Development of Annexin V Technology for Removal of Dead
Jian ZHANG Qi QI Yonghou LIANG Zhimin DONG Gui WANG Xueyuan ZHOU



Spermatozoa from Bovine Spermatozoa and Its Application in Frozen Sexed Semen Production
Abstract [Objectives] This study was conducted to develop a molecular marker immunomagnetic bead sorting technology method that can specifically identify dead spermatozoa. [Methods] This study first confirmed the specific binding of Annexin V to dead bovine spermatozoa, and tried to remove dead spermatozoa in semen combining with the immunomagnetic bead technology, so as to improve the separation efficiency of target spermatozoa in the process of sex-controlled semen preparation on a flow cytometer. [Results] The spermatozoon motility, membrane integrity and mitochondrial activity after sorting and the rate of dead spermatozoa during the on-machine X/Y separation were all improved to different degrees (P<0.05), indicating that the technical process design could effectively remove some dead spermatozoa, and there was no significant effect on frozen sexed semen prepared from the separated X or Y spermatozoa (P>0.05), indicating that the technical process did not cause additional damage to the spermatozoa. [Conclusions] Combining the specificity of Annexin V with the immunomagnetic bead method could effectively remove dead spermatozoa from bovine spermatozoa, and significantly reduce the rate of dead spermatozoa in bovine permatozoa during sex-controlled separation (P<0.05). The method developed can effectively improve the production efficiency of frozen sexed semen of dairy cows, reduce the production cost, and promote the industrial application of the product.
Key words Bovine spermatozoon; Annexin V; Immunomagnetic beads; Frozen sexed semen
Received: November 5, 2021 Accepted: January 6, 2022
Supported by Targeted Poverty Alleviation Special Project of Hetao College (HYZX201955); Introduced Talent Scientific Research Start-up Fund of Hetao College (HYRC2019002).
Jian ZHANG (1987-), male, P. R. China, lecturer, devoted to research about mammalian reproductive biology and biotechnology.
*Corresponding author.
The sex-controlled breeding technology of frozen sexed bovine semen combined with artificial insemination can rapidly increase the number of high-quality cows and improve the economic benefits of cow breeding, and it is the most successful and effective breeding biotechnology method for the promotion and application of dairy cow breeding industrialization. At present, the rate of cow calves obtained by sex-controlled separation technology in the development of the dairy industry is as high as 85%-95%[1-2], and the sex-controlled separation technology has also achieved great success in the application of other species[3-4]. The yield of some production traits affected by sex can be fully utilized by artificially regulating the sex ratio through the sex control technology, thereby achieving the greatest economic benefits. And compared with traditional mating methods, frozen semen artificial insemination technology has the advantages of being convenient for long-term storage and transportation. However, compared with ordinary frozen semen, the high separator production cost and patent royalties are the reasons for the restriction to further promotion and application of frozen semen technology in the dairy industry. Therefore, maximizing the use efficiency of separators is the key to reducing the production cost of frozen sexed semen. With the latest high-speed flow cytometers, X or Y spermatozoon sorting with accuracy>90% can be achieved in most species, but because the technology must evaluate the DNA content of each spermatozoon, the total number of spermatozoa sorted by a single machine does not exceed 200 million per hour[5-7]. Meanwhile, in order to ensure the activity of target spermatozoa after sorting, dead and live spermatozoa are stained in different colors, and dead spermatozoa are removed after being identified by flow cytometry during the sorting process, which further limits the efficiency of target spermatozoon separation. With the increase of the rate of dead spermatozoa, the separation efficiency of spermatozoa by flow cytometry will decrease accordingly. On the contrary, reducing the rate of dead spermatozoa before separation on a flow cytometer will greatly improve the efficiency of spermatozoon separation by flow cytometry. Therefore, reducing the rate of dead spermatozoa before separation on a flow cytometer is of great significance to improve the separation efficiency and reduce the cost of frozen sexed semen technology.
Many studies have confirmed the existence of molecular markers in spermatozoa that are specific for dead spermatozoa, and spermatozoa with these markers cannot fertilize normally in vivo or in vitro. Phosphatidylserine (PS), which is turned from the inside of the normal spermatozoon membrane to the surface of the spermatozoon membrane, is a special molecular marker[8-9]. This marker can be recognized by the specific binding of Annexin V (AnnexinV) to PS. Because Annexin V is a class of phospholipid-binding proteins, it has high affinity with PS. However, normal PS does not have the function of passing through the complete cell membrane, so it is believed that the binding of annexin V to PS can only occur outside the spermatozoon plasma membrane, thereby specifically identifying dead spermatozoa[10-11]. Based on this hypothesis, in this study, a molecular marker immunomagnetic bead sorting technology that can specifically identify dead spermatozoa, effectively remove dead spermatozoa and reduce the rate of dead spermatozoa in spermatozoon samples during the separation on a flow cytometer, was developed, so as to further improve the separation efficiency of target spermatozoa.
Materials and Methods
Reagents
Unless otherwise mentioned, the reagents used in this study were from Sigma.
Semen collection
The semen of Holstein bulls (Breeding Bull Station of Inner Mongolia Saikexing Reproductive Biotechnology (Group) Co., Ltd.) was collected by the artificial vagina method. The spermatozoon motility rate and deformity rate were evaluated by phase contrast microscopy, and the spermatozoon density was measured by a spectrophotometer.
