soybean sterile line JLCMS9A and its homotype maintainer line JLCMS9B provided by Soybean Research Institute, Jilin Academy of Agricultural Sciences were used as experimental materials, which were denoted as 9A and 9B, respectively. The seeds were sown in the Horqin sandy land test field of Inner Mongolia University for Nationalities in May 2019. The experiment was set up in three plots, each of which was 20 m2, and planted with 1∶1 equal row spacing. The spacing in the rows and between rows was 15 cm×60 cm, and each plot was planted with 6 rows. The experimental materials were sown in the same period, and the same management measures were adopted for field management during the growth period.
Sample collection
Sampling was started at the full-bloom stage, and the flower?buds, alabastrums, and mature flowers above the fourth node of the main stem were sampled respectively, with three replicates. The samples were put into liquid nitrogen for quick freezing, and then stored in a -80 ℃ refrigerator.
Determination items and methods
Soluble protein content was determined by Coomassie brilliant blue G-250 staining method; soluble sugar and starch were determined by sulfuric acid-anthrone colorimetry; free proline content was determined by acidic ninhydrin method; and CAT activity was determined by ultraviolet absorption method; SOD activity was determined by nitroblue tetrazolium (NBT) photoreduction method; POD activity was determined by guaiacol method; and MDA content was determined by thiobarbituric acid (TBA) method.
Indirect enzyme-linked immunosorbent assay (ELISA) was adopted to determine the contents of auxin (IAA), gibberellin (GA3), isopentenyl adenosine (iPA) and abscisic acid (ABA) at different developmental stages of floral organs of the sterile line and homotype maintainer line. Each sample was determined in three replicates.
Data analysis
The significance of differences between the determined index data was analyzed by software DPS16.05, and histograms were drawn in Excel2016.
Results and Analysis
Changes of peroxidase activity at different stages of floral organ development
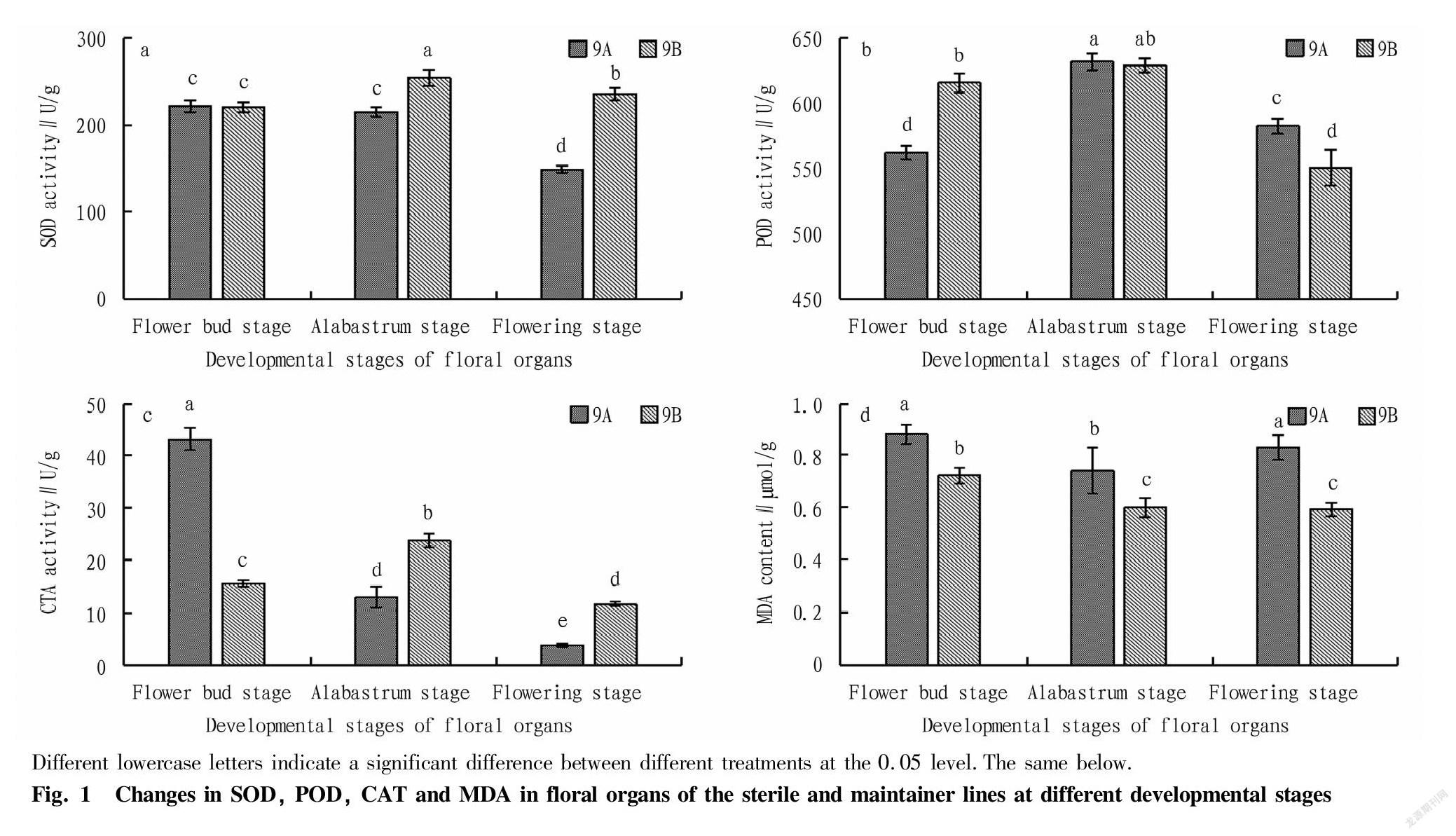
As shown in Fig. 1, during the development of floral organs, the SOD activity of sterile line 9A showed a downward trend, and reached a significant difference at the flowering stage. The SOD activity of maintainer line 9B first increased and then decreased, and reached the highest value (254.58 U/g FW) at the alabastrum stage, when a significant difference was reached. The SOD activity was significantly higher in maintainer line 9B than in sterile line 9A with a significant difference at both the alabastrum stage and the flowering stage, and the SOD activity of the maintainer line was 1.18 and 1.58 times that of the sterile line, respectively. At the flowering stage, the SOD activity content of sterile line 9A dropped sharply. It was speculated that the flowering stage might be the period when abortion occurred.
