Allelopathic Effects of Cedrus deodara Needle Extracts on Seed
Menghong HU Xun LYU Man SUN Baoquan ZHANG Yuan LIU
Germination and Early Growth of Three Turfgrasses
Abstract [Objectives] This study was conducted to provide reference for plant landscaping, optimization of planting structure and rational allocation of species in Cedrus deodara gardens. [Methods] With three common garden plants in northern China, Trifolium repens L., Poa pratensis L. and Trifolium pratense L., as receptors, the effects of four concentrations (0.025, 0.05, 0.075 and 0.10 g/ml) of C. deodara needle extract on seed germination and early seedling growth of the three turfgrasses were studied by the Petri dish filter paper method, using a clear water treatment (0 g/ml) as control check (CK). Data were subjected to analysis of variance (ANOVA) and multiple comparisons (Duncan) using SPSS16.0. [Results] Different concentrations of C. deodara needle extract had significant inhibitory effects on seed germination, radicle and seedling height growth of T. repens, T. pratense and P. pratensis (P<0.05), and the inhibitory effects increased with the increase of concentration. When the concentration reached 0.075 g/ml, seed germination and seedling growth of T. repens stopped, and radicle and seedling growth of T. pratense and P. pratensis also stopped. When the concentration was equal to 0.10 g/ml, germination of T. pratense and P. pratensis seeds was inhibited, and the absolute values of allelopathic index was the largest, that is, the inhibitory effects were the largest. And with the increase of concentration, the absolute value of synthetical allelopathic index also increased. The extract of C. deodara needles contained main allelochemicals leading to the scarcity of understory vegetation. [Conclusions] This study provides a theoretical basis for reasonable garden plant configuration during C. deodara greening and garden landscaping.
Key words Cedrus deodara L.; Needle extract; Seed germination; Early seedling growth; Allelopathic effect
Received: October 21, 2021 Accepted: December 23, 2021
Supported by Gansu Provincial Forestry and Grassland Bureau Science and Technology Project (2019KJ089); Tianshui Science and Technology Program Support Project (2020NCK2106).
Menghong HU (1965-), male, P. R. China, senior engineer, devoted to research about improved seed base management and spruce hybrid seed production.
*Corresponding author. E-mail: 648257429@qq.com.
Cedrus deodara, Pseudolarix amabilis, Araucaria cunninghamii, Sequoiadendron giganteum and Sciadopitys verticillata are known as the world’s five major garden trees, and are widely used in afforestation and landscaping of barren hills. Trifolium repens L., Trifolium pratense L. and Poa pratensis L. are the main grass species of temperate cool-season lawns, with the characteristics of long green period, beautiful grass appearance, long life, strong trampling resistance, and good overwintering performance. They are often planted with C. deodara for greening of roads and cities and garden landscaping. However, the growth of herbaceous plants in pure C. deodara forests or under a single C. deodara plant is severely inhibited, which leads to sparse vegetation and low biodiversity, and the soil under trees is largely bare, which seriously affects the effects of greening and garden landscaping. The reason for the sparse vegetation under C. deodara forests is not only related to the influence of light, but also related to the allelopathy of plants[1]. Allelopathy mainly refers to the fact that plants release their own chemical substances into the environment through stem and leaf volatilization, stem and leaf leaching, root exudation, and the decomposition of plant stubble, and disperse them into the air or soil, and plants use secondary metabolites as a medium to establish a stable chemical interaction relationship with the surrounding biological community, which has favorable or unfavorable effects on other plants or microorganisms in the surrounding environment[2-5]. Studies have shown that phenolic substances are a class of substances with strong allelopathic activity; C. deodara branches and leaves, litter, roots and rhizosphere soil contain phenolic substances, and the branches and leaves contain 52.78% of terpenes[1]. Terpenoids are common allelochemicals in nature, and they enter the environment through volatilization, leaching, secretion and decomposition of plant residues. Most allelochemicals are eventually integrated into the soil, and after a series of transformations, they have an effect on plant seeds and the soil ecological environment, causing significant changes in biological communities and ecosystems[1,6-9].
Seed germination and early seedling formation are the most fragile and sensitive stages in the life cycle of plants[10-11]. Allelopathic effect is mainly manifested in the impact on seed germination and seedling growth[12], resulting in changes in biodiversity, population structure, material cycling, ecosystem stability and ecological functions[13]. Seed germination rate, germination potential, germination index, radicle and seedling height growth and other indicators are the reflection of seed vigor, biodiversity and potential developmental ability of populations, and are the most commonly used indicators for allelopathy detection. Their laboratory determination and field test results are of significant significance for garden landscaping, adjustment and optimization of planting structure, and disease and pest control. Extracts of C. deodara litter, rhizosphere soil, branches and leaves have inhibitory effects on seed germination and seedling growth of Brassica rapa pekinensis and Lactuca sativa, and the branches and leaves have the strongest inhibitory effects[1]. Furthermore, the inhibitory effect of C. deodara leaf extract on seed germination and seedling growth of Lolium perenne L and other turfgrasses increased with the increase of the concentration[14]. It shows that the allelopathic effects of plants not only have a concentration effect, but also the allelopathic effects of different organ extracts are different[15]. In order to find out the effect of fresh needle extract of C. deodara in vigorous growth period on seed germination and early seedling growth of T. repens, T. pratense, and P. pratensis, the effects of different concentrations of C. deodara needle extract on seed germination and early growth of the three species of plants were measured in the laboratory, aiming to provide a reference for the rational selection of C. deodara landscaping species.
