Classification and morphology of circulating haemocytes in the razor clam Sinonovacula constricta
Dng Hi guyen , Donghong iu , Zhiyi Chen , Moxio Peng , g Thi Trn ,Jile Li ,*
a Key Laboratory of Genetic Resources for Freshwater Aquaculture and Fisheries, Shanghai Ocean University, Ministry of Agriculture, Shanghai, 201306, China
b Research Institute for Aquaculture No.1, Dinh Bang, Tu Son, Bac Ninh, 16000, Viet Nam
Keywords:Sinonovacula constricta Immune response Haemocytes Flow cytometry
ABSTRACT The razor clam Sinonovacula constricta is widely distributed in the intertidal zones and estuarine waters along the coast of western Pacific Ocean and is extensively cultured. Even though haemocytes are known to play an important role in the immune mechanisms of bivalves, these cells are poorly studied in S. constricta. We researched the morphology and immunological activities of haemocytes in S. constricta using light and electron microscopy and flow cytometry. Three major subpopulations of haemocytes were identified in the haemolymph:granulocytes, semigranulocytes, and hyalinocytes. These subpopulations were divided using flow cytometry, but not satisfactorily. Therefore, the flow cytometry findings were combined with the light and electron microscopy findings, as well as Percoll density-gradient centrifugation findings, to classify and distinguish between the cell types more effectively. The combined findings showed that granulocytes was larger cells, while semigranulocytes was smaller and more abundant. Further, granulocytes had numerous granules in the cytoplasm, semigranulocytes contained fewer and smaller granules, and hyalinocytes was smaller and less abundant with no or a few granules. Both granulocytes and semigranulocytes had greater phagocytotic activity and a higher lysosomal content than hyalinocytes. The results declared that granulocytes and semigranulocytes were the main haemocytes involved in the cellular defence mechanism in S. constricta.
1. Introduction
The razor clamSinonovacula constricta(Lamarck 1818), which is a popular cultivated bivalve mollusc, is one of the four major cultured clams in China (Niu et al., 2008).Sinonovacula constrictais widely distributed in the intertidal areas and estuarine zones of Korea, Japan,China (Wang et al., 1997) and Vietnam (Phan, 1999). In China, the production of S. constricta has increased steadily in recent years,reaching 793,708 tons and accounting for 30% of the total mudflat shellfish production in 2015 (Yuan et al., 2016). It was one of the five principal aquatic mollusc species in 2014, with a global production of 786,828 tons (FAO, 2014, p. 2014). However, in recent years, the razor clam has been also hampered by the degradation of germplasm resources and pollution as a result of high-density aquaculture, as well as pathogenic infection (Feng et al., 2010).
As an aquatic invertebrate organism,S. constrictahas an open circulatory system that is exposed to the environment. Numerous studies on the structure and function of haemocytes in bivalves have proved that they play a key role in various physiological functions including internal defence (Bayne, 1973, 1983, pp. 407–466; Sparks, 1972), shell/tissue repair (Pauley et al., 1967, 1969; Ruddell, 1971), transport of nutrients(Cappucci, 1978; Cheng et al., 1976). Haemocytes in invertebrates have been investigated since 1971 (Feng et al., 1971), but the classification of these haemocytes is still on-going. Various subpopulations of haemocytes have been described in several marine molluscs (Cheng, 1984; Ray et al., 2013). In 1983, hyalinocytes, semigranulocytes and granulocytes were first reported in the crustacean Carcinus maenas (S¨oderh¨all et al.,1983). In the mussel Mytilus edulis, leucocytes were classified as granular and agranular leucocytes in 1985 (Rasmussen et al., 1985). In bivalves, three haemocyte populations—large granular haemocytes(granulocytes), small granular haemocytes (semigranulocytes), and agranulocytes (hyalinocytes)—have been mentioned by many researchers (Fdhila et al., 2016; García et al., 2008; Parisi et al., 2008; Ray et al., 2013; 2016; Wang et al., 2012, 2017).
Although haemocyte classifications have been conducted for many bivalves, studies on haemocytes ofS. constrictaare still very limited.Until now, no research has investigated the structure, morphology,function and classification of haemocytes inS. constricta. It is important to understand the roles of haemocytes inS. constrictaand clearly define distinct cell types to better assess the immune mechanism ofS. constricta.We aim to characterize haemocytes according to their type and morphology in the adult bivalve molluscS. constricta. This is the first time the three main types of haemocytes (granulocytes, semigranulocytes, and hyalinocytes) present in the haemolymph of the razor clam have been identified and classified.