Spermatozoon motility test
The semen to be tested for motility was adjusted to 5.0×108 spermatozoa/ml, and then 10 μl was used for spotting and making slides, and the system was microscopically inspected and recorded. Two parallel samples were detected each time, and five fields of view were recorded for each sample, that is, one field of view in the middle, and four fields of view around, up, down, left, and right. Then, the spermatozoon motility of the semen to be tested at this time was obtained according to the ratio of the total number of linearly moving spermatozoa and the total number of spermatozoa in the 10 videos taken.
Annexin V-FITC staining of spermatozoa
Dead and live spermatozoa were separated and collected on a flow cytometer. Then, 20 μl of Annexin V-FITC was added to the collected dead and live spermatozoon samples, and after standing at room temperature for 10 min, they were washed once with PBS, and then re-suspended with 100 μl of PBS. Then, 10 μl was put on a clean glass slide, which was then covered with a coverglass, and observed for whether the spermatozoa were obviously stained under a fluorescent microscope, and the proportion of fluorescently stained spermatozoa was recorded. More than 200 spermatozoa were counted in each group of samples.
Removal of dead spermatozoa from bovine spermatozoa with different motility by immunomagnetic beads
Three bulls were selected, respectively, for each of the different spermatozoon motility ranges (<50%, 50%-70%, >70%), and semen was collected by the artificial vagina method. The semen samples were washed three times with PBS within 4 h of semen collection, and then the spermatozoon density was adjusted with PBS to a concentration of 107/ml. To a 100 μl of sample, 20 μl of Annexin V was added, and the obtained sample was mixed well, and stood at room temperature for 15 min. Then each group of samples was supplemented with 900 μl of PBS, followed by 10 μg of anti-Annexin V monoclonal antibody, and incubated at room temperature on a shaker for 30 min. The washed magnetic beads were added to each group of semen samples, 50 μl per group, and the samples were incubated at room temperature for 30 min. Then, each group of samples was adsorbed with magnets for 2 min, and the supernatant was transferred to obtain target spermatozoa obtained by separation. After sorting, indexes related to spermatozoa (motility, deformity rate, membrane integrity, mitochondrial activity and acrosome integrity rate) were then tested.
Spermatozoon deformity rate detection
Sperm deformity rate was counted by smearing according to Standard for Quality Inspection of Frozen Bovine Semen, and more than 200 spermatozoa were counted each time.
Spermatozoon membrane integrity test
First, 20 μl of the semen sample to be tested was pipetted into 80 μl of the 6-CFDA/PI staining liquid prepared. The obtained liquid was incubated in a water bath at 37 ℃ for 10 min in the dark, and then centrifuged at 2 000 rpm for 5 min. Then, the spermatozoa were re-suspended with 40 μl of 0.01 M PBS, and 10 μl of the re-suspended spermatozoon mixture was pipetted onto a clean glass slide, which was then covered and observed under a fluorescence microscope. Whether the spermatozoon membrane was complete was distinguished according to the fluorescence color of the spermatozoa. Spermatozoa with intact membranes were stained green by 6-CFDA, while spermatozoa with damaged membranes were stained red by PI. More than 200 spermatozoa were counted each time.
Spermatozoon mitochondrial activity assay
First, 20 μl of the semen sample to be tested was pipetted into an 80 μl of ready-made MITO staining liquid. The mixture was incubated in a water bath at 37 ℃ for 20 min in the dark. Then, 500 μl of PBS buffer was added into the above stained semen, and the mixture was centrifuged at 500 g for 5 min. The supernatant was discarded, and the spermatozoa were re-suspended with 40 μl of 0.01 M PBS, and 10 μl of the re-suspended liquid was transferred to a clean slide, which was then covered and observed under a fluorescence microscope to determine whether the spermatozoa had mitochondrial activity according to whether the mitochondrial region in the middle of the spermatozoon tail was significantly stained. More than 200 spermatozoa were counted each time.
Acrosome integrity test
First, 50 μl of the semen sample to be tested was pipetted and added into a centrifuge tube containing 2 ml of 3% isothermal PVP solution, and the mixture was centrifuged at 800 g for 15 min. The supernatant was discarded, and the precipitated spermatozoa were re-suspended with PBS at 37 ℃. The spermatozoon density was adjusted to (1-2)×106/ml. Then, 30 μl of the spermatozoon suspension was smeared on a slide, dried naturally in the air, and fixed with pure methanol for 10 min. And 30 μl of FITC-PNA staining liquid was added, and the mixture was incubated at 37 ℃ for 30 min in a dark and humid environment. Next, the culture was rinsed with PBS solution, dried naturally in air, and added with a little optical brightener, and slides were made and sealed with colorless nail polish. The slides were observed with a 1 000× fluorescence microscope as soon as possible to determine the acrosome integrity rate through fluorescence observation of acrosome morphology. For each time of detection, more than 200 spermatozoa were counted per slide.
Sex-controlled separation of spermatozoa by flow cytometry after sorting
Spermatozoa with motility >55% after sorting were selected for sex-controlled separation by flow cytometry. The sorted spermatozoa were collected and diluted to 3 ml with Staining Talp, and the obtained liquid was centrifuged at 360 g for 5 min. Then, 2 ml of the supernatant was pipetted and added with 4 ml of Staining Talp containing 75 μl of Hoechst33342 fluorescent dye, and the mixture was gently rotated and mixed, and heated in a 34 ℃ water bath for 45 min. Next, an equal volume of 4% Egg-Yolk Talp and Staining Talp were added. The mixture was filtered after shaking well, and prepared for the separation on a machine.