Materials and Methods
Experimental materials
In mid-June, southing branches of 1-2 years old were selected from the middle of 30-year-old C. deodara canopies, and C. deodara needles were picked (the needles were cut with scissors or a single-sided blade from the base close to the branches, put in plastic bags, sealed and brought back to the laboratory). The collected fresh C. deodara needles were washed with distilled water and dried naturally. From the dry sample, 100 g was weighed and crushed into small pieces of 3-4 mm. The crushed sample was added with 1 000 ml of distilled water, sealed with plastic wrap, and soaked at room temperature for 48 h, during which the sample liquid was shaken twice. After soaking, the liquid was filtered with gauze (sterilized filtration) to obtain a mother liquor with a concentration of 0.1 g/L, which was put into a sterilizing chamber for 2 h of sterilization. The extract was diluted with distilled water to 0.025, 0.050, 0.075, and 0.100 g/ml in turn, and the obtained solutions were stored in a refrigerator at 4 ℃ for later use.
Three kinds of herbal seeds, T. repens, T. pratense and P. pratensis, were purchased from the seed market of Gansu Academy of Agricultural Sciences (sold by Gansu Seed Industry Co., Ltd.).
Experimental methods
After soaking the seeds of T. repens, T. pratense and P. pratensis purchased from the market in clean water for 48 h, they were taken out and then sterilized with 0.1% KMnO4 solution for 15 min, and rinsed with sterile distilled water. Thirty full seeds of the same size were selected and put in petri dishes with a diameter of 9 cm sterilized by high temperature and high pressure and laid with two layers of filter paper, and extracts of different concentrations were added until the filter paper was completely soaked. The seeds treated with an equal amount of sterile distilled water were used as control check (CK), and each treatment was set with 3 replicates. The seeds were put in a light incubator set with a temperature of 25 ℃ and a light intensity of 2 000 Lux, and cultured with 12 h of irradiation (7:00-19:00) and 12 h of dark (19:00-7:00 the next day) every day. They were observed and recorded for the number of germinated seeds after every 24 h (seeds germinated when the radicle or hypocotyl broke through the seed coat by 2 mm), and browned or moldy seeds were picked out at any time until the germination rate of the seeds in the CK group did not change. The corresponding concentration of extract solution or the same amount of distilled water should be supplemented every day, and the supplementary amount should be judged as the amount when the filter paper was completely soaked and there was no water film around the tested seeds (about 1 ml). After 5 d of seed germination, 30 seedlings from each treatment were randomly selected, and their radicle growth and seedling height growth were measured with an electronic vernier caliper (the seedlings were all measured when the number of seedlings was less than 30) (0.01 mm).
Data processing
The absolute germination rate (GR), absolute germination potential (SP), germination index (GI) and the index of allelopathic effect (RI) of seeds treated in each batch were calculated. The index of allelopathic effect (RI) was calculated according to the calculation method of Williamson et al.[16]. SPSS16.0 was used for analysis of variance (ANOVA) and multiple comparisons (Duncan). Percentage data were arcsine transformed for ANOVA. The main calculation formulas and variance analysis models were:
GR=N1/(N-N0)×100%(1)
SP=Nt/(N-N0)×100%(2)
GI=∑(Gt/Dt)(3)
RI=1-C/T(T>C) RI=T/C-1(T≤C)(4)
∑RI=RIGR+RIGI+RISP+RIL+RIH(5)
xij=μ+αi+εij(6)
In the formulas: N, N1, N0, and Nt are the number of test seeds, the number of all germinated seeds, the number of browned and moldy seeds, and the number of germinated seeds when the seed germination reaches its peak, respectively; Gt is the germination number of the test seeds on day t, and Dt is the germination day corresponding to Gt; C is the value of the CK, and T is the treatment value; and xij is the jth observation value of the ith treatment, μ is the population mean, αi is the observation value of the ith treatment, and εij is the random error. RI<0 indicates inhibition, and if RI>0, it will be promotion. And the greater the absolute value of RI, the greater the intensity of the allelopathic effect[6].