2. Materials and methods
2.1. Bivalves and haemolymph preparation
In October 2016, clams (body length, 55 ±3.6 mm; dry weight, 9.2± 0.23 g) were collected from Sanmen, Zhejiang, China. After transportation to the research laboratory, only healthy clams that exhibited no shell damage and fast reflexes were selected. The clams were housed in tanks containing water that was obtained by dissolution of aquarium sea salts in filtered water, and were closely monitored to ensure the wellbeing of selected animals. The tank conditions approximated the extreme conditions within the present natural variability in normoxic regions at the sampling site (temperature: 24.5◦C; salinity: 24.5‰; pH:8.1).Sinonovacula constrictaindividuals were allowed to acclimatize to laboratory conditions for one week before experimentation. Aeration was constantly provided during the acclimation period. The clams were fed twice a day with microalgae ofChlorellaspp. (at 9 a.m. and at 8 p.m.).
The shells were removed carefully and approximately 500 μL of haemolymph was obtained directly from the heart using a 1000 μL pipette with cut tips. The collected haemolymph was directly transferred into a 1.5 mL tube maintained at low temperature in order to minimize cell aggregation. All analyses were carried out on individual clams.
2.2. Light microscopic observation
The collected haemolymph samples were mounted on glass slides using a micropipette, air dried, fixed in methanol for 3 min, and stained with the Wright-Giemsa stain (Zhuhai Beishuo Biotech Ltd. Co.). The morphology of the stained haemocytes were observed and analysed under a Leica light microscope, and the diameters of the cell and nucleus were measured using an image analysing software (Image J 1.43u;Wayne Rasband, National Institutes of Health, USA). Counting of the haemocytes from each clam was conducted in triplicate. The nucleus/cytoplasm ratio of each type of haemocyte was calculated for 50 cells.
For determining the different haemocyte counts, at least 100 stained cells were counted and their diameters were calculated under a 1000 ×magnification to determine size differences between haemocyte morphotypes. Profiles containing a nucleus were counted, and the different cell types were classified using on size and cytoplasmic granule content.
The total haemocyte count (THC) was determined instantly by loading freshly withdrawn haemolymph into a haemocytometer and counting the haemocytes under a Leica light microscope.
2.3. Transmission electron microscopy
Haemolymph samples were collected using the same procedure as described in Section 2.1. Haemocyte pellets were fixed with 2.5% pentylene glycol at 4◦C for 2 h. The pellets were washed three times with phosphate-buffered saline for 15 min each time and then transferred to a 1% osmic acid solution for 2–3 h. Following this, the pellets were dehydrated with ethanol and acetone and embedded. Ultrathin slices of 50–80 nm thickness were sliced using a Leica A-1170 ultra-microtome and double-stained with uranyl acetate and lead citrate. These sections were observed and photographed with a JEOL 2000 transmission electron microscope (JEOL Co., Tokyo, Japan) operated at 80 kV.
2.4. Flow cytometry analysis of haemocytes
Haemocytes collected from a single clam, using the procedure described in Section 2.1, were immediately used for cell sorting in a BD Accuri C6 flow cytometer (Accuri Cytometers Inc., USA) with laser absorbance/excitation at 488/640 nm. At least 20,000 events were recorded for each sample. Both relative cell size and relative cell complexity were determined using forward scatter (FSC) and side scatter(SSC), respectively. Cellular distributions were displayed as dot plots in electronic windows. Instrument calibration was conducted using the software Cflow plus (Accuri Cytometers Inc., USA). The gates were established based on the FSC and SSC of haemocyte distribution. Each process was run at least three times. The THC of each sample was determined utilizing the flow speed and the number of events counted in 1 min. The data for THC are presented as cells/ml haemolymph.
2.5. Lysosome content
The lysosome content of haemocytes was determined using the commercial kit LysoTracker Yellow HCK-123 (1 mM in DMSO, Invitrogen). LysoTracker (1 μL) was added to 400 μL haemolymph and the aliquot was incubated in the dark at ambient temperature. After 2 h, the aliquot was put in ice to stop the reaction.