Sterile line 9A and maintainer line 9B both showed a trend of first increasing and then decreasing in POD activity during the floral organ developmental period, and both reached the highest values at the alabastrum stage at the same time, but the differences were not significant. At the flower bud stage, the POD activity of 9B was significantly higher than that of 9A, and 9B was 9.5% higher than that of 9A. At the flowering stage, the POD activity of 9B decreased significantly, and was significantly lower than that of 9A (94.5% of that of 9A). The POD activity of the sterile line and maintainer line was significantly lower at the flowering stage than at the alabastrum stage, but the sterile line and maintainer line showed the same inverted U-shaped change pattern throughout the development period. Therefore, it was speculated that POD activity had little correlation with soybean male sterility.
The CAT activity showed different trends in sterile line 9A and maintainer line 9B. 9A showed a gradually decreasing trend, while 9B exhibited an inverted U-shaped trend. With the development of 9B’s floral organs, the content of CTA activity at the flowering stage was lower than that at the flower bud stage, and the difference reached the significant different level. When comparing 9A and 9B, 9A was 2.75 times of 9B at the flower bud stage, and in turn, 9B was 3.03 times of 9A at the flowering stage. It indicated that with the development of floral organs, the activity of CAT was gradually decreasing, and sterile line 9A was significantly lower than maintainer line 9B.
From the flower bud stage to the flowering stage, the MDA content of sterile line 9A showed a trend of first decreasing and then increasing, while maintainer line 9B showed a decreasing trend. At the flower bud stage, alabastrum stage and flowering stage, sterile line 9A and maintainer line 9B all reached significant differences, and the MDA content of sterile line 9A was 1.22, 1.23 and 1.41 times that of maintainer line 9B, respectively. It indicated that the MDA content at the flowering stage had a greater effect on fertility.
Comparison on nutrient contents of floral organs in different developmental stages
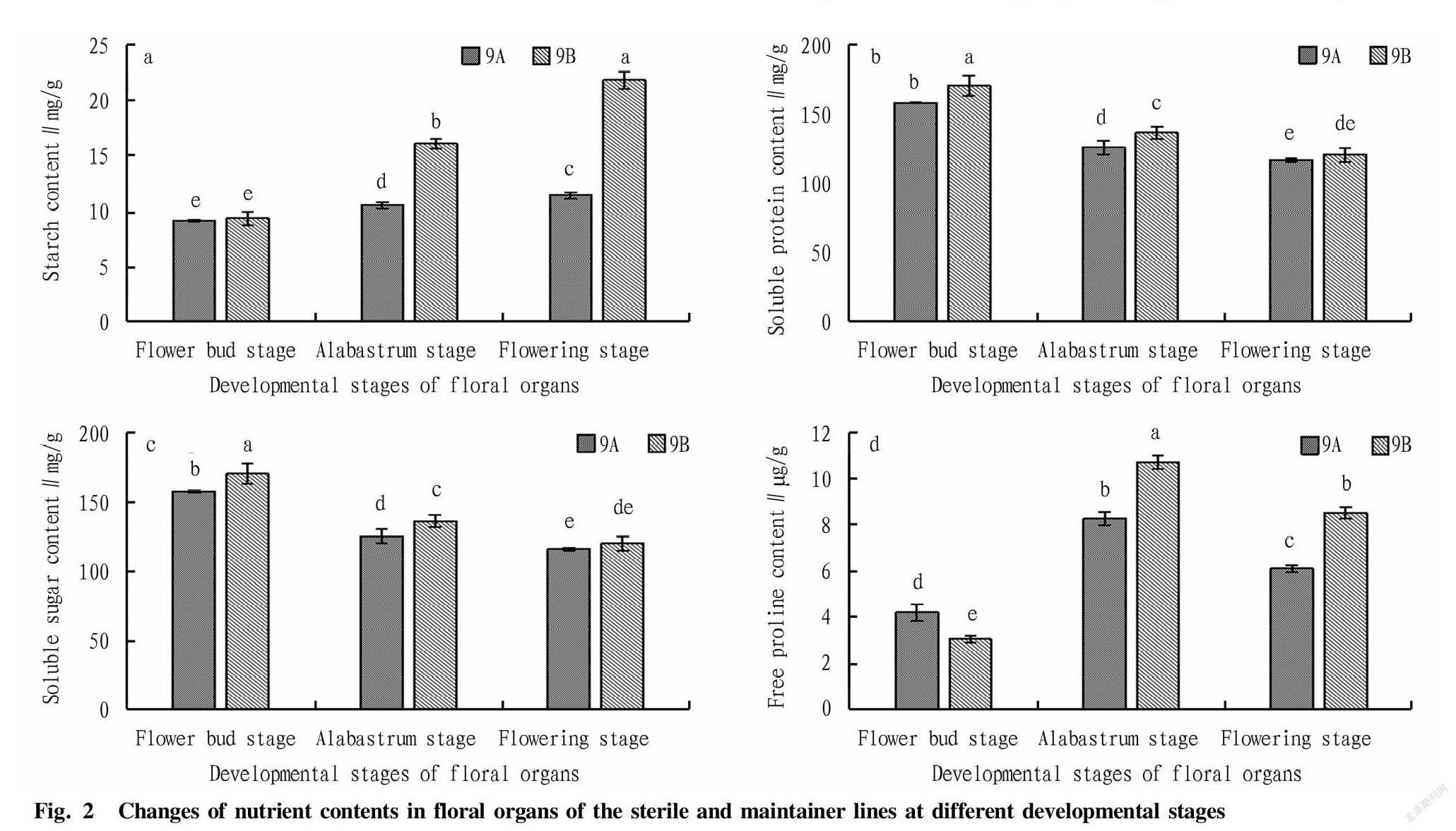
It can be seen from Fig.2 that the starch contents of sterile line 9A and maintainer line 9B both showed an increasing trend, and both reached at the flowering stage their highest values, which were 11.41 and 21.76 mg/g FW, respectively. In the process of floral organ development, the starch content of 9B was always higher than that of 9A, and the difference was significant at the alabastrum stage and the flowering stage, reaching a significant level of difference. The starch content of 9B was 52.4% and 90.7% higher than that of 9A at the alabastrum stage and flowering stage, respectively, indicating that with the development of floral organs, starch content played a key role in soybean fertility. The lack of starch in the later stage caused the lack of energy and nutrient supply in the development of floral organs, which led to abnormal development of floral organs, resulting in male sterility.