Preparation of frozen sexed semen
The semen samples were collected on the machine and equilibrated at 4 ℃ for 90 min. Then, centrifugation was performed at 850 g for 20 min at 4 ℃, and the supernatant was discarded. The semen was diluted to 5.0×108 spermatozoa/ml with Tris-A and further diluted with an equal volume of Tris-B (by two equal volumes, 15 min apart). The diluted semen was filled into 0.25 ml polyvinyl chloride plastic thin tubes, which were arranged horizontally in parallel on a serrated freezing rack. The semen was frozen with a programmed temperature-controlled freezer, and preserved in liquid nitrogen for later use.
Thawing of frozen sexed semen
The semen to be tested was taken out from the liquid nitrogen tank, and quickly put into a 37 ℃ water bath to thaw (the time from taking out of the liquid nitrogen tank to putting to the water bath should not exceed 5 s). One minute later, the semen in the frozen semen fine tubes was transferred to 1.5 ml centrifuge tubes preheated at 37 ℃, and incubated in a 37 ℃ water bath. Meanwhile, the motility, deformity rate, membrane integrity, mitochondrial activity and acrosome integrity rate of frozen sexed semen after thawing were detected.
Data analysis
Statistical analysis was performed using SAS 9.2 software, and Duncan’s method was used for multiple comparisons.
Results and Analysis
Study results of dead and live bovine spermatozoa labeled with Annexin V-FITC
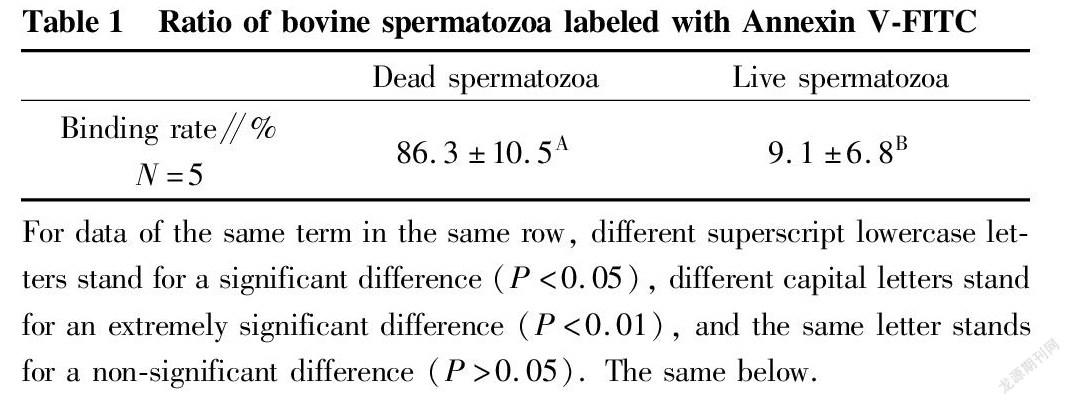
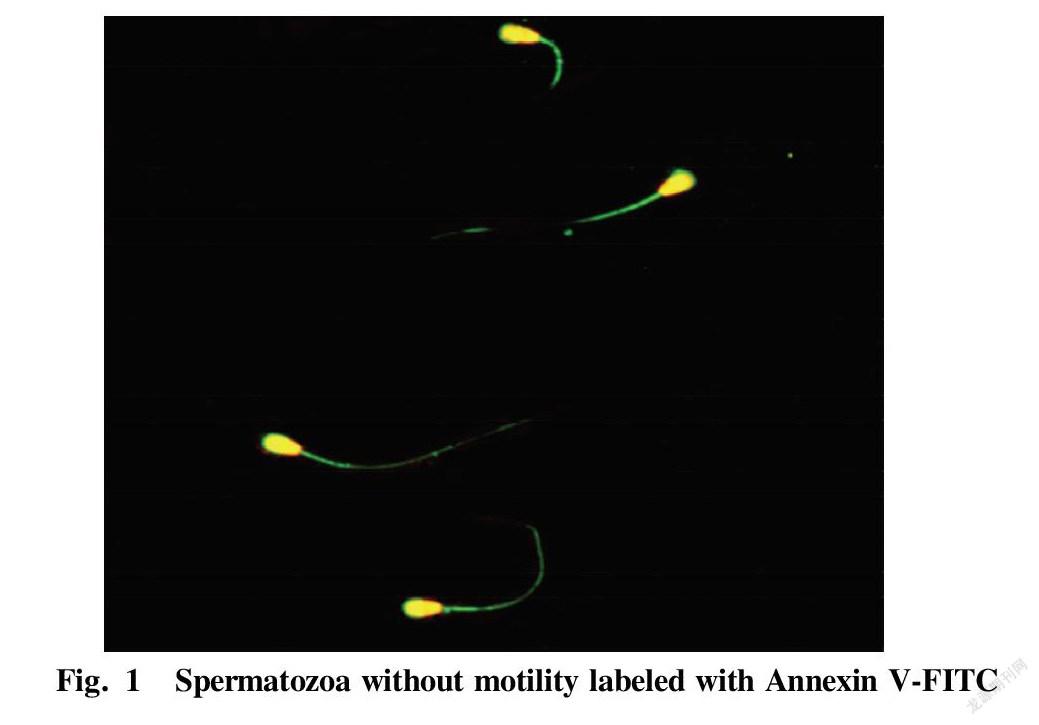
Table 1 shows the proportions of dead and live spermatozoa bound to Annexin V-FITC, respectively, after collecting dead and live spermatozoon samples by flow cytometry. The proportion of dead spermatozoa bound to Annexin V-FITC was (86.3±10.5)%, while the proportion of live spermatozoa bound to Annexin V-FITC was only (9.1±6.8)%. The statistical comparison showed an extremely significant difference (P<0.01). Fig. 1 shows the binding of dead spermatozoa to Annexin V-FITC.