2.6. Phagocytosis
Phagocytosis was assessed using an in vitro assay with FluoSpheres carboxylate-modified microspheres with yellow-green fluorescence(diameter, 1.0 μm Invitrogen) (Gagnaire, Thomas-Guyon, et al., 2006).Haemolymph (400 μL) was incubated with 10 μL of the one-tenth diluted fluorescent microspheres at ambient temperature in the dark for 1 h. Activity was then measured as the difference between the percentage of haemocytes.
2.7. Percoll density-gradient centrifugation
A commercial Percoll solution (Pharmacia) was used for the preparation of 70%, 50%, 30% and 10% solutions with modified Alsever’s solution. Two hundred microlitres of the 90% Percoll solution was placed at the bottom of a 1.5-mL centrifuge tube, and 200 μL each of the 70%, 50%, 30% and 10% Percoll solutions were gently layered one over the other. An aliquot of 200 μL of haemolymph was layered on the top,and the tubes were centrifuged for 5 min at 4◦C at 600g. After centrifugation, the three supernatant layers located at the interface and the upper layer of the different gradients were carefully absorbed as separate samples and examined with a haemocounter under a light microscope.
2.8. Statistical analysis
One-way analysis of variance (ANOVA) and Duncan’s range test were performed to compare the morphological and functional characteristics of the different types of haemocytes. The percentage data were transformed as the arc sine of the square root before ANOVA was conducted. All results in the figures are presented as the mean ± standard error values. SPSS software (18.0) was used for statistical analyses.
3. Results
3.1. Haemocyte classification and characterization by light microscopy and flow cytometry
Under the light microscope, the razor clamS. constrictahaemocytes stained with Wright-Giemsa could be observed and classified into three major types based on their morphological features: granulocytes, semigranulocytes, and hyalinocytes (Fig. 1) (see Fig. 2).
Using flow cytometry, theS. constrictahaemocytes were separated into three clustered dot clouds (P1, P2, and P3) based on their relative size (FSC values) and granularity (SSC values). The bead plots representing small and large haemocytes were very close to each other and partly overlapped, whereas the dot plot for the bigger haemocytes could be clearly distinguished. P1 represents a group of larger cells with more complexity, while P2 and P3 represent two groups of smaller cells with similar low FSC values but dissimilar SSC values. The cell complexity of P3 was significantly higher than that of P2. The percentage of haemocyte subpopulations in P1 was 26.2%; in P2, it was 34.5%; and in P3, it was 21.50%. By linking the results of light and transmission electron microscopy described above, P1, P2, and P3 were found to represent granulocytes, semigranulocytes and hyalinocytes, respectively. No quantitative comparisons were conducted because the beads overlapped each other.
The number of circulating haemocytes (THC) in the razor clam was 5.5 ×106cells/ml.
Semigranulocytes were the most abundant haemocytes (34.5%) in the haemolymph, followed by granulocytes (26.2%) and hyalinocytes(21.5%). Granulocytes were the biggest cells (diameter: 10–12 μm) and their shape varied between round and ovoid. The cytoplasm was filled with large granules and a few small granules that sometimes obscured the nucleus almost entirely. There were fewer organelles, but some large cytoplasmic vacuoles were observed. Semigranulocytes and hyalinocytes were characterized by a smaller diameters (7–10 μm). Semigranulocytes contained many small and a few larger granules (smaller than those in the granulocytes) (Fig. 1B). They were oval or round in shape and had more euchromatin distribution and a lower nucleus/cytoplasm ratio (0.42) than hyalinocytes (0.57). The hyalinocytes contained either a few small granules or no granules and were often round in shape. Their nuclei was centered and nearly filled up the entire cell,but they contained little cytoplasm. The hyalinocytes were slightly smaller than semigranulocytes, but the difference was not considerable as per the results of flow cytometry. The hyalinocytes, which were the smallest circulating haemocytes (ranging in size from 9 to 15 μm) had the highest nucleus-to-cytoplasm ratio (about 0.57). The nucleus/cytoplasm ratio was useful for identifying haemocyte sub-types.
3.2. Transmission electron microscopy
In order to identify and confirm the three distinct cell populations,the haemocytes were evaluated under a transmission electron microscope, which revealed many specifics about the haemocytes that were not detectible in the light micrographs. Granulocytes and semigranulocytes were the most frequently recognized cells under the transmission electron microscope (Fig. 3). Two distinct granule types were observed and used to classify granulocytes and semigranulocytes.