Tianyu CUI et al. Changes in Physiological and Biochemical Characteristics of Floral Organ Development in a Soybean Cytoplasmic-nuclear Male Sterile Line
In the whole process of floral organ development, the soluble protein content of sterile line 9A and maintainer line 9B showed a gradual downward trend during floral organ development, and the soluble protein content of sterile line 9A and maintainer line 9B both reached at the flower bud stage their maximums, which were 157.25 and 169.86 mg/g FW, respectively, and (a significant difference was reached). With the development of floral organs, the soluble protein content gradually decreased, and there was no significant difference at the flowering stage, but sterile line 9A was lower than maintainer line 9B at the flower bud stage and the alabastrum stage, with a significant difference, and the soluble protein content of 9B was 1.08 times and 1.09 times of that of 9A, respectively. It showed that the flower bud stage and the alabastrum stage might be the period when sterility occurred.
From the flowering bud stage to the flowering stage, the soluble sugar contents of sterile line 9A and maintainer line 9B showed the same trend, i.e., an increasing trend. In the process of floral organ development, the soluble sugar content of 9B was higher than that of 9A, but the difference was not significant at the flower bud stage and alabastrum stage; and the values of the two reached at the flowering stage their maximums, as high as 11.33 and 16.15 mg/g FW, respectively, showing a significant difference, and the value of 9B was 1.43 times that of 9A. It indicated that soluble sugar played a key role in fertility in the later period of floral organ development, and was related to the period when sterility occurred.
During the whole development process of floral organs, the free proline contents of sterile line 9A and maintainer line 9B both showed an inverted U-shaped change trend. With the development of floral organs, the content of free proline in the alabastrum stage was higher than those in the flower bud stage and alabastrum stage, with significant differences. Moreover, At the flower bud stage, 9A was significantly higher than 9B, and the value was 1.37 times that of 9B, while at the alabastrum stage and the flowering stage, 9B was significantly higher than 9A, and the value was 1.30 and 1.40 times that of 9A, respectively, indicating that the significant reduction of free proline content in the sterile line might lead to male sterility.