For data of the same term in the same row, different superscript lowercase letters stand for a significant difference (P<0.05), different capital letters stand for an extremely significant difference (P<0.01), and the same letter stands for a non-significant difference (P>0.05). The same below.
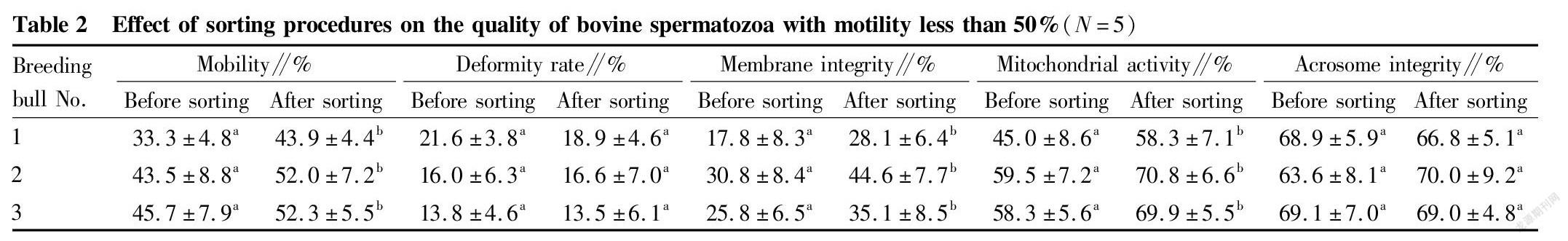
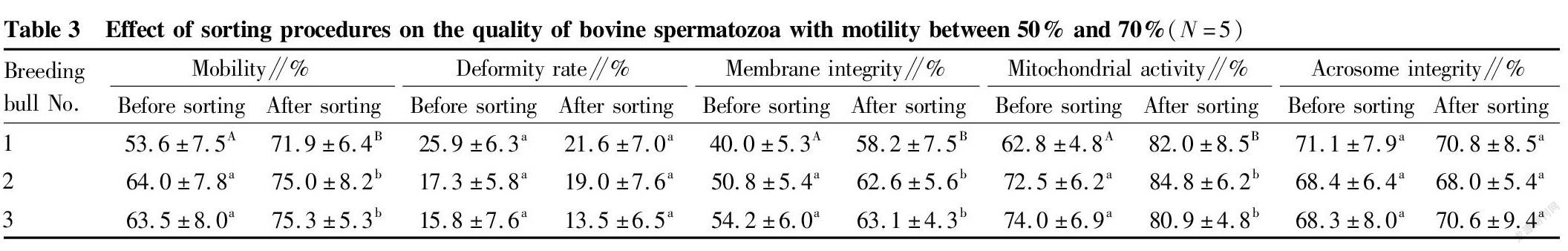
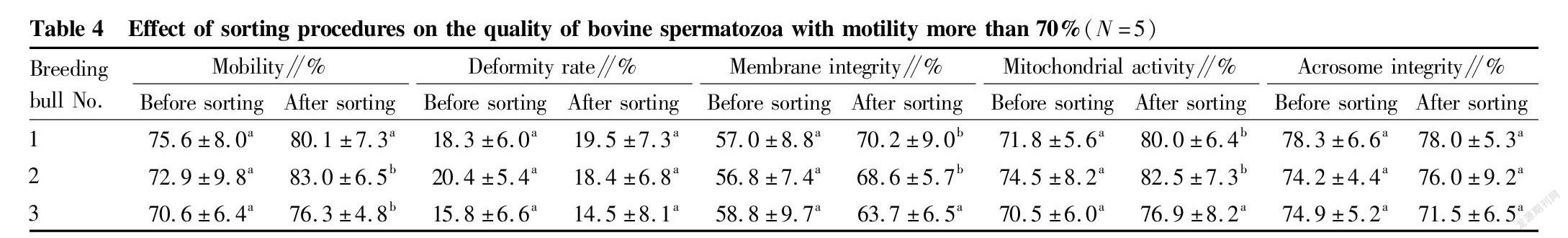
Study results of the removal of dead spermatozoa from bovine spermatozoa with different motility by immunomagnetic beads
Immunomagnetic beads combined with annexin V were used to sort bovine spermatozoa with different motility. The changes in spermatozoon quality after sorting are shown in Tables 2, 3 and 4. After sorting, the motility of bovine spermatozoa with different motility ranges in the three groups were improved, and most of them showed significant differences (P<0.05). Bull No. 1 in the 50%-70% group had an extremely significant increase in motility after sorting (P<0.01); and the change trends of spermatozoon membrane integrity and mitochondrial activity after sorting were consistent with the change trend of motility. Except bull No. 3 in the group with motility>70% before sorting, the spermatozoon membrane integrity and mitochondrial activity of other groups were significantly improved after sorting (P<0.05); and no significant changes were found in the spermatozoon deformity rate and acrosome integrity rate after sorting in the three groups. Finally, PI staining was performed on the spermatozoa adsorbed on immunomagnetic beads to confirm that the adsorbed spermatozoa were indeed positive dead spermatozoa (Fig. 2).
In summary, the method of removing dead spermatozoa from bull sperm by immunomagnetic beads combined with annexin V is effective for sperm in different motility ranges, and can effectively remove dead spermatozoa and make the spermatozoon motility, membrane integrity and mitochondrial activity after sorting improved.