Results and Analysis
Effects of different concentrations of C. deodara needle extract on seed germination of the three turfgrasses
Effects on germination rate
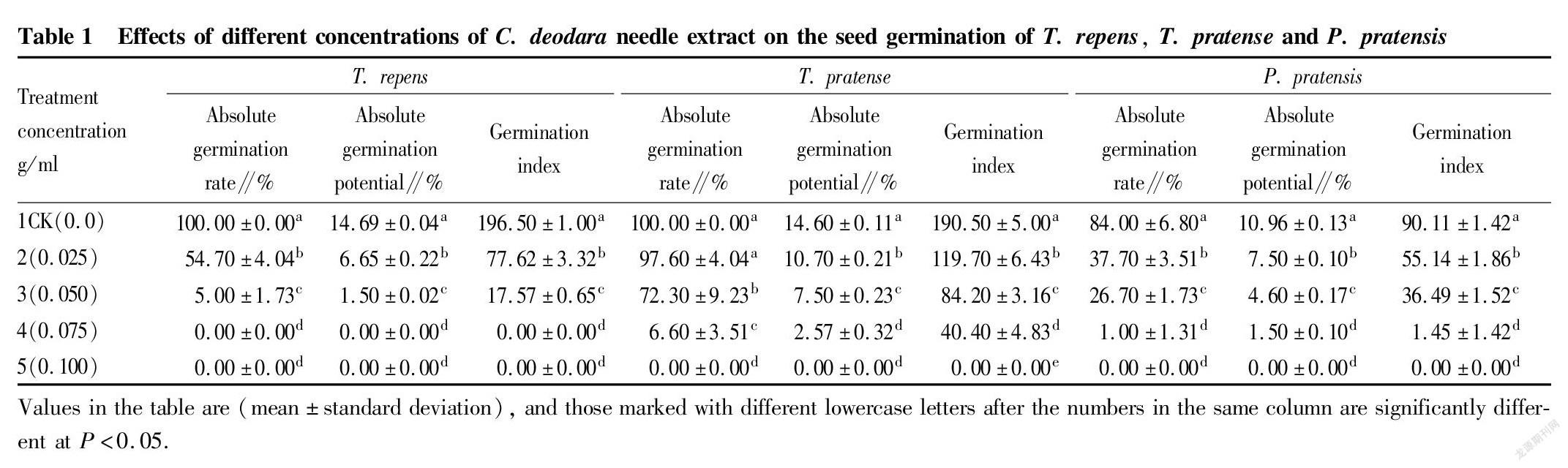
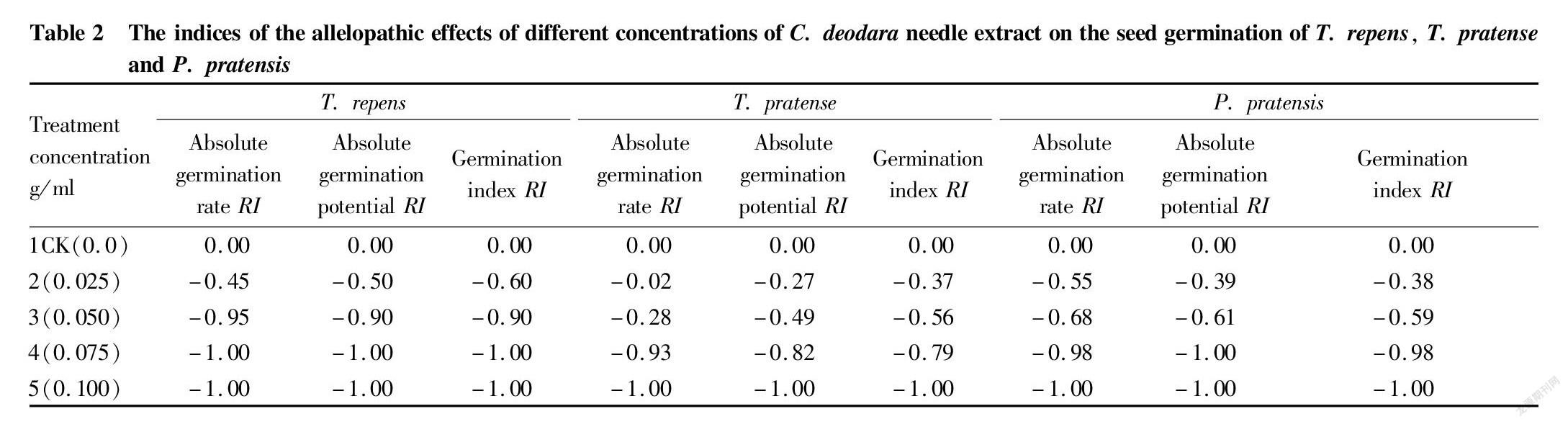
Seeds are the basis of plant reproduction, and their germination rate is one of the important indicators of seed quality. The level of germination rate reflects the potential ability of population growth to a certain extent. A higher germination rate indicates more viable seeds, and more seedlings will emerge after sowing, which is more conducive to the development of the population. The results of variance analysis showed that the effects of different concentrations of C. deodara needle extract on the absolute germination rates of T. repens, T. pratense and P. pratensis were significantly different (P<0.05) (Table 1). Multiple comparisons showed that except that the difference between treatment 2 and the CK of T. pratense was not significant and the differences between treatment 4 and treatment 5 of T. repens and P. pratensis were not significant (P>0.05), there were significant differences between other treatments. With the increase of the concentration of the extract, the absolute germination rates showed a decreasing trend, and the absolute values of allelopathic index showed an increasing trend with the increase of the concentration. When the concentration reached 0.075 g/ml, the absolute germination rate of T. repens was 0.0, and the allelopathic effect reached its maximum. When the concentration reached 0.10 g/ml, the absolute germination rates of T. pratense and P. pratensis were 0.0 (Table 2), that is, the allelopathic effects reached the maximum, and seed germination was inhibited. The results showed that different concentrations of C. deodara needle extract had inhibitory effects on the absolute germination rates of the three kinds of turfgrass seeds, and the higher the concentration, the stronger the inhibitory effects. When the concentration reached a certain level, it directly inhibited the germination of seeds.