Changes of endogenous hormone contents at different stages of floral organ development
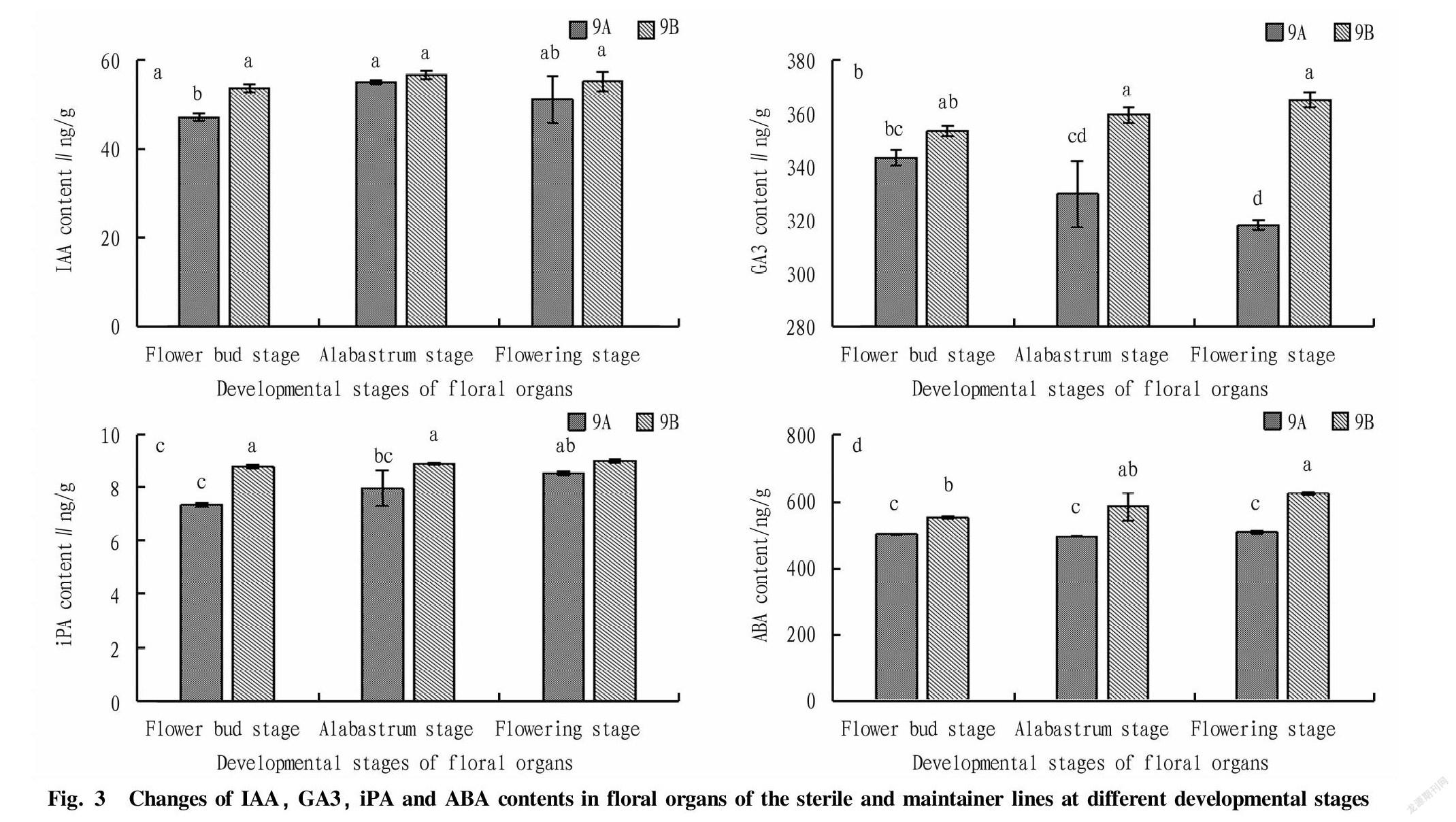
As shown in Fig. 3, the IAA contents of sterile line 9A and maintainer line 9B showed the same change trend, i.e., an inverted U-shaped change, and both reached at the alabastrum stage their maximum values, which were 55.12 and 56.73 ng/g·FW, respectively, but the difference was not significant. In the process of floral organ development, the IAA content of 9B was higher than that of 9A, and a significant difference was reached at the flower bud stage, when the IAA content of 9B was 1.14 times that of 9A, but there were no significant differences at the alabastrum stage and the flowering stage. It was inferred that the lack of IAA at the flower bud stage might lead to the occurrence of sterility.
During the development of floral organs, the contents of GA3 in sterile line 9A and maintainer line 9B showed different trends. 9A showed a gradually decreasing trend, while 9B showed a gradually increasing trend. In the process of floral organ development, the GA3 content of 9B was higher than that of 9A, and there were significant differences at the alabastrum stage and the flowering stage, when 9B was 1.09 times and 1.15 times of 9A, respectively, indicating that the content of GA3 played an important role in the fertility of soybean, especially in the later stage of floral organ development, which might be the period when sterility occurred.
The iPA content of sterile line 9A and maintainer line 9B showed a gradual upward trend during the floral organ development period, and both reached at the flowering stage their maximum values, without a significant difference, but the difference was significant at the flower bud stage and the alabastrum stage. Specifically, the value of 9B was 1.20 times that of 9A at the flower bud stage; and the value of 9B reached 1.11 times that of 9A at the alabastrum stage, indicating that the male sterility of soybean might occur at the flower bud stage or alabastrum stage.
From the flower bud stage to flowering stage, the ABA contents of sterile line 9A and maintainer line 9B showed completely different trends. 9A showed a U-shaped change trend, while 9B gradually increased with the development of floral organs. During the development of floral organs, the ABA content of 9B was significantly higher than that of 9A, and 9B was 1.10 times, 1.18 times and 1.23 times of 9A at the flower bud stage, alabastrum stage and flowering stage, respectively, while sterile line 9A had no significant difference in the development of floral organs, indicating that the accumulation of ABA affected the normal development of the floral organs of sterile line 9A at the later stage, resulting in male sterility, and the transformation of fertility affected the accumulation of ABA in plants.