Results of sex-controlled separation of dead spermatozoa by flow cytometry after immunomagnetic bead sorting of bovine spermatozoa with different mobility
Considering the effect of spermatozoon motility on the sorting efficiency of sex-controlled spermatozoon separation by flow cytometry, spermatozoa with spermatozoon motility greater than 55% after immunomagnetic bead sorting were selected for sex-controlled separation on the machine. According to the obtained experimental results, the bovine spermatozoa with initial motility of 50%-70% and >70% were selected for the next separation experiment on the machine.
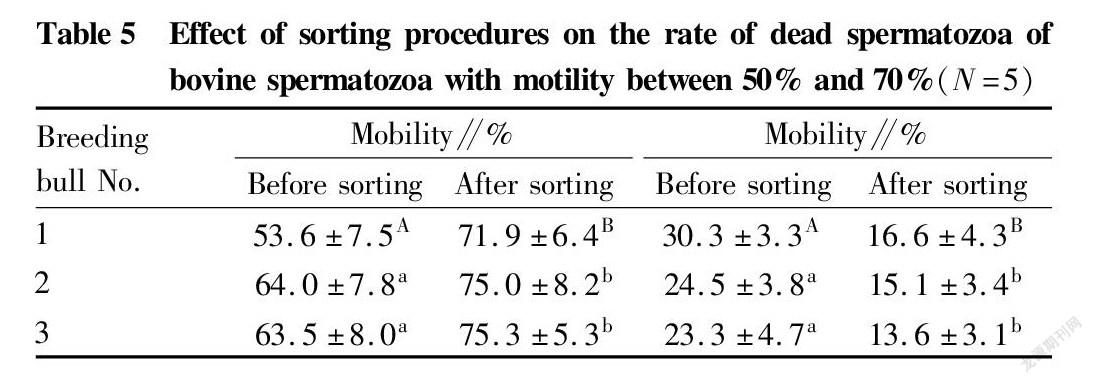
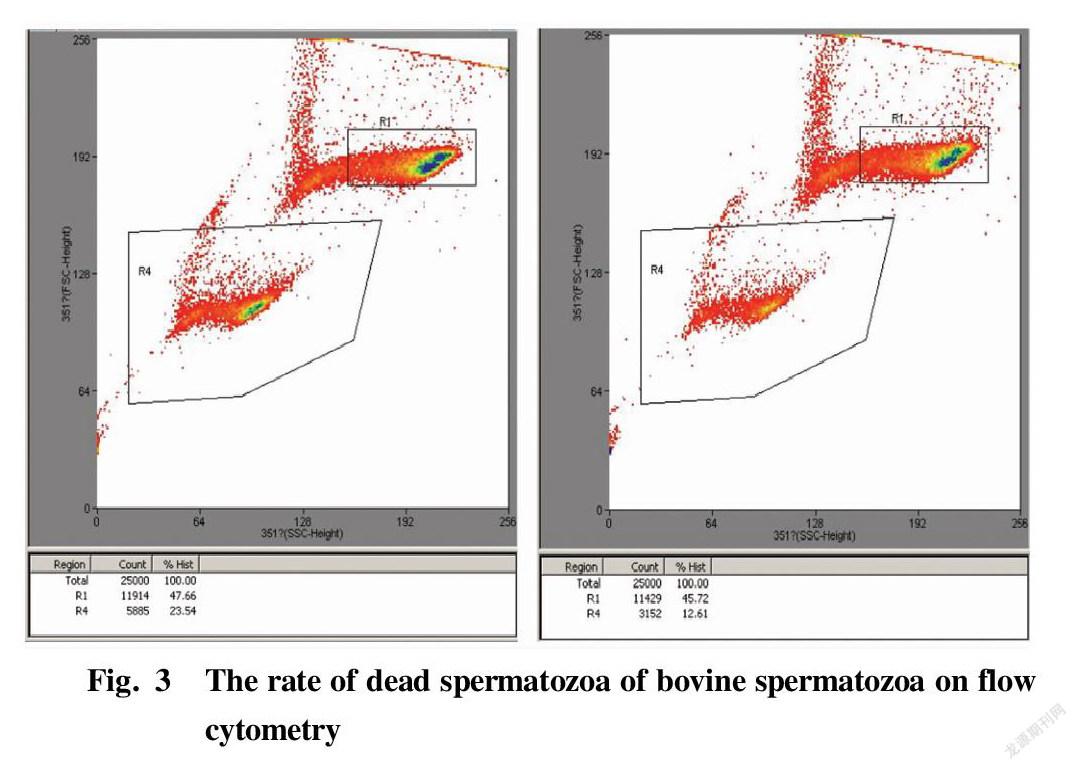
Fig. 3 shows the separation display interfaces of the flow cytometer when the spermatozoa of a bull had not been sorted (left) and when the spermatozoa of the same bull had been sorted on the machine to remove some dead spermatozoa (right), where R4 represents the death rate at the time of sorting. The results of each 5 times of separation were statistically analyzed. The rates of dead spermatozoa in bovine spermatozoa with different mobility subjected to immunomagnetic bead sorting for removing part of dead spermatozoa and separation by flow cytometry are shown in Table 5 and Table 6, and the values were significantly lower than the rates of dead spermatozoa when the samples were directly separated on the machine without sorting (P<0.05). Such effect was extremely significant in bull No. 1 in the group with mobility of 50%-70% before sorting (P<0.01).