Agricultural Biotechnology2022
Effects on germination potential
The absolute germination potential of seeds is also one of the indicators reflecting the quality of seeds. Under normal circumstances, when the germination rate is the same, the level of germination potential determines the uniformity of emergence. Higher germination potential means stronger vitality of the seeds, and seedlings will emerge uniformly and grow consistently, otherwise the seedlings are non-uniform. The results of variance analysis showed that the effects of different concentrations of C. deodara needle extract on the absolute germination potential of the three kinds of turfgrass seeds were significantly different (P<0.05). The results of multiple comparisons showed that there were no significant differences between treatments 4 and 5 of the three kinds of turfgrass seeds, but other treatments had significant differences, and the absolute germination potential of the CK was the highest. The absolute germination potential of the CK of T. repens was 8.79 times that of treatment 3, and the absolute germination potential of the CK of P. pratensis and T. pratense was 6.31 and 4.68 times that of treatment 4, respectively, and significantly higher than other treatments. With the increase of the concentration of the extract, the absolute germination potential of the three kinds of turfgrass seeds showed a decreasing trend (Table 1). When the concentration reached 0.075 g/ml, the absolute germination potential of T. repens was 0.0, and the absolute value of allelopathic effect reached the maximum. When the concentration reached 0.10 g/ml, the absolute germination potential of P. pratensis and T. pratense was 0.0, and the absolute values of the allelopathic effect reached a maximum value of 1.0 (Table 2). Different concentrations of C. deodara needle extract inhibited the absolute germination potential of the three kinds of turfgrass seeds. Such inhibitory effect increased with the increase of the extract concentration, and when the concentration reached a certain level, the inhibitory effect reached its maximum and directly inhibited the germination of seeds.
Effects on germination index
The germination index is also one of the important indicators reflecting seed vigor. The higher the germination index, the higher the seed vigor. The results of variance analysis and multiple comparison showed that the effects of different concentrations of C. deodara needle extract on the germination index of the three kinds of turfgrass seeds were significantly different (P<0.05). The results of multiple comparisons showed that the germination index of the CK was significantly higher than that of other treatments. There were no significant differences in germination index between T. repens and P. pratensis treatments (P>0.05), but there were significant differences between other treatments (Table 1). There were no significant differences in germination index between treatments 4 and 5 of T. repens and P. pratensis (P>0.05), but there were significant differences between other treatments (Table 1). When the concentration reached 0.075 g/ml, the germination potential of T. repens was 0.0, and the absolute value of allelopathic effect reached a maximum of 1.0 (Table 2). When the concentration was equal to 0.10 g/ml, the germination indexes of T. pratense and P. pratensis seeds were 0.0, and the value of the index of allelopathic effect was the largest (Table 2). With the increase of the concentration of the extract, the allelopathic effect was enhanced, and when the concentration reached a certain level, the seed germination index tended to be 0.0, which directly inhibited the vigor of the seeds.