Balanced relationship between endogenous hormones at different stages of floral organ development
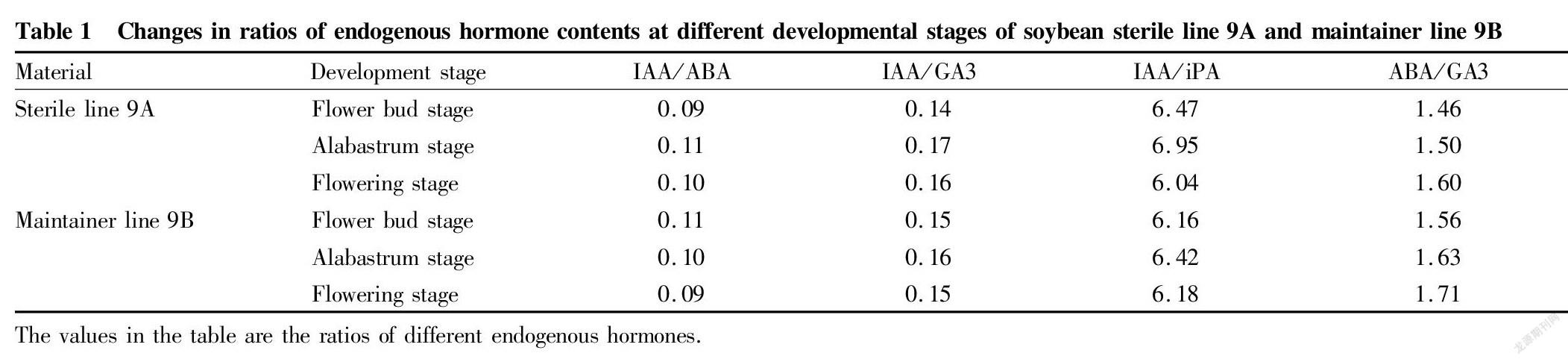
It can be seen from Table 1 that with the development of floral organs, the ratio of IAA/ABA of sterile line 9A first increased and then decreased, and the change trend of the ratio of maintainer line 9B was significantly different from that of 9A, and was a gradually decreasing trend. The ratio of IAA/ABA of 9B was higher than that of 9A at the flower bud stage, but lower than that of 9A at other two stages.
For IAA/GA3, the ratios of sterile line 9A and maintainer line 9B both reached their maximum values at the alabastrum stage, and the change trends of the two were the same, and were both increasing first and then decreasing. The IAA/GA3 value of sterile line 9A was lower than that of maintainer line 9B at the flower bud stage, but higher than that of maintainer line 9B at the alabastrum stage and flowering stage, though the differences were small. The average difference was 0.01.
The IAA/iPA values of sterile line 9A and maintainer line 9B both increased first and then decreased during the floral organ development, and the ratios of the two increased significantly at the alabastrum stage; the ABA/iPA value of sterile line 9A was the lowest at the flowering stage, while that of maintainer line 9B was the lowest at the flower bud stage; and the ABA/iPA value of sterile line 9A had a significant difference between the flowering bud stage and flowering stage, while the difference in the ratio of maintainer line 9B was smaller, at 0.02. The IAA/iPA value of sterile line 9A was significantly higher than that of maintainer line 9B at the flower bud stage and alabastrum stage, and the IAA/iPA value of sterile line 9A dropped sharply at the flowering stage, and was significantly lower than that of maintainer line 9B.
With the development of floral organs, the ABA/GA3 values of sterile line 9A and maintainer line 9B both reached at the flowering stage their maximums, which were 1.60 and 1.71, respectively. The ABA/GA3 values of the two both increased gradually with the development of floral organs. The ABA/GA3 value of sterile line 9A was lower than that of maintainer line 9B at various stages.
Conclusions and Discussion
Relationship between peroxidase activity and male sterility
Research reports indicate that SOD, POD and CAT have the effect of scavenging toxic oxygen in plants, thereby maintaining the dynamic balance in plants and enabling plants to carry out normal physiological and biochemical activities and normal development[22-24]. Jiang et al.[25] studied the physiological and biochemical characteristics of the sterile plants of Camellia crassicolumna Chang, and found that the POD activity of sterile plants showed an upward trend during the development of flower buds, and was higher than that of fertile plants, and the activity of SOD and CAT in sterile plants increased first and decreased then, and was significantly lower than fertile plants. The study of Wang et al.[26] found that the activity of SOD and POD in sterile watermelon plants was higher than that in fertile plants, while the activity of CAT was lower than that in fertile plants. Yang et al.[27] studied the flower buds of a sterile soybean line and found that the POD activity of the sterile line increased significantly and was significantly higher than that of the homotype maintainer line. Zhang et al.[28] studied the sterile lines of maize and found that the activity of SOD and POD in sterile plants were significantly higher than those in fertile plants, and the CAT activity was lower than that in fertile plants. The results of this study showed that during the development of soybean floral organs, the activity of SOD and CAT in the sterile line showed a downward trend, and the activity of SOD and CAT in the homotype maintainer line showed a trend of first increasing and then decreasing; the activity of SOD and CAT in the sterile line was higher than that of the maintainer line at the flower bud stage, but lower than that of the maintainer line at the alabastrum stage and flowering stage; and the POD activity of the sterile line was lower than that of the maintainer line at the flower bud stage, but significantly higher than that of the maintainer line at the alabastrum stage and flowering stages. The results are similar to the results of Jiang et al., who found that the POD activity of sterile C. crassicolumna plants was lower than that of fertile plants during flower bud development[25]. However, the results of this study found that the POD activity of the sterile line at the alabastrum stage and flowering stage was higher than that of the maintainer line. It is speculated that it may be related to different fertility of different crops, and the specific reasons need to be further studied.