Study results of related indexes in frozen sexed semen after sorting of bovine spermatozoa with different mobility
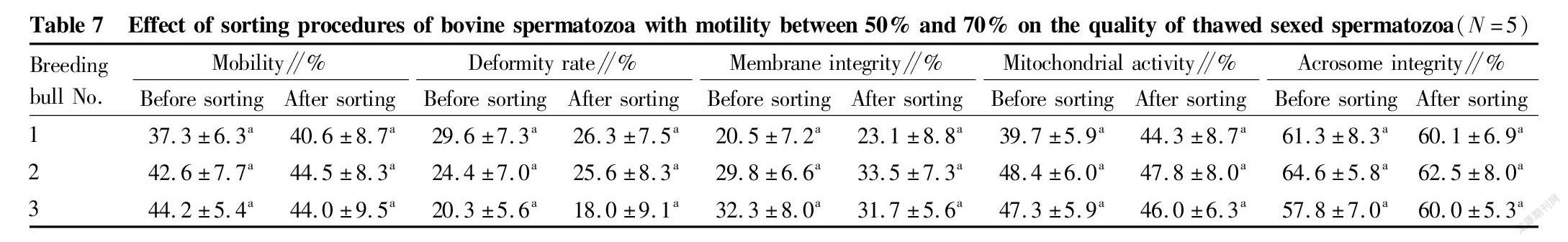
Table 7 and Table 8 show the quality changes of frozen sexed semen after removal of some dead spermatozoa by immunomagnetic bead sorting. The spermatozoon motility of frozen sexed semen prepared by removing part of dead spermatozoa did not increase significantly regardless of whether the motility before sorting was 50%-70% or >70% (P>0.05). Comparing the results without and with sorting, the spermatozoon deformity rate, membrane integrity, mitochondrial activity and acrosome integrity rate did not change significantly after removing some dead spermatozoa (P>0.05).
Conclusions and Discussion
Annexin V is a class of phospholipid-binding proteins with a molecular weight of about 35 kDa. It is an important component of the cell membrane of macrophages or other types of phagocytes, and has a high affinity with phosphatidylserine (PS)[11]. In most living eukaryotic cells, negatively charged PS exists in the cytoplasmic layer of the lipid bilayer of the cell membrane. However, in some necrotic cells, PS is exposed outside cells because it is turned from the inner side of the lipid bilayer to the outer side due to the destruction of the cytoplasmic membrane[12]. The same occurs in activated platelets[13], certain cellular abnormalities such as sickle cell anemia[14], senescent red blood cells[15], degranulated giant cells[16] and B cells at the stage of differentiation[17]. To test the applicability of this principle to bovine spermatozoa, dead and live spermatozoon samples were collected by flow cytometry to detect the proportions of dead and live spermatozoa bound to Annexin V, respectively. The results showed that the proportion of dead spermatozoa bound to Annexin V was (86.3±10.5)%, while the proportion of live spermatozoa bound to Annexin V was only (9.1±6.8)%, showing an extremely significant difference (P<0.01). It indicated that Annexin V could specifically bind dead spermatozoa, which is consistent with previous results[18-23]. However, for some dead spermatozoa that were not specifically stained by Annexin V-FITC and a few live spermatozoa were also stained by Annexin V-FITC, we believe that the possible reason is that there were some errors in spermatozoon sorting by flow cytometry. Because the proportion of X spermatozoa or Y spermatozoa obtained by the sex-controlled separation technology of flow cytometry based on different amounts of DNA carried by X spermatozoa and Y spermatozoa is not 100%, more than 90% can meet the sorting requirements in most species[24]. It shows that there is a certain error in the accuracy of flow cytometry sorting technology itself, resulting in that the proportions of dead and live spermatozoa after sorting did not reach the ideal 100%, so the results and expectations had a certain deviation.
Several unconventional methods have recently become increasingly accepted for spermatozoon sorting to improve spermatozoon quality[25]. Although great progress has been made in spermatozoon sorting technology, the sorting effect is still not an ideal target[26]. The immunomagnetic bead technology used in this study has been widely used to separate various types of cells from cell mixtures[27-28], and meanwhile, based on the principle of binding antibodies to sperm-specific antigens, immunomagnetic beads have also been used to specifically isolate spermatozoa from cell mixtures[29-30]. In this study, the use of a sorting strategy combining paramagnetic magnetic beads with the marker (Annexin V) that specifically recognizes dead spermatozoa to remove dead spermatozoa in bovine spermatozoa, reduced the rate of dead spermatozoa in original spermatozoa, thereby improving the efficiency of sex-controlled spermatozoon separation by flow cytometry, which is of great significance for reducing the production cost of frozen sexed semen. At present, the principle of sorting X spermatozoa or Y spermatozoa by high-speed flow cytometry is to use Hoechst 33342 dye that specifically labels DNA to stain the spermatozoon DNA, and then detect spermatozoon fluorescence intensity according to the differences in DNA contents of X spermatozoa or Y spermatozoa by flow cytometry, which present two groups, high and low, of which the group with high fluorescence intensity is X spermatozoa, and the group with low fluorescence intensity is Y spermatozoa. Based on this, the DNA content of each spermatozoon must be measured by fluorescence intensity, resulting in a relatively fixed total number of spermatozoa that does not exceed 200 million per hour per machine. Meanwhile, in order to ensure the mobility of spermatozoa after sorting, dead spermatozoa also need to be removed by a special staining method during the sorting process, while the separation method based on the staining of dead and live spermatozoa into different colors also requires discrimination and sorting by flow cytometry. Therefore, there is a trade-off between the two. With the increase of dead spermatozoa, the separation efficiency of target spermatozoa will decrease accordingly, and vice versa, the separation efficiency of target sperm will be improved.