Effects of different concentrations of C. deodara needle extract on the early growth of the three kinds of turfgrass seedlings
Effects on radicle growth
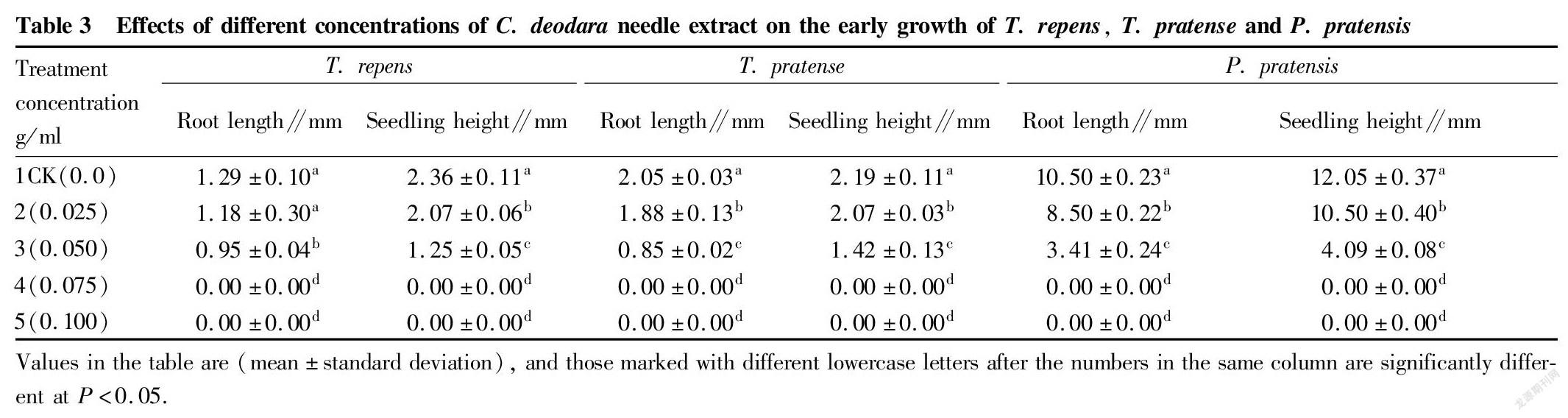
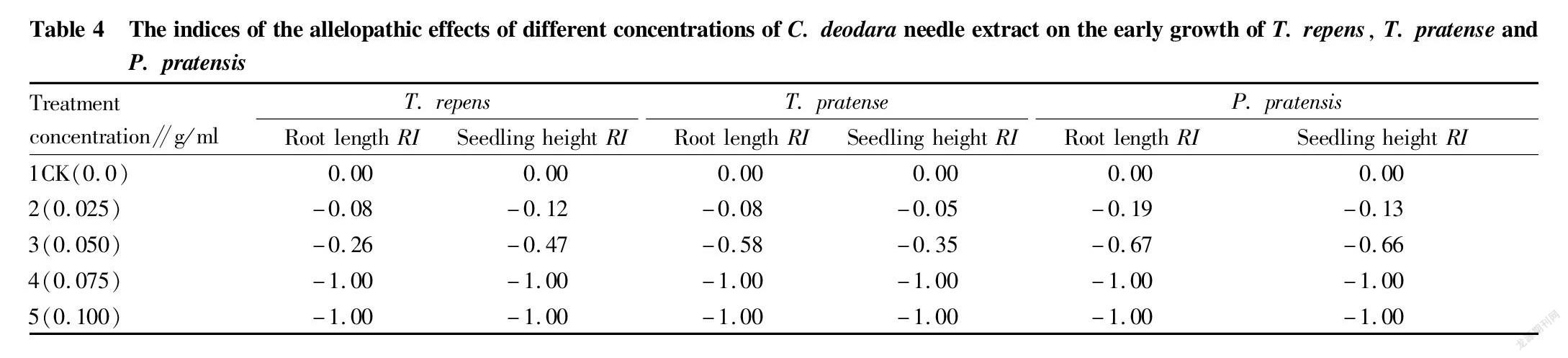
Radicles are an important vegetative organ in the process of plant growth, and they are the premise of the vegetative growth of plants. The growth and development of radicles directly affects the water absorption and fertility of plants, as well as the future growth and development of plants and their competitiveness in the ecosystem. The results of variance analysis and multiple comparisons showed that different concentrations of C. deodara needle extracts caused no significant differences in the effects on the radicle growth of the three turfgrasses between treatments 4 and 5, and no significant difference between treatments 1 and 2 of T. repens (P>0.05), but significant differences between other treatments (P<0.05). The results of multiple comparison showed that the radicle growth of the CK of T. repens, T. pratense and P. pratensis was the largest, reaching (1.29±0.10), (2.05±0.03) and (10.5±0.23) mm, respectively, which were significantly higher than treatment 3 by 0.36, 1.41 and 2.08 times (Table 3). The radicle growth of the three turfgrasses decreased with the increase of the concentration of the extract, and the allelopathic effects increased with the increase of the concentration of the extract. When the concentration reached 0.075 g/ml, the allelopathic indexes of the radicle growth of the three turfgrasses reached a maximum value of 1.0 (Table 4), which inhibited the growth of radicles of the three turfgrasses, and the radicle growth was 0.0 (Table 3). The inhibition of radicle growth will lead to poor root development and smaller and shorter root system with reduced water and fertilizer absorption capacity, resulting in short and weak plants and reduced competitiveness for light, which will directly affect the future growth and development of plants and their position and role in plant communities.
Effects of seedling height
The results of variance analysis and multiple comparisons showed that the effects of different concentrations of C. deodara needle extracts on the growth of the three kinds of turfgrass seedlings had significant differences. Except for treatment 4 and treatment 5 of the three kinds of turfgrass seedlings, which showed no significant differences (P>0.05), and there were significant differences between other treatments (P<0.05). The seedling height of the CK was significantly higher than that of other treatments, and the seedling heights of the CK of T. pratense, T. repens and P. pratensis were significantly higher than those of treatment 3 by 6.0%, 14.0% and 15.0%, respectively, and significantly higher than those of treatment 4 by 54.0% and 89.0% and 195.0%, respectively (Table 3). With the increase of the concentration of the extract, the growth of seedling height tended to decrease, and the allelopathic effects of different concentrations of the extract on the growth of seedlings tended to increase with the increase of the concentration. When the concentration was equal to 0.075 g/ml, the absolute values of the allelopathic index were the largest (Table 4), and the growth of the seedling height was 0.0, that is, the growth was stopped. When the growth of seedling height is inhibited, the growth is reduced or even stops, and when the growth of seedlings is poor, the competition of participating species for light, heat, water, and fertilizer will be weakened, and the growth of plants and the ability to compete and survive in the ecosystem will be reduced, which will lead to a decline in the importance and diversity of species in the population.