Relationship between nutrient contents and male sterility
In the development of plant floral organs, proteins, amino acids, malondialdehyde, starch and saccharides are important substances, which have an important impact on the formation of sterile lines. Abnormalities and abortions of anthers and pollen in plant floral organs are related to the deficiency of these nutrients[29]. The soluble protein content and proline content of sterile pepper lines were significantly lower than those of fertile lines[30], which is consistent to the result of this study that the soluble protein content of the sterile soybean line was lower than that of its homotype maintainer line. The proline content was higher than that of the maintainer line at the flower bud stage, but lower than that of the maintainer line at the alabastrum stage and the flowering stage, which might be related to the crop type and sampling period. Zhang et al.[28] found that the soluble sugar content of sterile maize plants was significantly lower than that of fertile plants, which is consistent with the result of this study. Wang et al.[31] found that the starch content of sterile plants of Medicago sativa L. was lower than that of fertile plants during alabastrum development, which is consistent with the result of this study, indicating that the deficiency of starch content can cause abnormal floral organ development, resulting in infertility. Based on this, it can be speculated that the deficiency of starch content during the floral organ development can lead to the abortion of soybean floral organs, thus leading to the occurrence of sterility. As the final product of lipid peroxidation, MDA can cause the polymerization of life macromolecules such as proteins and nucleic acids, making cells toxic, thereby affecting the fertility of floral organs[32]. In this study, it was found that in the flower bud stage, alabastrum stage and flowering stage, the accumulation of MDA in the sterile line was more, and it gradually increased with the development of floral organs, and was higher than that of the maintainer line, especially in the flowering stage, when the content of MDA was 1.41 times that of the maintainer line, indicating that the content of MDA was closely related to the fertility of floral organs, and high accumulation was more likely to cause the abortion of floral organs.
Relationship between endogenous hormone contents and male sterility
Auxin, gibberellin and isopentenyl adenosine all have the effects of promoting cell growth and regulating nutrients in cells, while abscisic acid can inhibit cell growth. The contents of the above hormones all play an important role in plant male sterility[33]. Wang et al.[26] found that the contents of IAA, GA3 and iPA in sterile watermelon plants were severely deficient, and were significantly lower than those in fertile plants during the development of microspores. Similar results have been obtained in male sterility studies on cabbage[34] and wheat[35]. The results of this study are similar to those of Wang et al. During the development of soybean floral organs, the contents of IAA, GA3 and iPA in the sterile line were lower than those in the homotype maintainer line, which resulted in the abortion of 9A. Wang et al.[36] found that the contents of IAA and GA3 in sterile cotton plants were relatively low, while the content of ABA was relatively high. The results of this study found that the content of ABA in the sterile line was lower than that of the homotype maintainer line at various stages. The difference may be related to the difference in the stage that sterility occurs.
Relationship between hormone ratios and male sterility
Hormones in plants are not individual individuals, but have mutual promotion and antagonism, and the interaction between hormones can lead to plant sterility[37]. The results of this study showed that the ratio of IAA/ABA in the sterile line was higher than that of the homotype maintainer line at each stage of floral organ development; the ratio of IAA/GA3 was lower in the sterile line than in the maintainer line at the flower bud stage, and it was just opposite at the alabastrum stage and flowering stage; the ratio of IAA/iPA was higher in the sterile line than in the maintainer line at the alabastrum stage and flower bud stage, but the opposite was true at the flowering stage; and the ratio of ABA/GA3 showed an increasing trend with the development of floral organs in both the sterile line and the maintainer line, and the sterile line was lower than that of the maintainer line. For ABA/GA3, the ratios of the sterile line and its homotype maintainer line were gradually increasing at each stage, and the ratio of the sterile line was lower than its homotype maintainer line at each stage. Sun et al.[38] analyzed the ratios between hormones in flower buds of sterile radish plants and found that the ratios of IAA/ABA, IAA/GA3 and GA3/ABA were inconsistent between sterile plants and fertile plants. Liu et al.[33] found through their study on sterile sesame plants that the change trends of IAA/ABA, IAA/SA and IAA/JA in sterile and fertile plants were quite different. It can be seen that the ratios of hormones are related to the type of crops, and the changing trends of the ratios of different crops were different.