It was found by comparing the changes of the motility of bovine spermatozoa before and after sorting with immunomagnetic beads combined with Annexin V and the change in the rate of dead spermatozoa during sex-controlled separation that the effects of sorting on spermatozoa in different motility ranges tended to be consistent. The mobility after sorting and the rate of dead spermatozoa during the sex-controlled separation on the machine were improved to different extents, indicating that the sorting strategy could effectively remove some dead spermatozoa and reduce the rate of dead spermatozoa during sex-controlled separation on the machine. For the differences between different bull individuals, sorting also had the effects of reducing the rate of dead spermatozoa and improving spermatozoon motility, but the increases were different. Most individuals showed a significant increase in spermatozoon motility after sorting (P<0.05), but no significant improvement was found in bull No. 1 in the group with motility>70% before sorting (P>0.05), and there was an extremely significant improvement (P<0.01) in bull No. 1 in the group with motility in the range of 50%-70%. According to the experimental results, it was speculated that the reason for the lack of significant improvement was that the spermatozoa of the bulls were relatively high in their own mobility before sorting, their initial mobility had reached (75.6±8.0)%, and the effect of later sorting optimization was not as obvious as other groups. For bull No. 1 in the group with mobility in the range of 50%-70% before sorting, the spermatozoon motility changed significantly after sorting. It was speculated that the reason might be that when the initial motility of spermatozoa was between 50% and 60%, and the increase in motility was greater than when the mobility was above 60%. When the initial mobility was less than 50%, their own mobility was low, indicating that there might be some developmental defects and they were relatively weak to external resistance, and then after a series of sorting operations, some spermatozoon damage would inevitably lead to a decrease in mobility, and thus reduced the effect of the part after sorting. In conclusion, we speculate that the optimal initial spermatozoon motility for the sorting method of immunomagnetic beads combined with Annexin V for removing dead spermatozoa is 50%-60%, and the effect after sorting is the best. The change in the rate of dead spermatozoa during the later sexed separation on the machine also supports this speculation.
In conclusion, the immunomagnetic bead sorting method combined with Annexin V can effectively remove dead spermatozoa from bovine spermatozoa, thereby reducing the rate of dead spermatozoa and providing a basis for ultimately improving the separation efficiency of target spermatozoa.
The sorted bovine spermatozoa were further subjected to sex-controlled separation, and then the spermatozoa were collected and prepared into frozen sexed semen. Considering the separation effect and efficiency of flow cytometry on X and Y spermatozoa in practical production applications, only bovine spermatozoa with an initial mobility of 50%-70% and >70% were selected for sex-controlled separation on a machine. By testing the indicators of the prepared frozen sexed semen, it was found that compared with the frozen sexed semen prepared directly without immunomagnetic bead sorting in the previous stage, there were no significant changes in all the indicators (P>0.05), which was inconsistent with the change trends after immunomagnetic bead sorting. The sorting operation had a certain improvement on spermatozoon motility, membrane integrity and mitochondrial activity before separation on the machine. However, the rate of dead spermatozoa was significantly reduced after sorting (P<0.05). It showed that the immunomagnetic bead sorting operation in the early stage played a role in removing dead spermatozoa in part of the later sex-controlled separation by flow cytometry. At the beginning of the design of sex-controlled separation by flow cytometry, in order to ensure the activity of target spermatozoa after sorting, it was also necessary to remove dead spermatozoa during the sorting process. Therefore, the part of dead spermatozoa in the bovine spermatozoa that had not been sorted in the early stage were also removed during the sexed separation on a machine, resulting in no obvious change in the quality of the frozen sexed semen prepared in the later stage. It shows that immunomagnetic bead sorting before sex-controlled separation on a machine is the premise to improve the efficiency of sexed separation by flow cytometry for target spermatozoa, especially for bovine spermatozoa with an initial motility between 50% and 60%. By comparing these two methods of spermatozoon removal, flow cytometry based on individual judgment and screening of each spermatozoon was more accurate, because there were still a certain percentage of dead spermatozoa from the bovine spermatozoa after immunomagnetic bead sorting. Therefore, future research needs to further optimize the immunomagnetic bead sorting process to achieve a more rational sorting effect.
References
[1] AMANN RP. Issues affecting commercialization of sexed sperm[J]. Theriogenology, 1999(52): 1441-1457.
[2] SEIDEL GE JR. Sexing mammalian spermatozoa and embryos-state of the art[J]. J Reprod Fertil, 1999(suppl 54): 475-485.
[3] RATH D, JOHNSON LA, DOBRINSKY JR, et al. Production of piglets preselected for sex following in vitro fertilization with X and Y chromosome-bearing spermatozoa sorted by flow cytometry[J]. Theriogenology, 1996(47): 795-800.
[4] LINDSEY AC, MORRIS LHA, ALLEN WR, et al. Hysteroscopic insemination of fresh and frozen unsexed and sexed equine spermatozoa. In: Proceedings of the 5th International Symposium on Equine Embryo Transfer. Saari, Finland, 2000.
[5] DE VRIES A, OVERTON M, FETROW, et al. Exploring the impact of sexed semen on the structure of the dairy industry[J]. J Dairy Sci, 2008(91): 847-856.
[6] SCHENK JL, CRAN DG, EVERETT RW, et al. Pregnancy rates in heifers and cows with cryopreserved sexed sperm: Effects of sperm numbers per inseminate, sorting pressure and sperm storage before sorting[J]. Theriogenology, 2009(71): 717-728.
[7] SCHENK JL, SUH TK, SEIDEL GE JR. Embryo production from superovulated cattle following insemination of sexed sperm[J]. Theriogenology, 2006(65): 299-307.
[8] OOSTERHUIS GJ, MULDER AB, KALSBEEK-BATENBURG E, et al. Measuring apoptosis in human spermatozoa: A biological assay for semen quality[J]. Fertil Steril, 2000(74): 245-250.