Synthetical allelopathic effect on seed germination and seedling growth
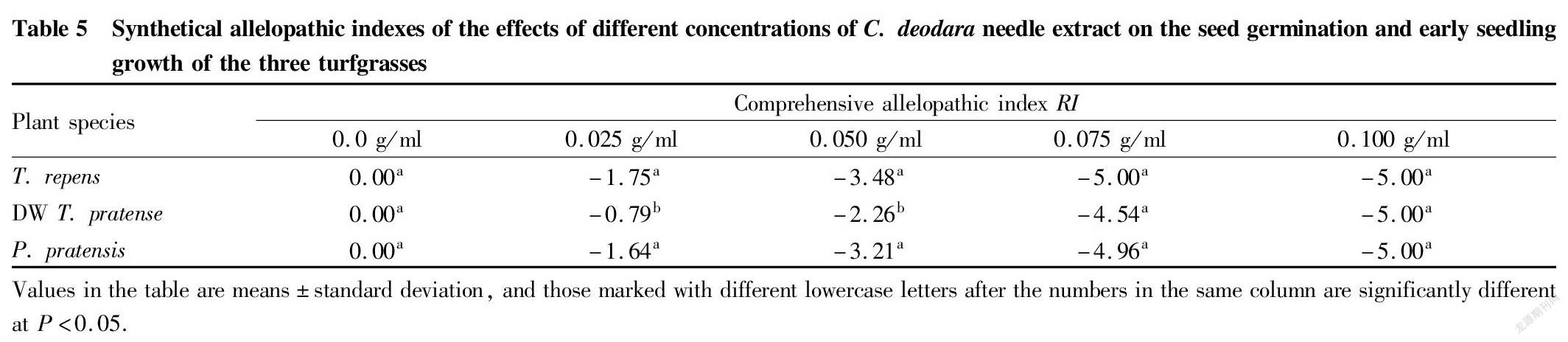
The value of the synthetical allelopathic index reflects the strength of the synthetical allelopathic effect on seed germination and early seedling growth. When the synthetical allelopathic index > 0.0, it has a promoting effect on seed germination and growth, and on the contrary, it has an inhibitory effect. Moreover, the larger the absolute value, the stronger the effect. Compared with the CK, the synthetical allelopathic indexes of the three turfgrasses were all less than 0.0, and with the increase of the concentration of the extract, the absolute value of the allelopathic index showed an increasing trend, indicating that with the increase of the concentration of the extract, the allelopathic effects on seed germination and growth of the three turfgrasses were enhanced. When the concentration of the extract reached 0.075 g/ml, the allelopathic effect on T. repens reached its maximum, and when the concentration was 0.100 g/ml, the allelopathic effects on T. pratense and P. pratensis reached their maximums. When the allelopathic effect reached a maximum, seeds would no longer germinate and seedling growth ceased (Table 5). When the concentration of the extract was equal to 0.025 and 0.075 g/ml, the synthetical allelopathic indexes of T. repens and P. pratensis were not significantly different (P>0.05), but had significant differences with T. pratense (P<0.05). It can be seen that in addition to the concentration effect of allelochemicals in donor plants, the sensitivity of different recipient plants to the same concentration of the same allelopathic substance is different.
Conclusions and Discussion
In recent years, with the increasing of population growth and the reduction of land resources, species invasion and biodiversity decline and other environmental problems have become increasingly prominent, and plant allelopathy has gradually attracted the attention of ecologists from all over the world, and has become one of the most active fields of research in agroecology and chemical ecology[17-19]. A large number of studies have been carried out on plant allelopathy at home and abroad, and China has also conducted in-depth studies in the fields of ecosystems such as agriculture[20], forestry[21], gardening[22], and grassland[23]. Plant allelochemicals interact with soil microorganisms and soil enzymes[24], which affects the degradation of soil organic matter and the mineralization of nutrient elements, thereby causing changes in soil physicochemical properties[25], affecting seed germination and growth[14,26], leading to changes in species diversity, vegetation succession, material cycle and ecosystem stability[27], and resulting in an impact on ecological diversity and ecological functions. Therefore, the study of allelopathic effects is of great significance to agroforestry management, optimization of planting structure, crop production, disease and pest control and other aspects[1,27]. Existing studies have confirmed that C. deodara branches, roots and rhizosphere soil all have allelopathic effects, which increase with the increase of the concentration, and the allelopathic effects of the branches and leaves were the strongest[1,14]. The results of this study showed that the fresh needle extract in the vigorous growth period of C. deodara had significant inhibitory effects on seed germination and early seedling growth of the three turfgrasses, and the inhibitory effects increased with the increase of the concentration. When the concentration reached a certain level, the germination of seeds and the growth of radicle and seedlings stopped, and the inhibitory effect reached the maximum, showing a significant concentration effect (P<0.05). The vigorous growth period of C. deodara is the period when the synthesis and transportation of photosynthetic products in branches and leaves are the most intensified and the exudation and leaching of allelochemicals is the most, and it is also the key period for the growth of understory plants. During the vigorous growth period of C. deodara, most of the allelochemicals exude and are leached from needles fall on the surface of understory plants, and some fall to the ground and merge into the soil. The allelopathic substances falling on the plant surface will be washed by rainwater, and some of them will fall into the soil. Allelochemicals falling into the soil interact with C. deodara roots and rhizosphere soil allelochemicals, causing changes in soil animals, soil microorganisms, soil enzyme activity, and soil physicochemical properties. They, together with the allelochemicals falling on the plant surface, cause sparse vegetation under C. deodara canopies and low diversity, and most of the land is bare. Therefore, it can be inferred that fresh coniferous extract during vigorous growth period is the key driver of the sparse vegetation under C. deodara canopies.