3.3. Density-gradient centrifugation with percoll
With the purpose of improving the classification of haemocytes,S. constrictahaemocytes were separated into three distinct layers by centrifugation using Percoll. The granulocyte layer gathered at the interface between the 70% and 50% Percoll gradients and more than 80% of the cells in this layer were granulocytes, with a small proportion(less than 20%) of semigranulocytes and hyalinocytes (Fig. 4). The second layer was present between the 50% and 30% Percoll gradients,which contained 55% semigranulocytes, 40% hyalinocytes and 3%granulocytes (Fig. 4). The top layer between the 30% and 10% Percoll gradients did not have many cells and only semigranulocytes (35%) and hyalinocytes (65%) were found here (Fig. 4).
3.4. Lysosome content
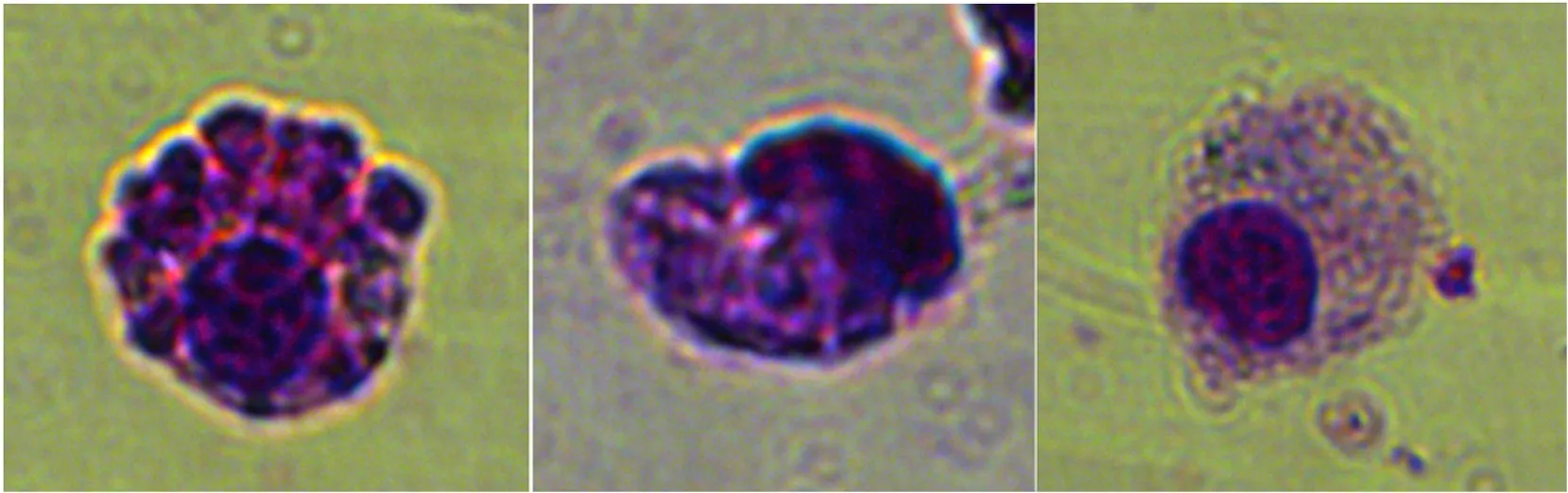
Fig. 1. Three types of haemocytes were identified: granulocytes (A), semigranulocytes (B) and hyalinocytes (C). Light microscopic observation of haemocytes from Sinonovacula constricta stained with Wright-Giemsa.
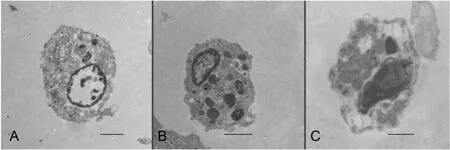
Fig. 3. Transmission electron micrograph of the three types of haemocytes identified in S. Constricta: A. Hyalinocytes. B. Semigranulocytes. C. Granulocytes. (Bar length: 2 μm).