In conclusion, the activity of SOD and CAT in the sterile soybean line was lower than that of the maintainer line during the floral organ development, while the POD activity gradually increased with the formation of floral organs; the accumulation of MDA in the sterile line was higher than that in the maintainer line; the contents of soluble sugar, soluble protein and starch were significantly lower than those in the maintainer line; and the content of free proline showed an increasing change; the contents of IAA, GA3, iPA and ABA in the sterile line were significantly lower than those in the maintainer line, and the hormone ratios were quite different, which caused the imbalance of hormones, so the abortion of sterile line 9A was related to the lack of hormones during the floral organ development. Therefore, it is speculated that the above results were affected by the cytoplasmic-nuclear male sterility of soybean. After the floral organs developed to the alabastrum stage, the contents of SOD, CAT, MDA, starch, soluble sugar, proline, GA3 and ABA and other physiological and biochemical indexes were significantly different between sterile line 9A and maintainer line 9B. The alabastrum stage may be the critical period for the occurrence of abnormal physiological and biochemical indexes in cytoplasmic-nuclear male sterile lines of soybean. The results of this study only reflect the trends of physiological and biochemical changes during the floral organ development process of the soybean cytoplasmic-nuclear male sterile line, aiming to provide a theoretical basis for further research on the genetic mechanism of soybean cytoplasmic-nuclear male sterility, which is of great significance to the practical application of soybean cytoplasmic-nuclear male sterile lines in the future.
References
[1] WANG SM, WANG YQ, LI JP, et al. Seed production of soybean cytoplasmic male sterile (CMS) line under field conditions[J]. Soybean Science, 2010, 29(3): 385-389. (in Chinese).
[2] MA XD, XING ZZ. Advanced study and utilization of the male sterility in cotton[J]. Cotton Science, 2006(5): 309-314. (in Chinese).
[3] CHEN M, YIN C, LIU Y, et al. Fertility conversion factors and physiological and biochemical characteristics in thermo-sensitive genic male-sterile (TGMS) rice[J]. Crop Research, 2013, 27(2): 164-168. (in Chinese).
[4] LU MH, SUN WC, KONG DJ, et al. Physiobiochemical characteristics and stamen development characteristics of LRCMS flower in winter rapeseed (Brassica campestris)[J]. Acta Botanica Boreali-Occidentalia Sinica, 2014, 34(3): 509-515. (in Chinese).
[5] LIU QY, LIU FH, HE KX, et al. A review of progress in cytomorphology, physiology and biochemistry of male sterility in tobacco[J]. Subtropical Plant Science, 2004(1): 69-72. (in Chinese).
[6] TANG WH, ZHANG SN, KONG YE, et al. Comparison on physiological and biochemical properties of pol CMS lines and their maintainer lines in different ploid materials of non-heading Chinese cabbage[J]. Acta Botanica Boreali-Occidentalia Sinica, 2009, 29(1): 80-84. (in Chinese).
[7] KONG YE, ZHANG HM, HAN YF, et al. Study on physiological and biochemical characters of tetraploid MtCMS and its maintainer line[J]. Acta Agriculturae Jiangxi, 2011, 23(7): 12-15. (in Chinese).
[8] WANG SQ, CUI HM, SHI GL. Analysis on physiological and biochemical characteristics of Ogura CMS line and its maintainer line in Brassica rapa L. ssp. pekinensis[J]. Xinjiang Agricultural Sciences, 2011, 48(1): 110-115. (in Chinese).
[9] ZOU J, LIN WH, LUO HB, et al. Comparison the activities of peroxidase, catalase, superoxide dismutase and peroxidase zymogram in C-cytoplasmic male sterility line on maize[J]. Journal of Maize Sciences, 2009, 17(6): 45-49. (in Chinese).
[10] HU XJ. Study on the interaction of several active substances with superoxide dismutase[D]. Hefei: Anhui?University, 2012. (in Chinese).
[11] FENG XL, FAN GY, SU X, et al. Advances in physiological and biochemical study on plant male sterility[J].Crops, 2012(3): 6-11. (in Chinese).
[12] HE CZ, XIAO LT, LIU ZM, et al. The research progresses in the relationship between phytohormones and plant male sterility[J]. Chinese Agricultural Science Bulletin, 2002, 18(3): 65-69. (in Chinese).
[13] YU JZ, JIAN L. Study on the allelism test of Shennong male sterile soybean L-78-387[J]. Journal?of Shenyang Agricultural University, 1985, 16(4): 19-24. (in Chinese).
[14] YANG SP, ZENG WY, DUAN MP, et al. SSR marker location of male sterile gene of nuclear male sterile mutant NJ89-1 in soybean[J]. Soybean Science, 2006, 25(4): 344-347. (in Chinese).
[15] ZHAO TJ, GAI JY. Detection and identification of soybean natural variation of sterility[J]. Scientia AgriculturA Sinica, 2006, 39(9): 1756-1764. (in Chinese).
[16] WEI BG. A preliminary report on the discovery of photo-and thermo-sensitive male sterile lines in soybean[J]. Zuowu Pinzhong Ziyuan, 1991(3): 12. (in Chinese).
[17] PENG YH, YANG GB, YUAN JZ, et al. Characteristic analysis of a planting timesensitive male sterile soybean[J]. Acta Agronomica Sinica, 1998, 24(6): 1010-1013. (in Chinese).
[18] WANG F, WEI BG, LI GQ, et al. A cytological observation of the pollen mother cells of the photoperiod-sensitive male sterile soybean plant of 88-428BY-827[J]. Scientia Agricultura Sinica, 2004, 37(8): 1110-1013. (in Chinese).
[19] SUN H, ZHAO LM, HUANG M. Study on the cytoplasmic-nuclear male sterile line of soybean[J]. Chinese Science Bulletin, 1993, 38(16): 1535-1536. (in Chinese).