The results of this study showed that when the concentration of C. deodara needle extract reached 0.075 g/ml, the seed germination and seedling growth of T. repens stopped, while when the concentration was equal to 0.100 g/ml, the seed germination and seedling growth of T. pratense and P. pratensis stopped. It can be seen that in addition to the concentration effect of allelochemicals of donor plants, the sensitivity of different recipient plants to different concentrations of the same allelochemical is different, and even for different indexes such as seed germination, radicle and seedling height growth of the same recipient plant, there are differences in the sensitivity to the concentration of allelochemicals, which is also concluded in the studies of Lycium barbarum Linn.[28], Gossypium spp[29], Camellia oleifera Abel.[30] and other species. Therefore, when choosing plant configuration for barren hills greening, ecosystem restoration, agroforestry and garden landscaping, it is necessary to select species with allelopathic substances that can promote their growth, and coniferous and broad-leaved mixed afforestation should be carried out. Moreover, the density should be adjusted appropriately to dilute the concentration of allelochemicals exuded or leached from C. deodara needles, and the concentration of water-soluble allelochemicals should be adjusted within a certain concentration range, so as to reduce the impact of allelochemicals on the survival and growth of plants under C. deodara forests and improve the greening and landscaping effects of barren hills.
In this study, the effects of C. deodara needle extracts with different concentrations on the seed germination and early growth of seedlings of three turfgrasses, P. pratensis, T. pratense and T. repens were investigated, in the vigorous growth period of C. deodara. Allelopathy is not only related to the concentration of allelochemical substances of donor plants and the species of recipient plants, but also to different tissues of donor plants, organ extracts, the extraction methods of extracts, and the extraction time of extracts[15,31]. Therefore, the effects of C. deodara needle extracts in different seasons on the germination and growth of understory plants should be studied in the future.
C. deodara needle extract had inhibitory effects on the seed germination and early seedling growth of P. pratensis, T. pratense and T. repens, and the inhibitory effects increased with the increase of concentration. When the concentration reached 0.075 g/ml, the early growth of the three turfgrasses stopped, and T. repens stopped germination. When the concentration was equal to 0.10 g/ml, the germination of T. pratense and P. pratensis seeds stopped, and the inhibitory effects reached their maximums. C. deodara needle extract is a key driver of understory vegetation scarcity.
References
[1] LI XF. Mechanism study on the effect of Cedrus deodara on the plant diversity under the trees[D]. Heifei: Anhui Agricultural University, 2015. (in Chinese).
[2] ZHAO LL, YANG TX, WEI AZ, et al. Allelopathy of aqueous extract of Zanthoxylum bungeanum leaves on four grass seeds[J]. Journal of Northwest Forestry University, 2017, 32(2): 150-154. (in Chinese).
[3] MALIK AU. Challenges and opportunities in allelopathy research: A bief overview[J].Journal of Chemical Ecology, 2000, 29(26): 2007-2009. (in Chinese).
[4] WARREN RJ, LABATORE A, CANDEIAS M. Allelopathic invasive tree (Rhamnus cathartica) alters native plant communities[J]. Plant Ecology, 2017(218): 1233-1241.
[5] HE F, CUI M, SUN Y, et al. Allelopathic effect of decomposed liquid of Robinia pseudoacacia leaf litter on three crops[J]. Journal of Northwest Forestry University, 2021, 3(2): 116-122. (in Chinese).
[6] MULLER CH, MULLER WH, HAINES BL. Volatile growth inhibitors production by shrubs[J]. Science, 1964(143): 471-473.
[7] ZHU ZC, LI XB, LI WY, et al. Allelopathic potential of soil with growth of alien plant species Melaleuca leucadendra Linn[J]. Ecological Science, 2019, 38(6): 131-135. (in Chinese).
[8] ZHAO XH, YANG DL, WANG H, et al. Effects of Flaveria bidentis invasion on soil nitrogen cycling and soil microbial biomass in different regions[J]. Acta Prataculturae Sinica, 2015, 24(2): 62-69. (in Chinese).
[9] HOU YP, LIU L, WANG X, et al. Allelopathic effects of aqueous extract of exotic plant Rhus typhina L. on soil micro-ecosystem[J]. Acta Ecologica Sinica, 2013, 33(13): 4041-4049. (in Chinese).
[10] GERALD RL. Bioassays in the study of allelopathy[J]. Journal of Chemical Ecological, 1984(10): 133-145.
[11] SONG WJ, GAO X, ZHANG FC, et al. Effects of aqueous extracts from 3 common plants on seed germination of Agropyron cristatum and Poa annua[J]. Journal of Northwest A&F University: Natural Science Edition, 2017, 45(3): 185-191. (in Chinese).