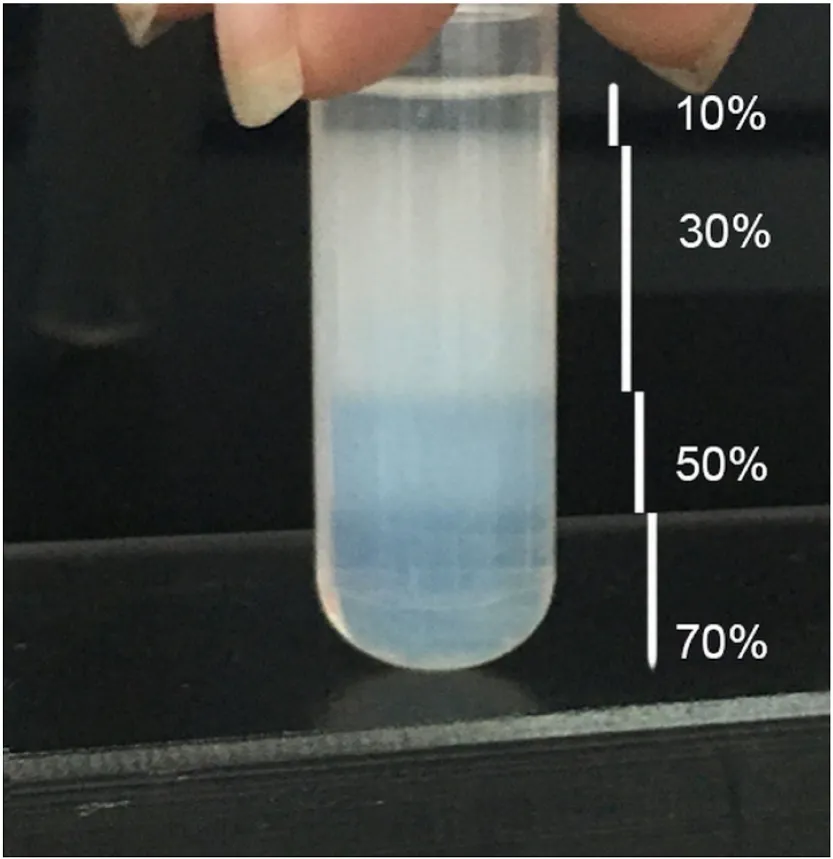
Fig. 4. Separation of haemocytes into three segments by Percoll densitygradient centrifugation.
Flow cytometry revealed that the cytoplasm ofS. constrictagranulocytes, granulocytes and hyalinocytes contained lysosomes. The lysosome content in granulocytes (412.8 A.U.) and semigranulocytes(326.1 A.U.) was significantly higher than that in hyalinocytes (59.3 A.U.) (p <0.05).
3.5. Phagocytotic capacity
As shown in the dot plots of cells that were associated or not associated with fluorescent microbeads (size vs. internal complexity, fluorescence vs. internal complexity; Fig. 5), both granulocytes and semigranulocytes displayed a certain level of phagocytic activity while hyalinocytes were less active. Further, the phagocytotic capacity of granulocytes (42%) and semigranulocytes (46%) was significantly higher than that of hyalinocytes (14%) (p <0.05).
4. Discussion
The findings from light microscopy, transmission electron microscopy, and flow cytometry analyses revealed the existence of three haemocyte subpopulations in the razor clamS. constricta. Although these haemocyte subpopulations were identified for this clam species,distinguishing between granulocytes, semigranulocytes, and hyalinocytes was difficult because of the widespread intersection between these cell types. This was also not possible using density-gradient centrifugation with Percoll, in agreement with those of Lopez et al. (L´opez,Carballal, Azevedo, & Villalba, 1997a,b). One reason might be that the cell dimensions do not relate directly with the cell density. To overcome this difficulty, the haemocyte types were distinguished based on the combined results of light and transmission electron microscopy.