[20] ZHANG L, DAI OH. Selection and breding of nucleo-cytoplasmic male sterile line W931A in soybean[J]. Scientia Agricultura Sinica, 1997(6): 90-91. (in Chinese).
[21] GAI JY, DING DR, CUI ZL, et al. Development and performance of the cytoplasmic-nuclear male sterile line NJCMS1A of soybean[J]. Scientia Agricultura Sinica, 1999, 32(5): 23-27. (in Chinese).
[22] ZHANG WJ, LIU L, HUANG ZL, et al. Effect of low temperature on antioxidative enzymes activity and endogenous hormone content in wheat root of rice-wheat rotation[J]. Journal of Triticeae Crops, 2016, 36(4): 501-506. (in Chinese).
[23] LIU SJ, ZHU Q, XING XJ, et al. Advanced research on male sterility of plant[J]. Chinese Agricultural Science Bulletin, 2014, 30(34): 46-50. (in Chinese).
[24] O’BRIEN JA, DAUDI A, BUTT VS, et al. Reactive oxygen species and their role in plant defence and cell wall metabolism[J]. Planta, 2012, 236(3): 765-779.
[25] JIANG HB, XU Y, SONG WX, et al. Physiological and biochemical changes during bud development in male sterile plant of Camellia crassicolumna[J]. Plant Physiology Communications, 2020, 56(9): 1807-1817, 1807-1817. (in Chinese).
[26] WANG YQ, YANG XZ, MO YL, et al. Analysis of the changes in antioxidant enzymes activities and endogenous hormones contents in watermelon male sterile line Se18 during bud development[J]. Acta Horticulturae Sinica, 2016, 43(11): 2161-2172. (in Chinese).
[27] YANG LS, LI JJ, HE TT, et al. Comparative analysis of physiological and biochemical characteristics between cytoplasmic-nuclear male sterile line and its maintainer in soybean[Glycine max (L.) Merr.][J]. Soybean Science, 2017, 36(3): 391-398. (in Chinese).
[28] ZHANG Q, JIN SH, FANG F, et al. Physiological and biochemical metabolism studies on the photo-thermo-sensitive male sterile line of maize CB1208-82[J]. Journal of Maize Sciences, 2019, 27(3): 48-53. (in Chinese).
[29] SHENG YY, CHANG W, JIAO SQ, et al. Stamen structure development and physiological and biochemical characteristics in male sterile melon[J]. Plant Physiology Journal, 2016, 52(7): 1028-1034. (in Chinese).
[30] FENG WP, ZHOU SD, YANG BZ, et al. Sterility characteristics and the genetic law of a reverse thermo-sensitive nuclear male sterile mutant E6421S in peppers[J]. Acta Horticulturae Sinica, 2019, 46(6): 1112-1122. (in Chinese).
[31] WANG Y, WANG YZ, XU AK, et al. A Comparative analysis of physiological and biochemical characteristics between alfalfa cytoplasmic male sterile line and maintainer line[J]. Chinese Journal of Grassland, 2018, 40(1): 24-34. (in Chinese).
[32] ZHENG BB, FANG YN, PAN ZY, et al. iTRAQ-based quantitative proteomics analysis revealed alterations of carbohydrate metabolism pathways and mitochondrial proteins in a male sterile cybrid pummelo[J]. J Proteome Res, 2014, 13(6): 2998-3015.
[33] LIU HY, WU K, YANG MM, et al. Variation of soluble sugar, starch and plant hormones contents in sesame dominant genic male sterile line during bud development[J]. Chinese Journal of Oil Crop Sciences, 2014, 36(2): 175-180. (in Chinese).
[34] WANG YQ. Analysis of fertility gene expression characteristics of Chinese cabbage dual-purpose nuclear sterile lines and isolation of microspore development-related genes[D]. Hangzhou: Zhejiang University, 2003. (in Chinese).
[35] ZHANG ZG, MA XF, ZHANG HX, et al. Relationship between fertility transition of thermo-photo-sensitive wheat male sterile line BNS and endogenous hormone contents in its developing ear[J]. Journal of Plant Genetic Resources, 2016, 17(5): 913-919. (in Chinese).
[36] WANG XD. Overview of the study and application of cytoplasmic male sterility in cotton[J]. Scientia Agricultura Sinica, 2019, 52(8): 1341-1354. (in Chinese).
[37] CONG Q, ZHANG Q, SONG LL, et al. Roles of phytohormones on cold response in plants[J]. Journal of Nuclear Agricultural Sciences, 2016, 30(3): 614-619. (in Chinese).
[38] SUN XL, XU XY, ZHANG LG. Analysis on the changes of phytohormones during flower bud development in male-sterile radish[J]. Northern Horticulture, 2011(19): 11-15. (in Chinese).
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Expression Analysis of Heat Shock Protein 70 Gene in Rice (Oryza sativa L.)
- Research Progress on Lonicera japonica Thunb. Affected by Environmental Stress
- Research Progress on Genetic Diversity of Snap Bean
- Allelopathic Effects of Cedrus deodara Needle Extracts on Seed
- Development Status and Countermeasures of Passiflora spp. Seedling Industry in Qinzhou, Guangxi
- Pathogen Identification and Phylogenetic Analysis of Sugarcane