[12] LIU YJ, MENG ZJ, CHANG XH, et al. Allelopathic effects of Stellera chamaejasme on seed germination and seedling growth of alfalfa and two forage grasses[J]. Acta Prataculturae Sinica, 2019, 28(8): 130-138. (in Chinese).
[13] HE F, CUI M, SUN Y, et al. Allelopathic effect of decomposed liquid of Robinia pseudoacacia leaf litter on three crops[J]. Journal of Northwest Forestry University, 2021, 3(2): 116-122. (in Chinese).
[14] QI YX, ZHU HY. Effects of aqueous extracts of cedar leaves on the seed germination and seedling growth of three turf grasses[J]. Prataculture & Animal Husbandry, 2017(3): 41-43. (in Chinese).
[15] LI XX, LAI JL, YUE JH, et al. Allelopathy of Phyllostachys pubescens extract on the seed germination of Chinese fir[J]. Acta Ecologica Sinica, 2018, 38(22): 8149-8157. (in Chinese).
[16] WILLIAMSON GB, RICHANDSON D. Bioassays for allelopathy: Measuring treatment response with independent controls[J]. Journal of Chemical Ecology, 1988, 14(1): 181-187.
[17] CHEN ZH, WANG CH, XIAO XM, et al. Allelopathic effects of decomposing garlic stalk on some vegetable crops[J]. African Journal of Biotechnology, 2011, 10(69): 15514-15520.
[18] MANUEL J REIGOSA, NURIA PRDROL, LIUS GONZALEZ. Allelopathy: A physiological process with ecological implications[M]. Netherlands: Springer, 2006.
[19] Inderjit. Soil microorganisms: An important determinant of allelopathic activity[J].Plant and Soil, 2005(274): 227-236.
[20] MAIMAITIAIZEZI·MUHETAER, WANG D. Effects of different Sophora alopecuroides treatments on seed germination and seedling growth of "Bixiekeqi" melon[J]. China Vegetables, 2021, 4(3): 57-60, 67. (in Chinese).
[21] HE F, CUI M, SUN Y, et al. Allelopathic effect of decomposed liquid of Robinia pseudoacacia leaf litter on three crops[J]. Journal of Northwest Forestry University, 2021, 36(2): 116-122. (in Chinese).
[22] LI K, YANG L. Allelopathic effects of aqueous extracts from rhizosphere soil of Robinia pseudoacacia Linn on seed germination and seedling growth of five kinds of common garden plants[J]. Seed, 2019, 38(6): 115-120. (in Chinese).
[23] SONG WJ, GAO X, ZHANG FC, et al. Effects of aqueous extracts from 3 common plants on seed germination of Agropyron cristatum and Poa annua[J]. Journal of Northwest A&F University: Natural Science Edition, 2017, 45(3): 185-191. (in Chinese).
[24] DANG YQ, LIU CF, LIN L. Effects of solvent extracts from Sophoraa lopecuroides L. on sucrose synthase and acid invertase activities of Jiashi melon[J]. Northern Horticulture, 2017(9): 24-29. (in Chinese).
[25] ZHU ZC, LI XB, LI WY, et al. Allelopathic potential of soil with growth of alien plant species Melaleuca leucadendra Linn[J]. Ecological Science, 2019, 38(6): 131-135. (in Chinese).
[26] SONG L, PAN KW, WANG JC. Review on action mechanism of effects of allelochemicals on seeds germination[J]. World Sci-Tech R & D, 2006, 28(4): 52-57. (in Chinese).
[27] ZHAO YJ. The Importance and application prospect of allelopathy in the cultivation of medicinal plant[J]. Chinese Traditional and Herbal Drugs, 2000, 31(8): 8-84. (in Chinese).
[28] LI M, YAN XF, MA L, et al. Allelopathic inhibition of phenolic acids on germination of wolfberry (Lycium barbarum Linn.)[J]. Acta Ecologica Sinica, 2020, 40(6): 2072-2079. (in Chinese).
[29] LI LL, LIU JG, YAN P, et al. Allelopathic effects of different combinations of phenolic acid allelochemicals on cotton seed germination and seedling growth[J]. Ecological Science, 2019, 38(6): 115-119. (in Chinese).
[30] LIU ST, WANG N, LI JA. Allelopathy of Camellia oleifera extract on two forage grass[J]. Molecular Plant Breeding, 2020, 18(10): 3373-3381. (in Chinese).
[31] GAO J, CHENG X, HU BX, et al. Effects of pine needle extract on germination of Glycyrrhiza uralensis seeds[J]. Shaanxi Journal of Agricultural Sciences, 2020, 66(5): 54-58. (in Chinese).
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Expression Analysis of Heat Shock Protein 70 Gene in Rice (Oryza sativa L.)
- Changes in Physiological and Biochemical Characteristics of Floral Organ
- Research Progress on Lonicera japonica Thunb. Affected by Environmental Stress
- Research Progress on Genetic Diversity of Snap Bean
- Development Status and Countermeasures of Passiflora spp. Seedling Industry in Qinzhou, Guangxi
- Pathogen Identification and Phylogenetic Analysis of Sugarcane