The morphological classes are categorized based on granule quantity and size, and on the nucleus/cytoplasm ratio determined by light microscopy and transmission electron microscopy. In the present study,three haemocyte subpopulations in the razor clam were identified and classified: hyalinocytes, the smallest circulating haemocytes with the highest nucleus-to-cytoplasm ratio (0.57); semigranulocytes, the most numerous haemocytes with a spindle, ovoid or round shape and a nucleus-to-cytoplasm ratio of 0.42; and granulocytes, with abundant large granules in the cytoplasm and a nucleus-to-cytoplasm ratio of 0.41.The classification in this study is similar to the results of previous research on bivalves, such as Perna,Meretrixlusoria andCrassostreagigas (Barracco et al., 1999; Preziosi et al., 2016). We sub-classified the granulocytes into granulocytes (large granular granulocytes) and semigranulocytes (small granular granulocytes) based on the dimension and number of granules present. Similar results have been reported in several bivalves such asCrassostrea gigas,Mytilusedulis andRuditapesdecussates (Chang et al., 2005; L´opez et al., 1997a,b; Pipe et al., 1997).Moreover, the variety of granules identified in this study is a common feature of various bivalve species, such asMytilusgalloprovincialisand Crassostreavirginica (Carballal et al., 1997; Feng et al., 1971).
Although the terminology used for the identified haemocyte types differs slightly among authors (S¨oderh¨all et al., 1992), these three haemocyte types are usually present in invertebrates (Clare et al., 1994;Hose et al., 1990). Thus, our findings on haemocyte types inS. constrictaare in agreement with those reported in the literature.
Granulocytes contain numerous large granules in their cytoplasm,something which has been found in many bivalves (Hine, 1999). This study presents similar results, with large granules usually >1 μm in diameter and occupying most of the cytoplasm. Nuclei, when recognizable, are regularly or erratically distributed. The nucleus-to-cytoplasm ratio is about 0.41. Granulocytes are recognized as a principal storage site for prophenoloxidase and show a crucial role in vitro fungal encapsulation (Hose et al., 1990). Granulocytes and semigranulocytes could be cytotoxic and cause the lysis of foreign eukaryotic cells.
Hyalinocytes form the major population of haemocytes in the haemolymph of several invetebrates, such as the blue shrimpL. stylirostris(Le Moullac et al., 1997), the white shrimp L. vannamei (Maeda et al.,2003), and the shore crab Carcinus maenas (Ghosh et al., 1995). Hyalinocytes also represent 46% ±6% of the total haemocytes in the Sydney rock oysterSaccostrea glomerata(Aladaileh et al., 2007), but they are rare in the razor clamS. constricta, where hyalinocytes covered only 21.5% of the haemocytes. On the other hand, the semigranulocytes were the most abundant circulating haemocytes (more than 50%) in M.rosenbergii (Barracco et al., 1998), P. leniusculus (Holmblad et al.,1999), and in the spiny lobster P. argus (Li et al., 2007). A high percentage of semigranulocytes were also seen in our study, in which semigranulocytes comprised 34.5% of the circulating haemocytes fromS. constricta.
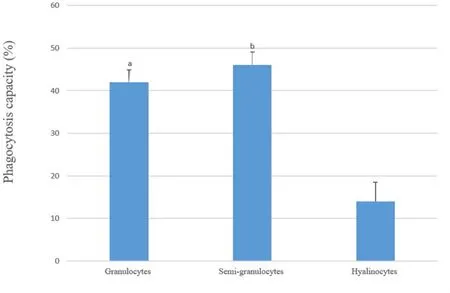
Fig. 5. Phagocytotic capacity of the three types of haemocytes obtained from the razor clam. Values are presented as mean ± SE. Different letters (a, b) in the columns represent significant (one-way ANOVA, Tukey’s test, P < 0.05) differences between the three cell types.
Lysosomes are the main bacteriolytic factor, which kill bacteria by hydrolysing components of the bacterial walls (LO´Pez et al., 1997). The lysosome content is a biomarker of the health status and vitality of the defence system in bivalves (Moore, 2002). Gagnaire et al. (Gagnaire,Thomas-Guyon, et al., 2006) hypothesized that a high number of lysosomes correlates with greater capacity of the cell to respond to an assault. The present results revealed that the lysosome content in granulocytes and semigranulocytes was significantly higher than that in hyalinocytes. These results indicate that granulocytes and semigranulocytes play a key role in the immune system of the razor clam, as supported by results forMya arenaria(Mateo et al., 2009).