[9] VERMES I, HAANEN C, STEFFENS-NAKKEN H, et al. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V[J]. J Immunol Methods, 1995(184): 39-51.
[10] GLANDER HJ, SCHALLER J. Binding of annexin V to plasma membranes of human spermatozoa: A rapid assay for detection of membrane changes after cryostorage[J]. Mol Hum Reprod, 1999(5): 109-115.
[11] VAN HEERDE WL, DE GROOT PG, REUTELINGSPERGER CP. The complexity of the phospholipid binding protein Annexin V[J]. Thromb Haemost, 1995(73): 172-179.
[12] KOOPMAN G, REUTELINGSPERGER CP, KUIJTEN GAM, et al. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis[J]. Blood, 1994(84): 1415-1420.
[13] THIAGARAJAN P, TAIT JF. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets[J]. J Biol Chem, 1990(265): 17420-17423.
[14] KUYPERS FA, LEWIS RA, HUA M, et al. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled Annexin V[J]. Blood, 1996(87): 1179-1187.
[15] SCHROIT AJ, ZWAAL RFA. Transbilayer movement of phospholipids in red cells and platelet membranes[J]. Biochimica et Biophysica Acta, 1991(1071): 313-329.
[16] DEMO SD, MASUDA E, ROSSI AB, et al. Quantitative measurement of mast cell degranulation using a novel flow cytometric Annexin-V binding assay[J]. Cytometry, 1999(36): 340-348.
[17] DILLON SR. Annexin V binds to positively selected B cells[J]. J Immunol, 166: 58-71.
[18] BOCHEV I, GAVRILOV P, KYURKCHIEV S, et al. 27th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE). Sperm motility improvement after magnetic-activated cell sorting in men with astheno/oligo-asthenozoospermia[J]. Hum Reprod, 2011, 26(suppl 1): i133.
[19] DE VANT RY ARRIGHI C, HERV L, CHARDONNENS D, et al. Removal of spermatozoa with externalized phosphatidylserine from sperm preparation in human assisted medical procreation: Effects on viability, motility and mitochondrial membrane potential[J]. Reprod Biol Endocrinol, 2009(7): 1-12.
[20] RAWE V, ALVARES SEDó C, URIONDO H, et al. ICSI outcome using annexin V columns to select non-apoptotic spermatozoa[J]. Fertil Steril, 2009(92 Suppl 3): S73-74.
[21] ROMANY L, GARRIDO N, FERNANDEZ JL, et al. 28th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE). Assisted reproduction results improvements due to magnetic activated cell sorting (MACS) depletion of apoptotic sperm is not mediated by sperm DNA fragmentation decrease[J]. Hum Reprod, 2012: 27(suppl 2): ii121-150.
[22] SAID TM, AGARWAL A, ZBOROWSKI M, et al. Utility of magnetic cell separation as a molecular sperm prepartion technique[J]. J Androl, 2008(29): 134-142.
[23] SAID TM, LAND JA. Effects of advanced selection methods on sperm quality and ART outcome: A systematic review[J]. Hum Reprod Updat, 2011(17): 719-733.
[24] RATH D, BARCIKOWSKI S, DE GRAAF S, et al. Sex selection of sperm in farm animals: Status report and developmental prospects[J]. Reproduction, 2013(145): 15-30.
[25] SCHULMAN JD, KARABINUS DS. Scientifi c aspects of preconception gender selection[J]. Reprod Biomed Online, 2005, 10(Suppl. 1): 111-115.
[26] HENKEL RR, SCHILL WB. Sperm preparation for ART[J]. Reprod Biol Endocrin, 2003(1): 108.
[27] COULAIS D, PANTERNE C, FONTENEAU JF, et al. Purification of circulating plasmacytoid dendritic cells using counterflow centrifugal elutriation and immunomagnetic beads[J]. Cytotherapy, 2012(14): 887-896.
[28] BJERKE T, NIELSEN S, HELGESTAD J, et al. Purification of human blood basophils by negative selection using immunomagnetic beads[J]. J Immunol Methods, 1993(157): 49-56.
[29] ANSLINGER K, BAYER B, DANILOV SM, et al. Application of sperm-specific antibodies for the separation of sperm from cell mixtures[J]. Forensic Science Int Genet Supplement Series, 2008(1): 394-395.
[30] MARSHALL P. Optimization of spermatozoa capture during the differential extraction process for STR typing with the potential for automation[J]. University of North Texas Health Science Center at Fort Worth 2002.
[31] WINDSOR DD. Mitochondrial function and ram sperm fertility[J]. Reprod Fert Develop, 1997(9): 279-284.
[32] GADEA G, MATAS C, LUCAS X. Prediction of porcine semen fertility by homologous in vitro penetration(hIVP) assay[J]. Animal Reprod Sci, 1998(54): 95-108.
[33] TARDIF S, LAFOREST JP, CORMIER N, et al. The importance of porcine sperm parameters on fertility in vivo[J]. Theriogenology, 1999(52): 447-459.
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Expression Analysis of Heat Shock Protein 70 Gene in Rice (Oryza sativa L.)
- Changes in Physiological and Biochemical Characteristics of Floral Organ
- Research Progress on Lonicera japonica Thunb. Affected by Environmental Stress
- Research Progress on Genetic Diversity of Snap Bean
- Allelopathic Effects of Cedrus deodara Needle Extracts on Seed
- Development Status and Countermeasures of Passiflora spp. Seedling Industry in Qinzhou, Guangxi