Phagocytosis is the leading mechanism of internal defense in molluscs. All major cell types of all bivalve species have been shown to have phagocytic ability. Granular cells have been recorded as the most actively phagocytic in numerous species (Foley et al., 1972; Pipe et al.,1997; Tripp, 1992). In the present study, the investigated structure of granulocytes and semigranulocytes holding a number of granules and organelles in the cytoplasm was similar to that of the granular haemocytes of Ruditapes decussates (L´opez et al., 1997a,b), eosinophilic granular haemocytes in the giant clam (Nakayama et al., 1997), and acidophilic granulocytes of the pacific oyster (Auffret, 1989). Further,the transmission electron microscope images in the present study showed that granulocytes and semigranulocytes possess several granules and organelles related to phagocytosis, but there are few to none of these in hyalinocytes.
In P. viridis, both granulocytes and hyalinocytes could overwhelm the fluorescent bead, but the hyalinocytes were shown to be less active with regard to phagocytosis (Wang et al., 2012). In recent studies, all hemocytes were able to phagocytize, although the granulocytes and semigranulocytes were shown to be more active phagocytic cells than hyalinocytes. The highest percentage of semigranulocytes inS. constrictamay be responsible for its highest phagocytic proportion compared with granulocytes and hyalinocytes. In agreement with previous studies concerningCerastoderma glaucum(Matozzo et al., 2007) andArgopecten irradians(Zhang et al., 2006) semigranulocytes and granulocytes are far more phagocytic than hyalinocytes. These results are in agreement with those reported for other crustaceans such as crayfish and crab. For example, it has been shown that hyalinocytes are involved in phagocytosis and granular cells (granulocytes and semigranulocytes) are involved in incapsulation, phagocytosis, storage and release of the proPO system, and cytotoxicity (Clare et al., 1994; Johansson et al.,2000; Wang et al., 2012). These results reinforce the belief thatimmune mechanisms of haemocytes in bivalves and crustaceans are closely related.
In conclusion, this study has confirmed that granulocytes and semigranulocytes are the main circulating haemocytes in the razor clamS. constricta, and that they have many morphological and functional characteristics in common with other bivalve species. Moreover, granulocytes and hyalinocytes display differences in their metabolism and phagocytotic activity, which implies that they play different physiological roles that need to be explored in the future. Based on their relative size (FSC values) and granularity (SSC values), haemocytes were divided into three separate dot clouds, but the distinction between the clusters was not clear. Thus, methods that can help to more clearly distinguish between these cell types would be useful in the future.
5. Credit author statement
In order to publish our paper in the Aquaculture and Fisheries,manuscript ID: AAF_2017_133, title “Classification and morphology of circulating haemocytes from the razor clamSinonovacula constricta”,corresponding Author Name/Email: Jiale Li, jlli2009@126.com.
We, the Authors of this paper, certify that:
5.1. Authorship responsibility, criteria and contributions
1. The manuscript is an original and valid work and neither this paper nor one with substantial similar content under our authorship has been published or is being considered for publication elsewhere;
2. Each Author has participated (design, drafting, data acquisition, data analysis and interpretation, statistical analysis, etc.) in the work represented by this manuscript to take public responsibility for the content;
3. Each Author has given final approval for the article submission;
4. Each Author qualifies for authorship by listing his/her name;
5. All Authors have agreed to allow the corresponding author to be the primary correspondent with the editorial office.
5.2. Financial disclosure
None of the authors of this article have any conflicts of interest,including specific financial interests and relationships relevant to the subject discussed in the manuscript.
We certify that all financial and material support for this research and manuscript are clearly mentioned in an acknowledgment section to be published with the article.
Acknowledgments
This work was supported by grants from the “863” Hi-tech Research and Development Program of China (2012AA10A400-3), the National Natural Science Foundation of China (31101897), the Shanghai Universities First-class Disciplines Project of Fisheries, and the Shanghai Universities Knowledge Service Platform (ZF1206).
 Aquaculture and Fisheries2022年1期
Aquaculture and Fisheries2022年1期
- Aquaculture and Fisheries的其它文章
- A review of gynogenesis manipulation in aquatic animals
- Tilapia Lake Virus (TiLV) disease: Current status of understanding
- Potential probiotic and health fostering effect of host gut-derived Enterococcus faecalis on freshwater prawn, Macrobrachium rosenbergii
- Utility of gillnets for selectively targeting penaeids off Iran
- Refining tickler chains for penaeid trawls
- The deformation characteristics and flow field around knotless polyethylene netting based on fluid structure interaction (FSI)one-way coupling
